A Recombinant Multivalent Vaccine (rCpa1) Induces Protection for C57BL/6 and HLA Transgenic Mice against Pulmonary Infection with Both Species of Coccidioides
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Culture
2.2. DNA Sequence Analysis
2.3. Mice
2.4. Vaccination Protocol, Animal Challenge, and Evaluation of Protection
2.5. Flow Cytometry Analysis
2.6. Statistical Analyses
3. Results
3.1. Variants of Vaccine Antigens among Clinical Isolates of Coccidioides
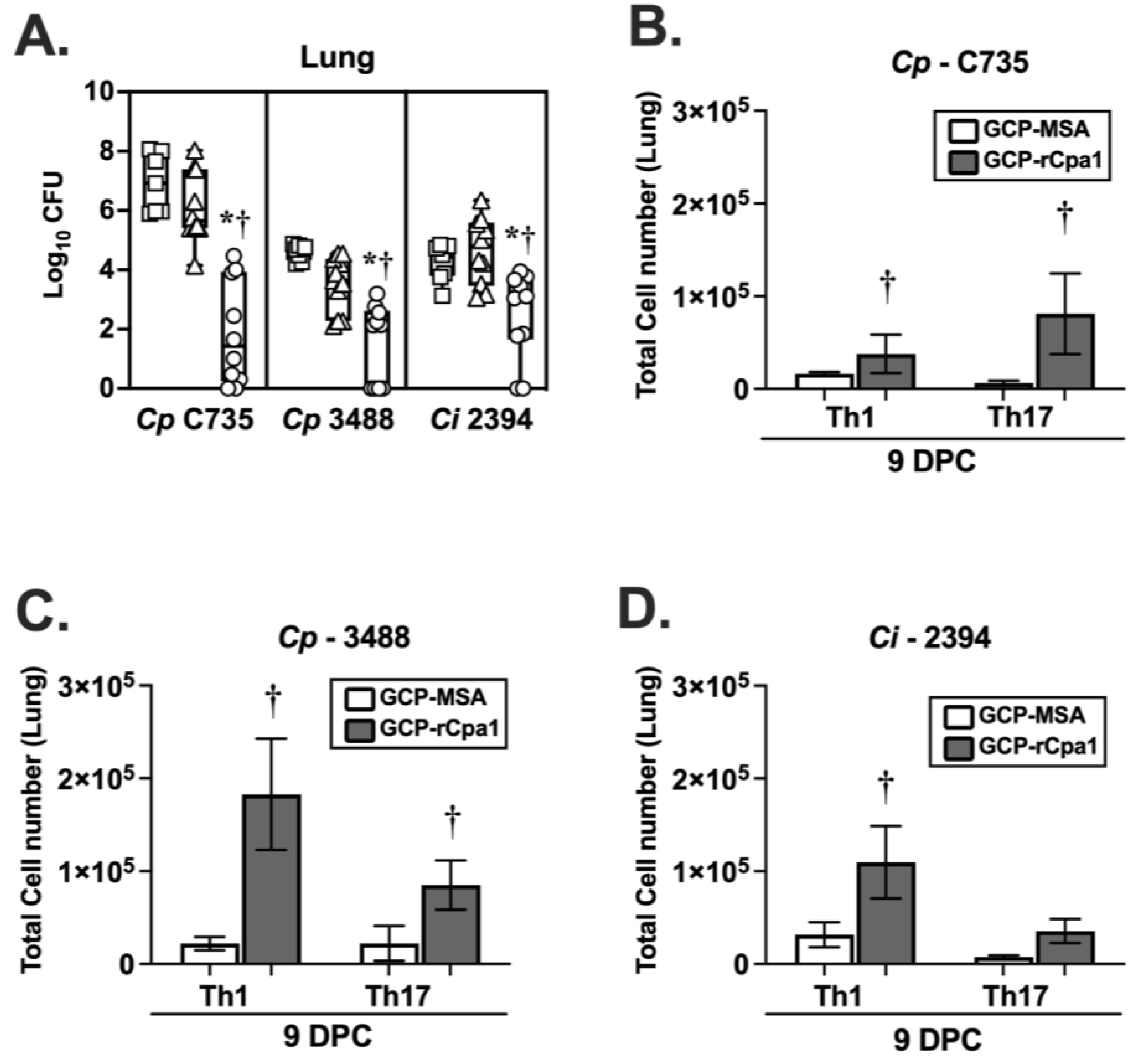
3.2. The GCP-rCpa1 Vaccine Is Protective against Multiple Clinical Isolates of Cp
3.3. GCP-rCpa1 Vaccine Cross-Protected C57BL/6 Mice against Ci Isolates
3.4. Vaccination with GCP-rCpa1 Resulted in Early Induction of a Mixed Th1 and Th17 Response but Not Th2 by Both Cp and Ci Infection
3.5. GCP-rCpa1 Vaccine Conferred Protection for HLA-DR4 Transgenic Mice against Both Cp and Ci
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirkland, T.N.; Fierer, J. Coccidioides immitis and posadasii; A review of their biology, genomics, pathogenesis, and host immunity. Virulence 2018, 9, 1426–1435. [Google Scholar] [CrossRef]
- Sun, S.H.; Cole, G.T.; Drutz, D.J.; Harrison, J.L. Electron-microscopic observations of the Coccidioides immitis parasitic cycle in vivo. J. Med. Vet. Mycol. 1986, 24, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Galgiani, J.N.; Ampel, N.M.; Blair, J.E.; Catanzaro, A.; Geertsma, F.; Hoover, S.E.; Johnson, R.H.; Kusne, S.; Lisse, J.; MacDonald, J.D.; et al. Executive Summary: 2016 Infectious Diseases Society of America (IDSA) Clinical Practice Guideline for the Treatment of Coccidioidomycosis. Clin. Infect. Dis. 2016, 63, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.A.; Magee, D.M. Coccidioidomycosis: Host response and vaccine development. Clin. Microbiol. Rev. 2004, 17, 804–839. [Google Scholar] [CrossRef] [PubMed]
