Going Forward: Potential Impact of Protein-Based COVID-19 Vaccination Coverage on Population Outcomes and Costs in the United States
Abstract
1. Introduction
2. Materials and Methods
2.1. Modeling Approach
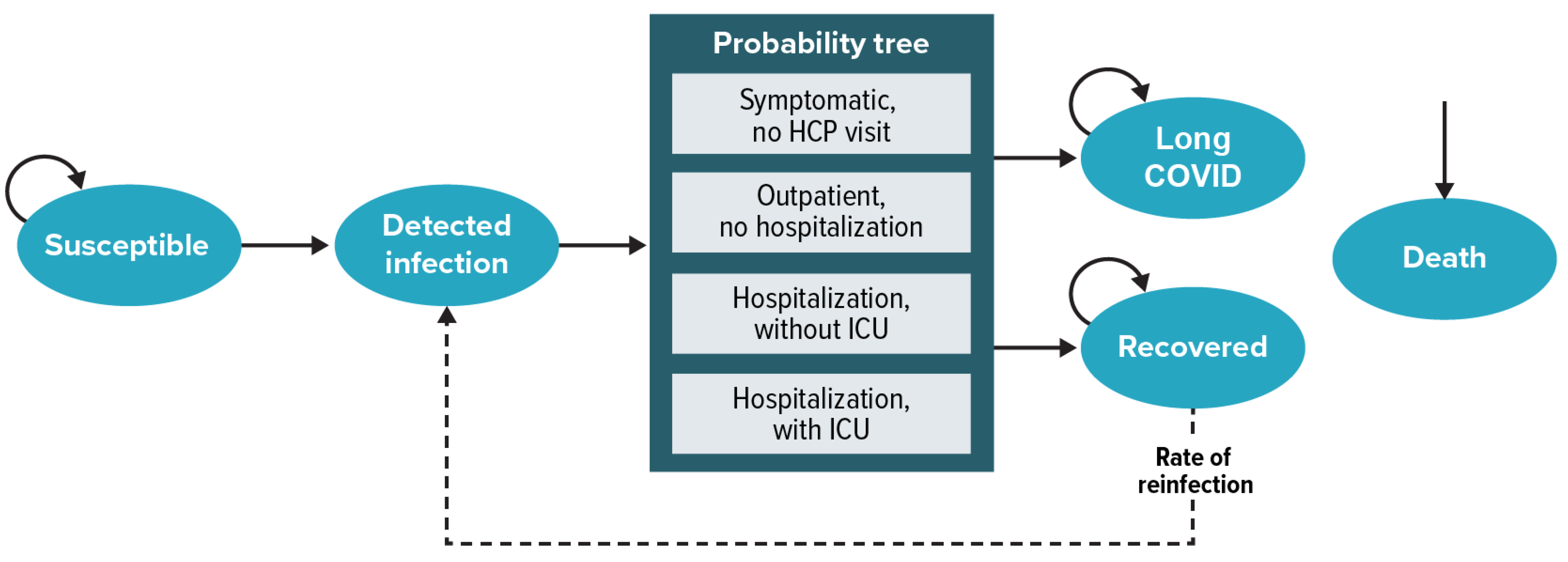
2.2. Model Structure
2.3. Model Parameters
2.3.1. Epidemiology
| 12–17 Years | 18–64 Years, Low Risk | 18–64 Years, High Risk | ≥65 Years | Sources and Notes | |
|---|---|---|---|---|---|
| Eligible population | |||||
| No. of persons per group (%) | 23,803,103 (8.6%) | 188,455,975 (67.7%) | 12,456,578 (4.5%) | 53,624,155 (19.3%) | Derived from [1,22] |
| Weekly probability of detected infection (annualized) | |||||
| Base case (post-Omicron dominance (December 2021–April 2023)) | 0.2341% (11.5%) | Derived from [19]; additional details provided in Table S1 | |||
| Low (most recent year of available data (May 2022–April 2023)) | 0.1384% (7.0%) | ||||
| High (first year post-Omicron dominance (December 2021–November 2022)) | 0.2971% (14.3%) | ||||
| COVID-19 severity distribution by highest level of care required | |||||
| Hospitalization, with ICU | 0.10% | 0.40% | 2.10% | 2.10% | Derived from [20,21,23] |
| Hospitalization, without ICU | 0.60% | 2.40% | 10.50% | 10.50% | |
| Outpatient, no hospitalization | 31.60% | 30.40% | 54.20% | 54.20% | |
| Symptomatic, no HCP visit | 67.70% | 66.80% | 33.20% | 33.20% | |
| COVID-19 mortality probability per event by highest level of care required | |||||