- Neafsey, D.E.; Barker, B.M.; Sharpton, T.J.; Stajich, J.E.; Park, D.J.; Whiston, E.; Hung, C.Y.; McMahan, C.; White, J.; Sykes, S.; et al. Population genomic sequencing of Coccidioides fungi reveals recent hybridization and transposon control. Genome Res. 2010, 20, 938–946. [Google Scholar] [CrossRef]
- Engelthaler, D.M.; Roe, C.C.; Hepp, C.M.; Teixeira, M.; Driebe, E.M.; Schupp, J.M.; Gade, L.; Waddell, V.; Komatsu, K.; Arathoon, E.; et al. Local Population Structure and Patterns of Western Hemisphere Dispersal for Coccidioides spp., the Fungal Cause of Valley Fever. mBio 2016, 7, e00550-16. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Barker, B.M. Use of Population Genetics to Assess the Ecology, Evolution, and Population Structure of Coccidioides. Emerg. Infect. Dis. 2016, 22, 1022–1030. [Google Scholar] [CrossRef]
- Sharpton, T.J.; Stajich, J.E.; Rounsley, S.D.; Gardner, M.J.; Wortman, J.R.; Jordar, V.S.; Maiti, R.; Kodira, C.D.; Neafsey, D.E.; Zeng, Q.; et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009, 19, 1722–1731. [Google Scholar] [CrossRef]
- Yu, J.J.; Holbrook, E.; Liao, Y.R.; Zarnowski, R.; Andes, D.R.; Wheat, L.J.; Malo, J.; Hung, C.Y. Characterization of an Uncinocarpus reesii-expressed recombinant tube precipitin antigen of Coccidioides posadasii for serodiagnosis. PLoS ONE 2019, 14, e0221228. [Google Scholar] [CrossRef]
- Yu, J.J.; Kirkland, T.N.; Hall, L.K.; Wopschall, J.; Smith, R.C.; Hung, C.Y.; Chen, X.; Tarcha, E.; Thomas, P.W.; Cole, G.T. Characterization of a serodiagnostic complement fixation antigen of Coccidioides posadasii expressed in the nonpathogenic Fungus Uncinocarpus reesii. J. Clin. Microbiol. 2005, 43, 5462–5469. [Google Scholar] [CrossRef]
- McCotter, O.Z.; Benedict, K.; Engelthaler, D.M.; Komatsu, K.; Lucas, K.D.; Mohle-Boetani, J.C.; Oltean, H.; Vugia, D.; Chiller, T.M.; Sondermeyer Cooksey, G.L.; et al. Update on the Epidemiology of coccidioidomycosis in the United States. Med. Mycol. 2019, 57, S30–S40. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, T.N. The Quest for a Vaccine Against Coccidioidomycosis: A Neglected Disease of the Americas. J. Fungi 2016, 2, 34. [Google Scholar] [CrossRef]
- Ampel, N.M. Coccidioidomycosis: Changing Concepts and Knowledge Gaps. J. Fungi 2020, 6, 354. [Google Scholar] [CrossRef]
- Castro-Lopez, N.; Hung, C.Y. Immune Response to Coccidioidomycosis and the Development of a Vaccine. Microorganisms 2017, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Tarcha, E.J.; Basrur, V.; Hung, C.Y.; Gardner, M.J.; Cole, G.T. Multivalent recombinant protein vaccine against coccidioidomycosis. Infect. Immun. 2006, 74, 5802–5813. [Google Scholar] [CrossRef] [PubMed]
- Shubitz, L.F.; Yu, J.J.; Hung, C.Y.; Kirkland, T.N.; Peng, T.; Perrill, R.; Simons, J.; Xue, J.; Herr, R.A.; Cole, G.T.; et al. Improved protection of mice against lethal respiratory infection with Coccidioides posadasii using two recombinant antigens expressed as a single protein. Vaccine 2006, 24, 5904–5911. [Google Scholar] [CrossRef] [PubMed]
- Herr, R.A.; Hung, C.Y.; Cole, G.T. Evaluation of two homologous proline-rich proteins of Coccidioides posadasii as candidate vaccines against coccidioidomycosis. Infect. Immun. 2007, 75, 5777–5787. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.Y.; Zhang, H.; Castro-Lopez, N.; Ostroff, G.R.; Khoshlenar, P.; Abraham, A.; Cole, G.T.; Negron, A.; Forsthuber, T.; Peng, T.; et al. Glucan-Chitin Particles Enhance Th17 Response and Improve Protective Efficacy of a Multivalent Antigen (rCpa1) against Pulmonary Coccidioides posadasii Infection. Infect. Immun. 2018, 86, e00070-18. [Google Scholar] [CrossRef]
- Campuzano, A.; Zhang, H.; Ostroff, G.R.; Dos Santos Dias, L.; Wuthrich, M.; Klein, B.S.; Yu, J.J.; Lara, H.H.; Lopez-Ribot, J.L.; Hung, C.Y. CARD9-Associated Dectin-1 and Dectin-2 Are Required for Protective Immunity of a Multivalent Vaccine against Coccidioides posadasii Infection. J. Immunol. 2020, 204, 3296–3306. [Google Scholar] [CrossRef]