| Hospitalization, with ICU | 0.50% | 2.20% | 5.60% | 5.60% | Derived from [21]; differentiation by ICU status not available |
| Hospitalization, without ICU | 0.50% | 2.20% | 5.60% | 5.60% | |
| Outpatient, no hospitalization | 0.00% | 0.00% | 0.00% | 0.00% | |
| Symptomatic, no HCP visit | 0.00% | 0.00% | 0.00% | 0.00% | |
| Long COVID proportion of cases (duration) | 7.2% (remainder of model time horizon) | [24] | |||
| Effectiveness against infection, all 2023–2024 COVID-19 vaccines | 56.0% | [5]; assumption | |||
| Monthly waning rate | 12.8% | Derived from [25] | |||
| Effectiveness against hospitalization, all 2023–2024 COVID-19 vaccines | 73.0% | [5]; assumption | |||
| Monthly waning rate | 6.0% | Derived from [26] | |||
| Vaccine coverage in eligible population | |||||
| Without updated protein-based COVID-19 vaccine in mix a | 7.56% | 14.47% | 42.44% | 42.44% | [27] |
| With updated protein-based COVID-19 vaccine in mix a | 9.51% | 18.20% | 53.37% | 53.37% | Assumption |
2.3.2. Vaccine Effectiveness and Coverage
2.3.3. Costs and Health-Related Quality of Life
| Input Parameter | Baseline Value | Sources and Notes |
|---|---|---|
| Direct costs per case | ||
| Hospitalization, with ICU | USD 37,429 | [31] |
| Hospitalization, without ICU | USD 13,282 | [30] |
| Outpatient, no hospitalization | USD 282 | Derived from [32] |
| Symptomatic, no HCP visit | USD 0 | Assumption |
| Average daily cost of lost productivity | ||
| 12–17 years | USD 0 | Assumption |
| 18–64 years (low and high risk) | USD 98.95 | Derived from data on income by age [33] |
| ≥65 years | USD 25.43 | |
| COVID-19–related disutility (duration) | ||
| Hospitalization, with ICU | 0.55 (22 days) | [34] |
| Hospitalization, without ICU | 0.30 (17 days) | |
| Outpatient, no hospitalization | 0.19 (10 days) | |
| Symptomatic, no HCP visit | 0.19 (10 days) | |
| Vaccine WAC price (CDC cost) | ||
| Spikevax (Moderna) | USD 128 (USD 81.60) | Adult COVID-19 Vaccine Price List [28] |
| Comirnaty (Pfizer) | USD 115 (USD 85.10) | |
| Novavax COVID-19 vaccine, adjuvanted (2023–2024 formula) | USD 130 (USD 58.00) | |
| Outcomes due to vaccination | ||
| Proportion with missed work (duration) | 40.9% (0.575 days) | Derived from [35] |
| Disutility (duration) | 0.04 (0.575 days) | Disutility derived from [36] |
| Discounted QALYs lost due to COVID-19 death | ||
| 12–17 years | 24.8 | Derived from [37,38] following the life table method [39] with a 3% discount rate [40] |
| 18–64 years (low and high risk) | 17.9 | |
| ≥65 years | 8.3 | |
| Long COVID | ||
| Disutility (duration) | 0.19 (up to 1 year) | Assumption |
| Direct costs per week | USD 51.60 | [41] |
| Total lost productivity | USD 1100 | [42] |
2.4. Model Outcomes and Analysis
2.4.1. Base Case Analysis
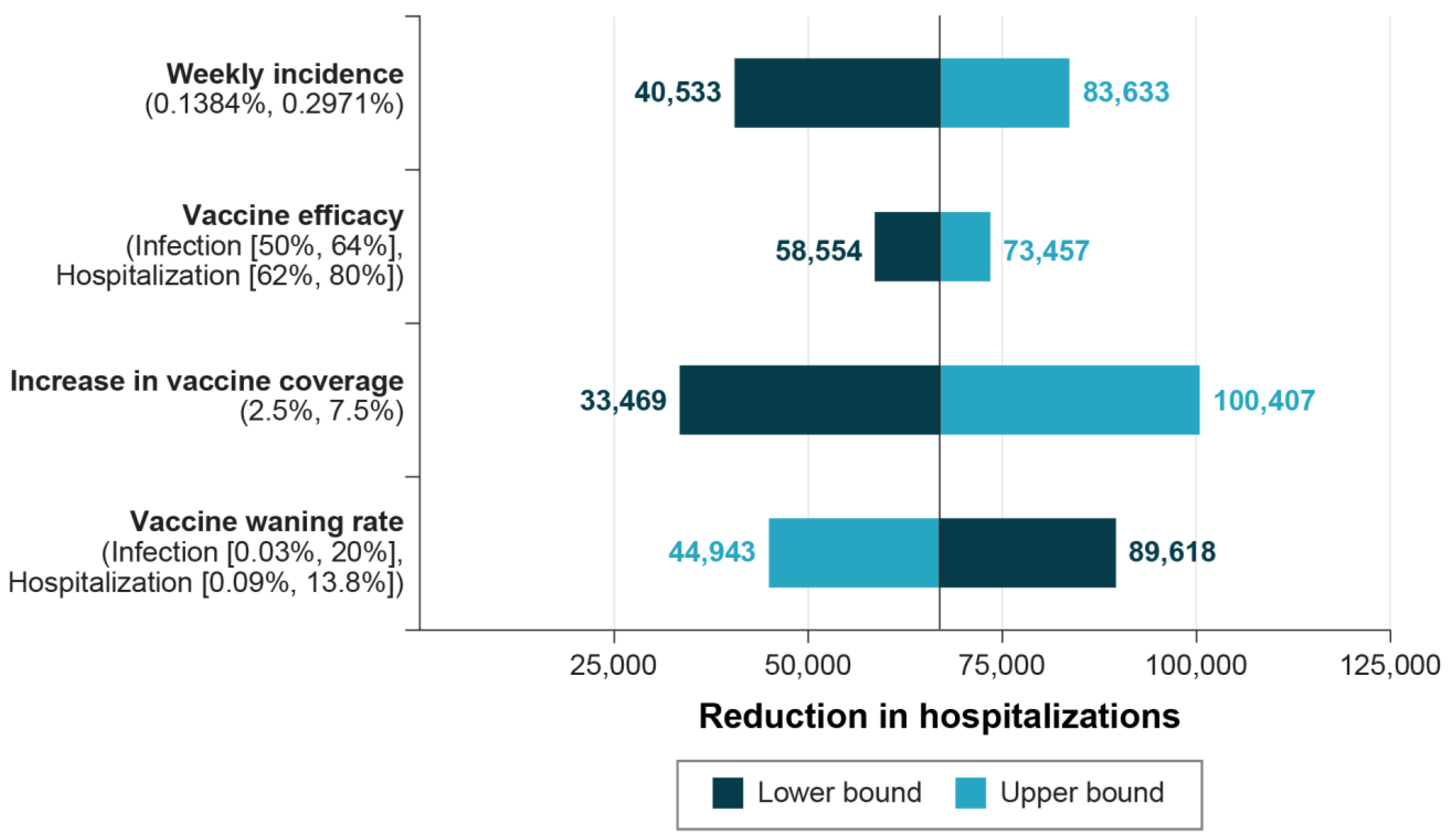
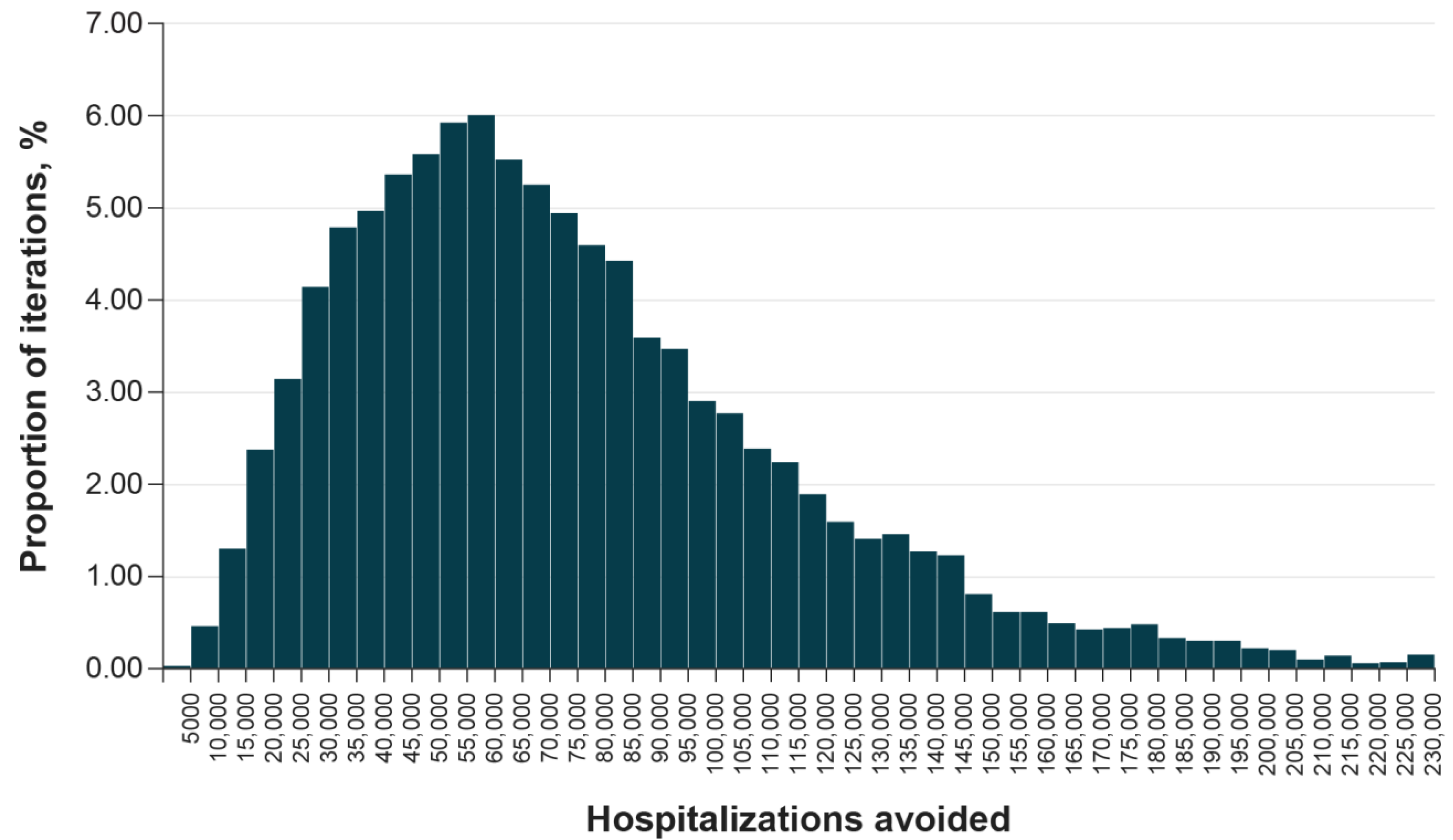
2.4.2. Sensitivity Analyses
3. Results
3.1. Base Case Results