- Orsborn, K.I.; Shubitz, L.F.; Peng, T.; Kellner, E.M.; Orbach, M.J.; Haynes, P.A.; Galgiani, J.N. Protein expression profiling of Coccidioides posadasii by two-dimensional differential in-gel electrophoresis and evaluation of a newly recognized peroxisomal matrix protein as a recombinant vaccine candidate. Infect. Immun. 2006, 74, 1865–1872. [Google Scholar] [CrossRef]
- Hurtgen, B.J.; Hung, C.Y.; Ostroff, G.R.; Levitz, S.M.; Cole, G.T. Construction and evaluation of a novel recombinant T cell epitope-based vaccine against Coccidioidomycosis. Infect. Immun. 2012, 80, 3960–3974. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Koenig, G.L.; White, T.J.; Taylor, J.W. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 2002, 94, 73–84. [Google Scholar] [CrossRef]
- Hung, C.Y.; Gonzalez, A.; Wuthrich, M.; Klein, B.S.; Cole, G.T. Vaccine immunity to coccidioidomycosis occurs by early activation of three signal pathways of T helper cell response (Th1, Th2, and Th17). Infect. Immun. 2011, 79, 4511–4522. [Google Scholar] [CrossRef]
- Ito, K.; Bian, H.J.; Molina, M.; Han, J.; Magram, J.; Saar, E.; Belunis, C.; Bolin, D.R.; Arceo, R.; Campbell, R.; et al. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J. Exp. Med. 1996, 183, 2635–2644. [Google Scholar] [CrossRef]
- Hung, C.Y.; Castro-Lopez, N.; Cole, G.T. Card9- and MyD88-Mediated Gamma Interferon and Nitric Oxide Production Is Essential for Resistance to Subcutaneous Coccidioides posadasii Infection. Infect. Immun. 2016, 84, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Chen, X.; Selby, D.; Hung, C.Y.; Yu, J.J.; Cole, G.T. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect. Immun. 2009, 77, 3196–3208. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.M.; Alvarado, P.; Roe, C.C.; Thompson, G.R., 3rd; Patané, J.S.L.; Sahl, J.W.; Keim, P.; Galgiani, J.N.; Litvintseva, A.P.; Matute, D.R.; et al. Population Structure and Genetic Diversity among Isolates of Coccidioides posadasii in Venezuela and Surrounding Regions. mBio 2019, 10, e01976-19. [Google Scholar] [CrossRef]
- Wuthrich, M.; Gern, B.; Hung, C.Y.; Ersland, K.; Rocco, N.; Pick-Jacobs, J.; Galles, K.; Filutowicz, H.; Warner, T.; Evans, M.; et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Investig. 2011, 121, 554–568. [Google Scholar] [CrossRef]
- Hurtgen, B.J.; Castro-Lopez, N.; Jimenez-Alzate, M.D.P.; Cole, G.T.; Hung, C.Y. Preclinical identification of vaccine induced protective correlates in human leukocyte antigen expressing transgenic mice infected with Coccidioides posadasii. Vaccine 2016, 34, 5336–5343. [Google Scholar] [CrossRef]
- Pappagianis, D. Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. The Valley Fever Vaccine Study Group. Am. Rev. Respir. Dis. 1993, 148, 656–660. [Google Scholar] [CrossRef]
- Narra, H.P.; Shubitz, L.F.; Mandel, M.A.; Trinh, H.T.; Griffin, K.; Buntzman, A.S.; Frelinger, J.A.; Galgiani, J.N.; Orbach, M.J. A Coccidioides posadasii CPS1 Deletion Mutant Is Avirulent and Protects Mice from Lethal Infection. Infect. Immun. 2016, 84, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Shubitz, L.F.; Robb, E.J.; Powell, D.A.; Bowen, R.A.; Bosco-Lauth, A.; Hartwig, A.; Porter, S.M.; Trinh, H.; Moale, H.; Bielefeldt-Ohmann, H.; et al. Deltacps1 vaccine protects dogs against experimentally induced coccidioidomycosis. Vaccine 2021, 39, 6894–6901. [Google Scholar] [CrossRef] [PubMed]
- Minor, P.D. Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479–480, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Dumpa, N.; Goel, K.; Guo, Y.; McFall, H.; Pillai, A.R.; Shukla, A.; Repka, M.A.; Murthy, S.N. Stability of Vaccines. AAPS PharmSciTech 2019, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.T.; Hung, C.Y.; Sanderson, S.D.; Hurtgen, B.J.; Wuthrich, M.; Klein, B.S.; Deepe, G.S.; Ostroff, G.R.; Levitz, S.M. Novel strategies to enhance vaccine immunity against coccidioidomycosis. PLoS Pathog. 2013, 9, e1003768. [Google Scholar] [CrossRef]