3.2. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US Census Bureau. Annual Estimates of the Resident Population for Selected Age Groups by Sex for the United States: April 1, 2020 to July 1, 2021. 2023. Available online: https://www.census.gov/topics/population/age-and-sex/data/tables.2021.List_897222059.html#list-tab-List_897222059 (accessed on 6 October 2023).
- Centers for Disease Control and Prevention. End of the Federal COVID-19 Public Health Emergency (PHE) Declaration. 2023. Available online: https://www.cdc.gov/coronavirus/2019-ncov/your-health/end-of-phe.html (accessed on 6 October 2023).
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef]
- Maqbool, I.; Riaz, M.; Siddiqi, U.I.; Channa, J.A.; Shams, M.S. Social, economic and environmental implications of the COVID-19 pandemic. Front. Psychol. 2023, 13, 898396. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Recommendation for the 2023–2024 formula of COVID-19. 2023. Available online: https://www.fda.gov/media/169591/download (accessed on 6 October 2023).
- Centers for Disease Control and Prevention. Updated COVID-19 Vaccine Recommendations Are Now Available. 2023. Available online: https://www.cdc.gov/respiratory-viruses/whats-new/covid-vaccine-recommendations-9-12-2023.html (accessed on 6 October 2023).
- US Food and Drug Administration Press Release. FDA Takes Action on Updated mRNA COVID-19 Vaccines to Better Protect against Currently Circulating Variants. 2023. Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-action-updated-mrna-covid-19-vaccines-better-protect-against-currently-circulating (accessed on 6 October 2023).