- Mead, H.L.; Hamm, P.S.; Shaffer, I.N.; Teixeira, M.M.; Wendel, C.S.; Wiederhold, N.P.; Thompson, G.R., 3rd; Muñiz-Salazar, R.; Castañón-Olivares, L.R.; Keim, P.; et al. Differential Thermotolerance Adaptation between Species of Coccidioides. J. Fungi 2020, 6, 366. [Google Scholar] [CrossRef]
- Shubitz, L.F.; Powell, D.A.; Trinh, H.T.; Lewis, M.L.; Orbach, M.J.; Frelinger, J.A.; Galgiani, J.N. Viable spores of Coccidioides posadasii Δcps1 are required for vaccination and provide long lasting immunity. Vaccine 2018, 36, 3375–3380. [Google Scholar] [CrossRef]
- Rixford, E. A Case of Protozoic Dermatitis. In Occidental Medical Times; Forgotten Books: London, UK, 1894; pp. 704–707. [Google Scholar]
- Barker, B.M.; Litvintseva, A.P.; Riquelme, M.; Vargas-Gastélum, L. Coccidioides ecology and genomics. Med. Mycol. 2019, 57, S21–S29. [Google Scholar] [CrossRef]
- Wang, H.; LeBert, V.; Hung, C.Y.; Galles, K.; Saijo, S.; Lin, X.; Cole, G.T.; Klein, B.S.; Wüthrich, M. C-type lectin receptors differentially induce th17 cells and vaccine immunity to the endemic mycosis of North America. J. Immunol. 2014, 192, 1107–1119. [Google Scholar] [CrossRef]
- Magee, D.M.; Cox, R.A. Interleukin-12 regulation of host defenses against Coccidioides immitis. Infect. Immun. 1996, 64, 3609–3613. [Google Scholar] [CrossRef]
- Magee, D.M.; Cox, R.A. Roles of gamma interferon and interleukin-4 in genetically determined resistance to Coccidioides immitis. Infect. Immun. 1995, 63, 3514–3519. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.T.; Xue, J.M.; Okeke, C.N.; Tarcha, E.J.; Basrur, V.; Schaller, R.A.; Herr, R.A.; Yu, J.J.; Hung, C.Y. A vaccine against coccidioidomycosis is justified and attainable. Med. Mycol. 2004, 42, 189–216. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Hung, C.Y.; Yu, J.J.; Cole, G.T. Immune response of vaccinated and non-vaccinated mice to Coccidioides posadasii infection. Vaccine 2005, 23, 3535–3544. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yu, J.J.; Hung, C.Y.; Lehmann, P.F.; Cole, G.T. Recombinant urease and urease DNA of Coccidioides immitis elicit an immunoprotective response against coccidioidomycosis in mice. Infect. Immun. 2001, 69, 2878–2887. [Google Scholar] [CrossRef] [PubMed]
- Shubitz, L.F.; Dial, S.M.; Perrill, R.; Casement, R.; Galgiani, J.N. Vaccine-induced cellular immune responses differ from innate responses in susceptible and resistant strains of mice infected with Coccidioides posadasii. Infect. Immun. 2008, 76, 5553–5564. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, E.P.; Hsu, A.P.; Pechacek, J.; Bax, H.I.; Dias, D.L.; Paulson, M.L.; Chandrasekaran, P.; Rosen, L.B.; Carvalho, D.S.; Ding, L.; et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J. Allergy Clin. Immunol. 2013, 131, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Vinh, D.C.; Schwartz, B.; Hsu, A.P.; Miranda, D.J.; Valdez, P.A.; Fink, D.; Lau, K.P.; Long-Priel, D.; Kuhns, D.B.; Uzel, G.; et al. Interleukin-12 receptor β1 deficiency predisposing to disseminated Coccidioidomycosis. Clin. Infect. Dis. 2011, 52, e99–e102. [Google Scholar] [CrossRef]
- Tsai, M.; Thauland, T.J.; Huang, A.Y.; Bun, C.; Fitzwater, S.; Krogstad, P.; Douine, E.D.; Nelson, S.F.; Lee, H.; Garcia-Lloret, M.I.; et al. Disseminated Coccidioidomycosis Treated with Interferon-γ and Dupilumab. N. Engl. J. Med. 2020, 382, 2337–2343. [Google Scholar] [CrossRef]
- Odio, C.D.; Milligan, K.L.; McGowan, K.; Rudman Spergel, A.K.; Bishop, R.; Boris, L.; Urban, A.; Welch, P.; Heller, T.; Kleiner, D.; et al. Endemic mycoses in patients with STAT3-mutated hyper-IgE (Job) syndrome. J. Allergy Clin. Immunol. 2015, 136, 1411–1413. [Google Scholar] [CrossRef]
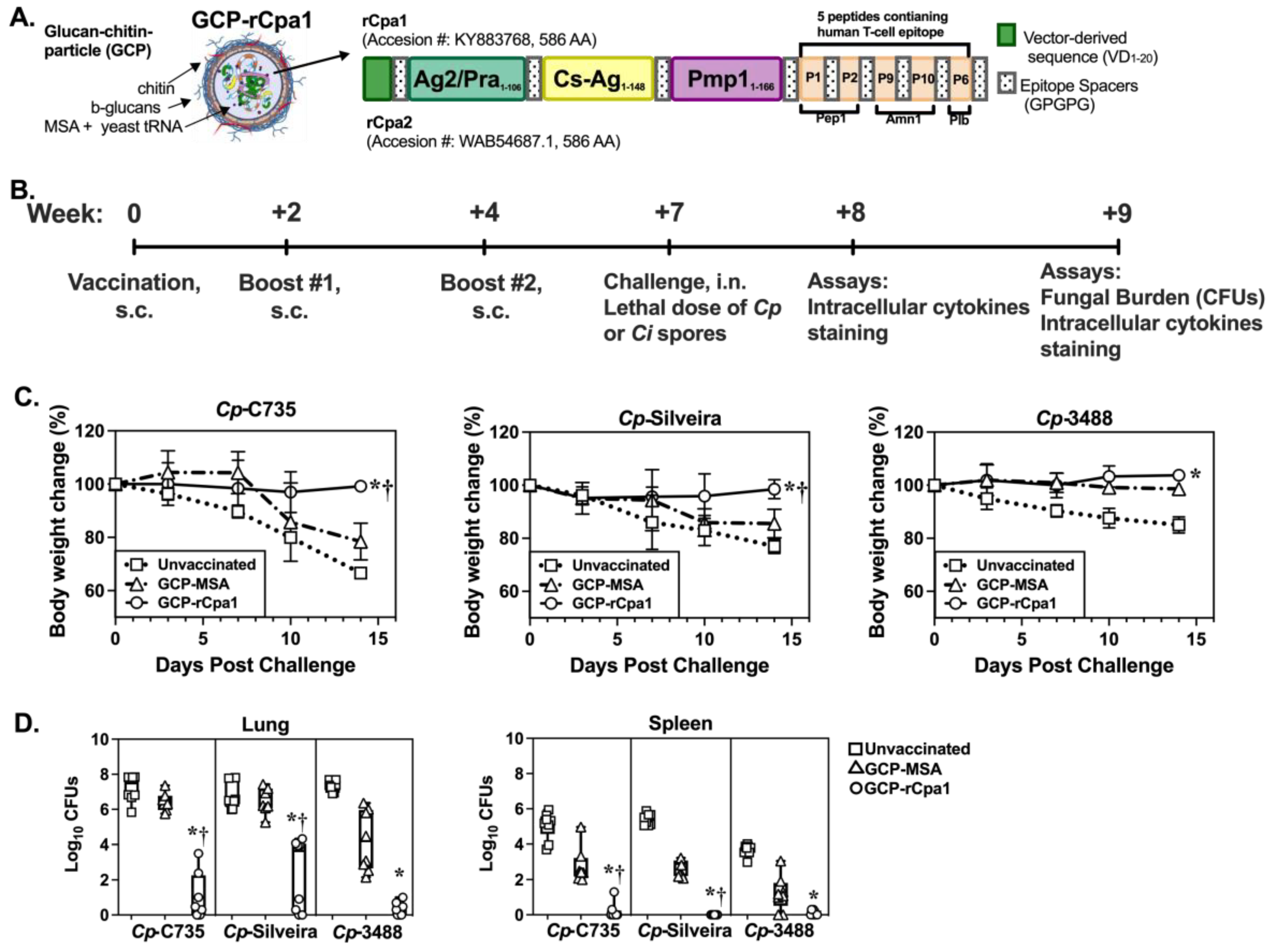