- US Food and Drug Administration Press Release. FDA Authorizes Updated Novavax COVID-19 Vaccine Formulated to Better Protect against Currently Circulating Variants. 2023. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-updated-novavax-covid-19-vaccine-formulated-better-protect-against-currently (accessed on 6 October 2023).
- Mackey, K.; Ayers, C.K.; Kondo, K.K.; Saha, S.; Advani, S.M.; Young, S.; Spencer, H.; Rusek, M.; Anderson, J.; Veazie, S. Racial and ethnic disparities in COVID-19–related infections, hospitalizations, and deaths: A systematic review. Ann. Intern. Med. 2021, 174, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.; Jin, L.; Kim, D.; Kim, J.; Teo, Y.Y.; Ho, T.-H. Assessing the impact of novelty and conformity on hesitancy towards COVID-19 vaccines using mRNA technology. Commun. Med. 2022, 2, 61. [Google Scholar] [CrossRef]
- Kutasi, K.; Koltai, J.; Szabó-Morvai, Á.; Röst, G.; Karsai, M.; Biró, P.; Lengyel, B. Understanding hesitancy with revealed preferences across COVID-19 vaccine types. Sci. Rep. 2022, 12, 13293. [Google Scholar] [CrossRef]
- Holm, M.R.; Poland, G.A. Critical aspects of packaging, storage, preparation, and administration of mRNA and adenovirus-vectored COVID-19 vaccines for optimal efficacy. Vaccine 2021, 39, 457–459. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Wyka, K.; White, T.M.; Picchio, C.A.; Rabin, K.; Ratzan, S.C.; Parsons Leigh, J.; Hu, J.; El-Mohandes, A. Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat. Commun. 2022, 13, 3801. [Google Scholar] [CrossRef] [PubMed]
- Izadi, R.; Hatam, N.; Baberi, F.; Yousefzadeh, S.; Jafari, A. Economic evaluation of strategies against coronavirus: A systematic review. Health Econ. Rev. 2023, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Padula, W.V.; Malaviya, S.; Reid, N.M.; Tierce, J.; Alexander, G. Economic value of treatment and vaccine to address the COVID-19 pandemic: A US cost-effectiveness and budget impact analysis. J. Med. Econ. 2021, 24, 1060–1069. [Google Scholar] [CrossRef]
- Santoli, G.; Nurchis, M.C.; Calabrò, G.E.; Damiani, G. Incremental net benefit and incremental cost-effectiveness ratio of COVID-19 vaccination campaigns: Systematic review of cost-effectiveness evidence. Vaccines 2023, 11, 347. [Google Scholar] [CrossRef]
- Joint Committee on Vaccination and Immunisation. Impact Assessment for the COVID-19 Autumn 2023 Booster Vaccination Programme. 2023. Available online: https://assets.publishing.service.gov.uk/media/650ade0f52e73c001254dc08/covid-19-autumn-2023-impact-assessment.pdf (accessed on 8 November 2023).
- Prosser, L. Economic analysis of COVID-19 vaccination. In Proceedings of the Meeting of the Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention, Atlanta, GA, USA, 12 September 2023; Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-12/06-COVID-Prosser-508.pdf (accessed on 6 October 2023).
- Centers for Disease Control and Prevention. Weekly United States COVID-19 Cases and Deaths by State. 2023. Available online: https://data.cdc.gov/Case-Surveillance/Weekly-United-States-COVID-19-Cases-and-Deaths-by-/pwn4-m3yp (accessed on 6 October 2023).
- Li, R.; Liu, H.; Fairley, C.K.; Zou, Z.; Xie, L.; Li, X.; Shen, M.; Li, Y.; Zhang, L. Cost-effectiveness analysis of BNT162b2 COVID-19 booster vaccination in the United States. Int. J. Infect. Dis. 2022, 119, 87–94. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Disease Severity among Hospitalized Patients. 2023. Available online: https://covid.cdc.gov/covid-data-tracker/#hospitalizations-severity (accessed on 22 September 2023).
- Patel, M.; Chen, J.; Kim, S.; Garg, S.; Flannery, B.; Haddadin, Z.; Rankin, D.; Halasa, N.; Talbot, H.K.; Reed, C. Analysis of MarketScan data for immunosuppressive conditions and hospitalizations for acute respiratory illness, United States. Emerg. Infect. Dis. 2020, 26, 1720. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, S.M.; Ferguson, M.C.; McKinnell, J.A.; O’Shea, K.J.; Wedlock, P.T.; Siegmund, S.S.; Lee, B.Y. The potential health care costs and resource use associated with COVID-19 in the United States. Health Aff. 2020, 39, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Montoy, J.C.C. Prevalence of symptoms ≤ 12 months after acute illness, by COVID-19 testing status among adults—United States, December 2020–March 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Menegale, F.; Manica, M.; Zardini, A.; Guzzetta, G.; Marziano, V.; d’Andrea, V.; Trentini, F.; Ajelli, M.; Poletti, P.; Merler, S. Evaluation of waning of SARS-CoV-2 vaccine–induced immunity: A systematic review and meta-analysis. JAMA Netw. Open 2023, 6, e2310650. [Google Scholar] [CrossRef]
- Wu, N.; Joyal-Desmarais, K.; Ribeiro, P.A.; Vieira, A.M.; Stojanovic, J.; Sanuade, C.; Yip, D.; Bacon, S.L. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: Findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 2023, 11, 439–452. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Summary of Historical COVID-19 Vaccinations in the United States as of May 10, 2023. 2023. Available online: https://covid.cdc.gov/covid-data-tracker/#vaccination-states-jurisdictions (accessed on 6 October 2023).
- Centers for Disease Control and Prevention. Adult COVID-19 Vaccine Price List. 2023. Available online: https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html (accessed on 22 September 2023).
- Institute for Clinical and Economic Review. Special Assessment of Outpatient Treatments for COVID-19. Draft Evidence Report. 2022. Available online: https://icer.org/wp-content/uploads/2021/08/ICER_COVID_19_Draft_Evidence_Report_020322.pdf (accessed on 16 February 2023).
- Sheinson, D.; Dang, J.; Shah, A.; Meng, Y.; Elsea, D.; Kowal, S. A cost-effectiveness framework for COVID-19 treatments for hospitalized patients in the United States. Adv. Ther. 2021, 38, 1811–1831. [Google Scholar] [CrossRef]
- Kohli, M.; Maschio, M.; Becker, D.; Weinstein, M.C. The potential public health and economic value of a hypothetical COVID-19 vaccine in the United States: Use of cost-effectiveness modeling to inform vaccination prioritization. Vaccine 2021, 39, 1157–1164. [Google Scholar] [CrossRef]
- Tsai, Y.; Vogt, T.M.; Zhou, F. Patient characteristics and costs associated with COVID-19–related medical care among Medicare fee-for-service beneficiaries. Ann. Intern. Med. 2021, 174, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- US Department of Labor. Consumer Price Index Tables. 2021. Available online: https://www.bls.gov/cpi/ (accessed on 23 July 2021).
- Wang, W.-C.; Fann, J.C.-Y.; Chang, R.-E.; Jeng, Y.-C.; Hsu, C.-Y.; Chen, H.-H.; Liu, J.-T.; Yen, A.M.-F. Economic evaluation for mass vaccination against COVID-19. J. Formos. Med. Assoc. 2021, 120, S95–S105. [Google Scholar] [CrossRef]
- Rousculp, M.; Beyhaghi, H.; Hollis, K.; Ziemiecki, R.J.B.L. Burden and impact of reactogenicity among United States and Canadian adults receiving COVID-19 vaccines: Vaccine Impact on Productivity (VIP) Study (poster). In Proceedings of the Ninth ESWI Influenza Conference, Valencia, Spain, 17–20 September 2023. [Google Scholar]
- Fens, T.; de Boer, P.; van Puijenbroek, E.; Postma, M. Inclusion of safety-related issues in economic evaluations for seasonal influenza vaccines: A systematic review. Vaccines 2021, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Arias, E.; Xu, J. United States Life Tables, 2017. National Vital Statistics Reports. 2019. Available online: https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_07-508.pdf (accessed on 23 July 2021).
- Janssen, B.; Szende, A. Population norms for the EQ-5D. In Self-Reported Population Health: An International Perspective Based on EQ-5D; Szende, A., Janssen, B., Cabases, J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 19–30. [Google Scholar]
- Briggs, A.H.; Goldstein, D.A.; Kirwin, E.; Meacock, R.; Pandya, A.; Vanness, D.J.; Wisløff, T. Estimating (quality-adjusted) life-year losses associated with deaths: With application to COVID-19. Health Econ. 2021, 30, 699–707. [Google Scholar] [CrossRef]
- Sanders, G.D.; Neumann, P.J.; Basu, A.; Brock, D.W.; Feeny, D.; Krahn, M.; Kuntz, K.M.; Meltzer, D.O.; Owens, D.K.; Prosser, L.A.; et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA 2016, 316, 1093–1103. [Google Scholar] [CrossRef]
- Kompaniyets, L.; Pennington, A.F.; Goodman, A.B.; Rosenblum, H.G.; Belay, B.; Ko, J.Y.; Chevinsky, J.R.; Schieber, L.Z.; Summers, A.D.; Lavery, A.M. Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, March 2020–March 2021. Prev. Chronic Dis. 2021, 18, E66. [Google Scholar] [CrossRef] [PubMed]
- Cutler, D.M. The costs of long COVID. JAMA Health Forum 2022, 3, e221809. [Google Scholar] [CrossRef]
- Caro, J.J.; Briggs, A.H.; Siebert, U.; Kuntz, K.M. Modeling good research practices—Overview: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Med. Decis. Mak. 2012, 32, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Áñez, G.; Adelglass, J.M.; Barrat Hernández, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef]
- Anzalone, A.J.; Sun, J.; Vinson, A.J.; Beasley, W.H.; Hillegass, W.B.; Murray, K.; Hendricks, B.M.; Haendel, M.; Geary, C.R.; Bailey, K.L. Community risks for SARS-CoV-2 infection among fully vaccinated US adults by rurality: A retrospective cohort study from the National COVID Cohort Collaborative. PLoS ONE 2023, 18, e0279968. [Google Scholar] [CrossRef]
- Mauskopf, J.; Talbird, S.; Standaert, B. Categorization of methods used in cost–effectiveness analyses of vaccination programs based on outcomes from dynamic transmission models. Expert Rev. Pharmacoecon. Outcomes Res. 2012, 12, 357–371. [Google Scholar] [CrossRef]
- Ferdinands, J.M.; Rao, S.; Dixon, B.E.; Mitchell, P.K.; DeSilva, M.B.; Irving, S.A.; Lewis, N.; Natarajan, K.; Stenehjem, E.; Grannis, S.J.; et al. Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: Test negative, case-control study. BMJ 2022, 379, e072141. [Google Scholar] [CrossRef]
- Regan, J.J. Use of updated COVID-19 vaccines 2023–2024 formula for persons aged ≥6 months: Recommendations of the Advisory Committee on Immunization Practices—United States, September 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.W.M.; Beinfeld, M.; Mohammed, R.; Wright, A.; Nhan, E.; Fluetsch, N.; Richardson, M.; Pearson, S.D. Special Assessment of Outpatient Treatments for COVID-19; Final Evidence Report and Meeting Summary. 2022. Available online: https://icer.org/assessment/covid-19-2022/ (accessed on 4 January 2024).
- Campbell, J.D.W.M.; Rind, D.M.; Pearson, S.D. Alternative Pricing Models for Remdesivir and Other Potential Treatments for COVID-19; Updated Report. 2020. Available online: https://icer-review.org/topic/covid-19/ (accessed on 4 January 2024).
- Gottlieb, S. The need for a US national clinical trial infrastructure in a public health crisis. JAMA Health Forum 2021, 2, e213223. [Google Scholar] [CrossRef]
- Covid Crisis Group. Lessons From the Covid War: An Investigative Report; Public Affairs: New York, NY, USA, 2023. [Google Scholar]
- Ball, P. What the COVID-19 pandemic reveals about science, policy and society. Interface Focus 2021, 11, 20210022. [Google Scholar] [CrossRef]
- Notarte, K.; Catahay, J.; Velasco, J.; Pastrana, A.; Ver, A.; Pangilinan, F.; Peligro, P.; Casimiro, M.; Guerrero, J.; Gellaco, M. Impact of covid-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. eClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef]
- Tannous, J.; Pan, A.P.; Potter, T.; Bako, A.T.; Dlouhy, K.; Drews, A.; Sostman, H.D.; Vahidy, F.S. Real-world effectiveness of COVID-19 vaccines and anti-SARS-CoV-2 monoclonal antibodies against postacute sequelae of SARS-CoV-2: Analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open 2023, 13, e067611. [Google Scholar] [CrossRef]
| Summary Results (Total in Target Population) | Mix with Updated Protein-Based Vaccine | Mix without Updated Protein-Based Vaccine | Incremental |
|---|---|---|---|
| Approximate US population | 331,893,745 | 331,893,745 | 0 |
| Number of eligible individuals | 284,481,964 | 284,481,964 | 0 |
| Number of individuals receiving vaccination | 73,558,971 | 58,485,411 | 15,073,560 |
| Health outcomes | |||
| COVID-19 cases | 30,513,657 | 31,015,349 | −501,692 |
| COVID-19 hospitalizations | 1,295,688 | 1,362,626 | −66,938 |
| COVID-19 deaths | 51,965 | 55,284 | −3319 |
| Long COVID cases | 2,136,565 | 2,171,882 | −35,317 |
| QALYs lost | |||
| Vaccine adverse events | 1905 | 1515 | 390 |
| Outpatient cases | 149,149 | 151,397 | −2248 |
| Hospitalizations | 21,716 | 22,849 | −1134 |
| COVID-19 deaths | 619,468 | 654,907 | −35,439 |
| Long COVID | 207,617 | 211,972 | −4355 |
| Total QALYs lost | 999,855 | 1,042,641 | −42,785 |
| Direct costs (in millions) | |||
| Vaccine costs | USD 9205.45 | USD 7248.10 | USD 1957.35 |
| Outpatient costs | USD 3096.97 | USD 3153.00 | USD −56.03 |
| Hospitalization costs | USD 22,142.00 | USD 23,292.80 | USD −1150.80 |
| Long COVID costs | USD 2856.82 | USD 2916.74 | USD −59.92 |
| Total direct costs | USD 37,301.23 | USD 36,610.64 | USD 690.59 |
| Indirect costs (lost productivity in millions) | |||
| Due to vaccination | USD 1144.09 | USD 909.64 | USD 234.44 |
| Due to COVID-19 | USD 14,128.26 | USD 14,394.33 | USD −266.07 |
| Due to long COVID | USD 2122.33 | USD 2159.93 | USD −37.60 |
| Total indirect costs | USD 17,394.68 | USD 17,463.91 | USD −69.23 |
| Third-party payer perspective (direct costs only) | |||
| Incremental cost per QALY gained (i.e., per QALY losses avoided) | USD 16,141 | ||
| Societal perspective (including indirect costs due to lost productivity) | |||
| Incremental cost per QALY gained (i.e., per QALY losses avoided) | USD 14,523 | ||
| Age and Risk Groups | Incremental Cases | Incremental Hospitalizations | Incremental Direct Costs (In Millions) | Incremental QALYs Lost | Incremental Cost per QALY Gained |
|---|---|---|---|---|---|
| 12–17 years | −16,037 | −206 | USD 59.60 | −237 | USD 251,338 |
| 18–64 years, low risk | −226,705 | −11,557 | USD 677.69 | −7669 | USD 88,364 |
| 18–64 years, high risk | −47,525 | −10,126 | USD −8.57 | −10,823 | Cost saving |
| ≥65 years | −211,425 | −45,049 | USD −38.13 | −24,056 | Cost saving |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paret, K.; Beyhaghi, H.; Herring, W.L.; Mauskopf, J.; Shane, L.G.; Rousculp, M.D. Going Forward: Potential Impact of Protein-Based COVID-19 Vaccination Coverage on Population Outcomes and Costs in the United States. Vaccines 2024, 12, 74. https://doi.org/10.3390/vaccines12010074
Paret K, Beyhaghi H, Herring WL, Mauskopf J, Shane LG, Rousculp MD. Going Forward: Potential Impact of Protein-Based COVID-19 Vaccination Coverage on Population Outcomes and Costs in the United States. Vaccines. 2024; 12(1):74. https://doi.org/10.3390/vaccines12010074
Chicago/Turabian StyleParet, Kyle, Hadi Beyhaghi, William L. Herring, Josephine Mauskopf, Lesley G. Shane, and Matthew D. Rousculp. 2024. "Going Forward: Potential Impact of Protein-Based COVID-19 Vaccination Coverage on Population Outcomes and Costs in the United States" Vaccines 12, no. 1: 74. https://doi.org/10.3390/vaccines12010074
APA StyleParet, K., Beyhaghi, H., Herring, W. L., Mauskopf, J., Shane, L. G., & Rousculp, M. D. (2024). Going Forward: Potential Impact of Protein-Based COVID-19 Vaccination Coverage on Population Outcomes and Costs in the United States. Vaccines, 12(1), 74. https://doi.org/10.3390/vaccines12010074