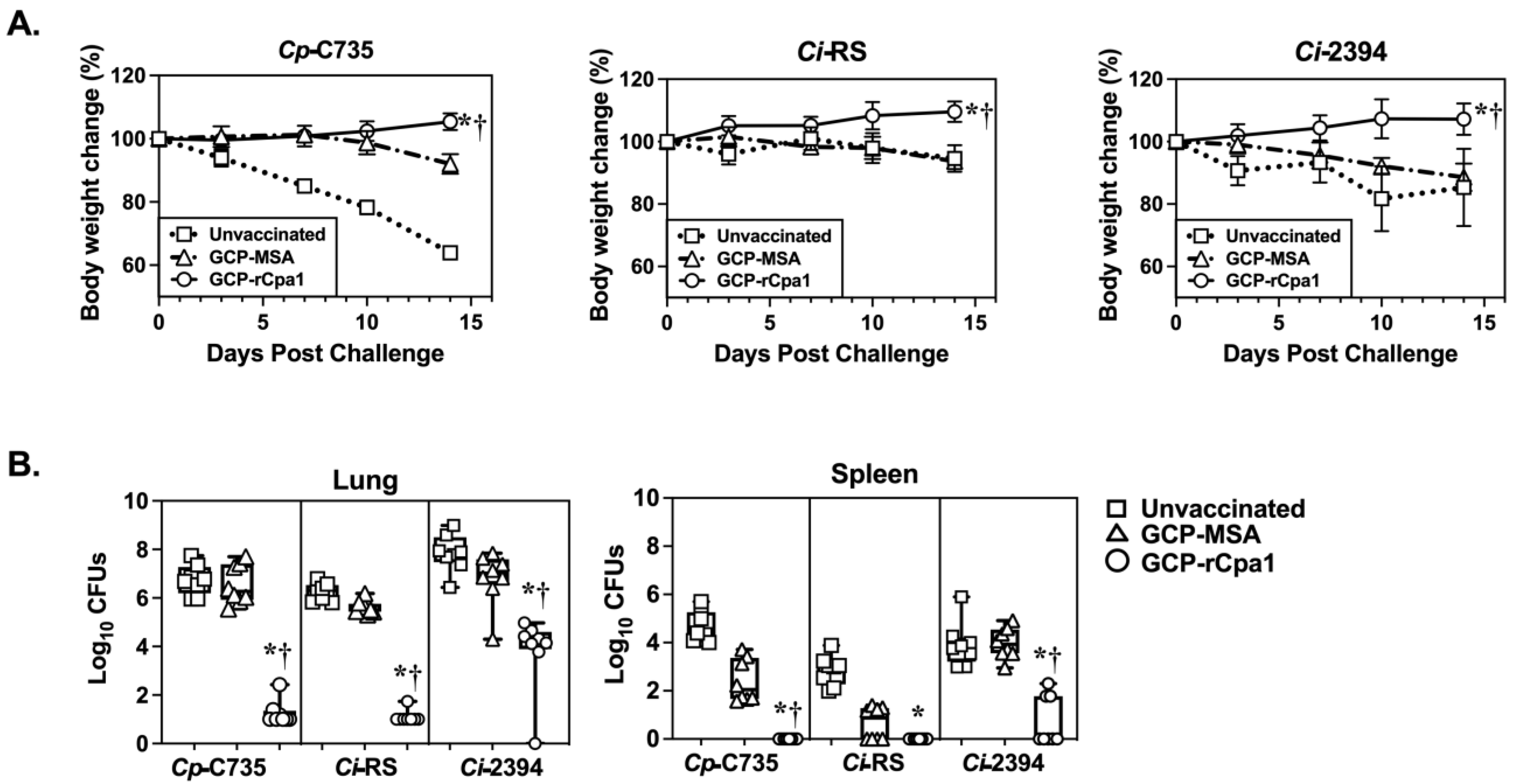
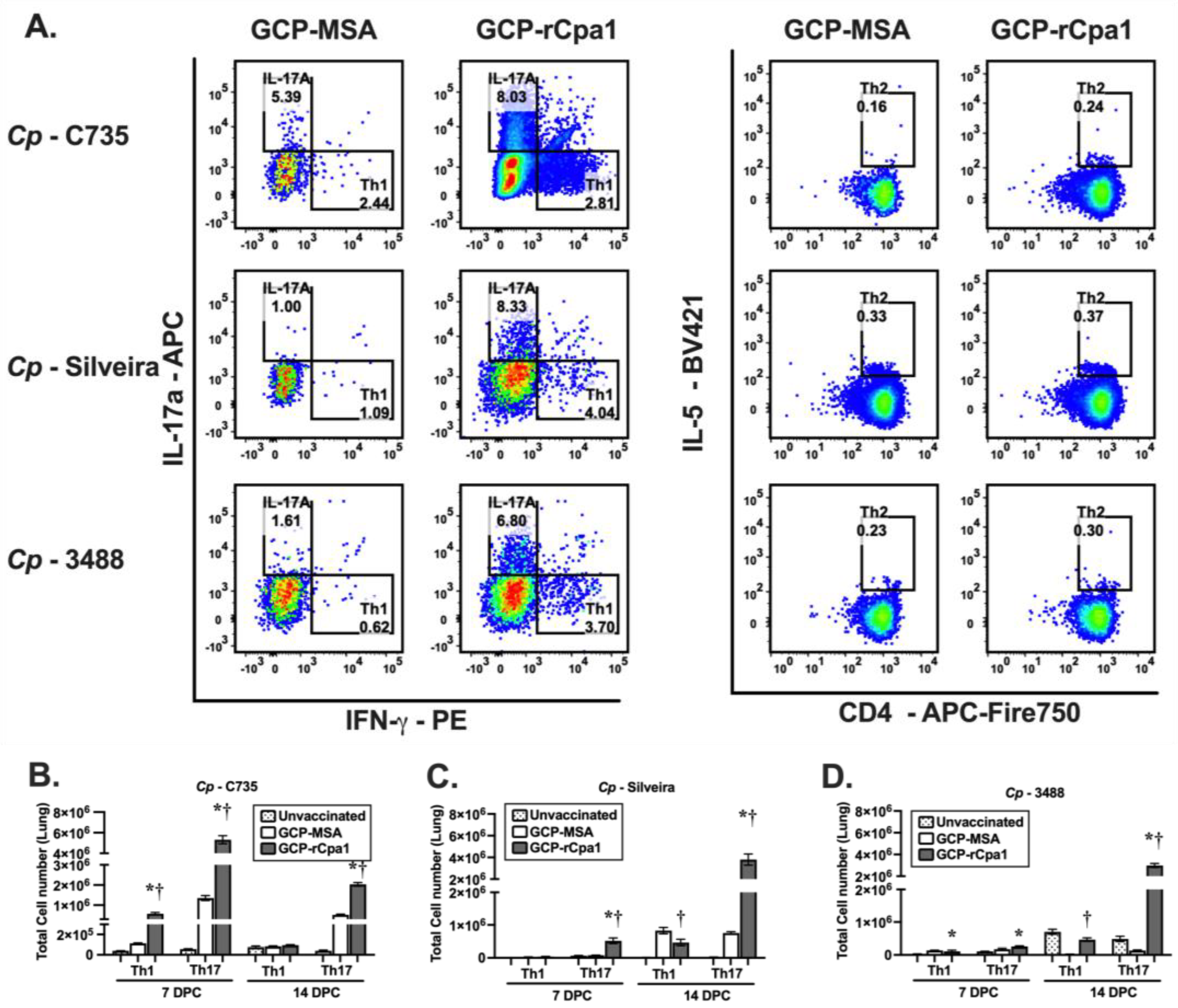
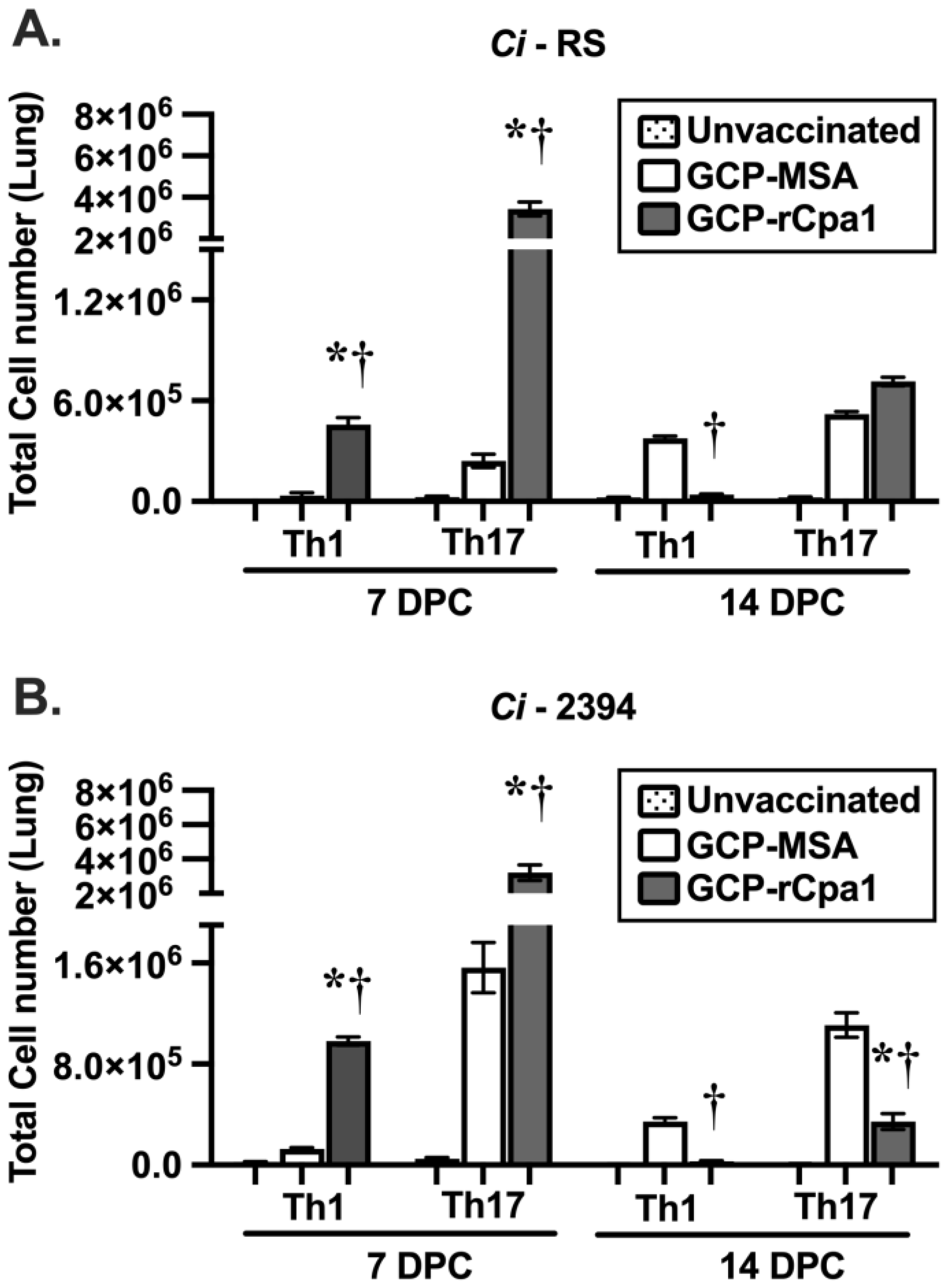
| Antigen/Epitope | Cp a (39 Isolates) b rCpa1 | Ci a (17 Isolates) c rCpa2 | GenBank no. Cp (aa Position) d | GenBank no. Ci (aa Position) d |
|---|---|---|---|---|
| Ag2/Pra | D117 | E117 | EER27008.1 (91) | XP_001240075.1 (91) |
| Cs-Ag | A164 | T164 | AAN73410.1 (27, 41) | XP_001247410.1 (27, 41) |
| P178 | A178 | |||
| Pmp1 | M424 | I424 | ABB42829.1 (136, 142, 163) | XP_001241932.1 (136, 142, 163) |
| Q430 | K430 | |||
| I451 | F451 | |||
| Plb-P6 | W576 | F576 | ABA12208.1 (522) | XP_001241137.2 (522) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campuzano, A.; Pentakota, K.D.; Liao, Y.-R.; Zhang, H.; Wiederhold, N.P.; Ostroff, G.R.; Hung, C.-Y. A Recombinant Multivalent Vaccine (rCpa1) Induces Protection for C57BL/6 and HLA Transgenic Mice against Pulmonary Infection with Both Species of Coccidioides. Vaccines 2024, 12, 67. https://doi.org/10.3390/vaccines12010067
Campuzano A, Pentakota KD, Liao Y-R, Zhang H, Wiederhold NP, Ostroff GR, Hung C-Y. A Recombinant Multivalent Vaccine (rCpa1) Induces Protection for C57BL/6 and HLA Transgenic Mice against Pulmonary Infection with Both Species of Coccidioides. Vaccines. 2024; 12(1):67. https://doi.org/10.3390/vaccines12010067
Chicago/Turabian StyleCampuzano, Althea, Komali Devi Pentakota, Yu-Rou Liao, Hao Zhang, Nathan P. Wiederhold, Gary R. Ostroff, and Chiung-Yu Hung. 2024. "A Recombinant Multivalent Vaccine (rCpa1) Induces Protection for C57BL/6 and HLA Transgenic Mice against Pulmonary Infection with Both Species of Coccidioides" Vaccines 12, no. 1: 67. https://doi.org/10.3390/vaccines12010067
APA StyleCampuzano, A., Pentakota, K. D., Liao, Y.-R., Zhang, H., Wiederhold, N. P., Ostroff, G. R., & Hung, C.-Y. (2024). A Recombinant Multivalent Vaccine (rCpa1) Induces Protection for C57BL/6 and HLA Transgenic Mice against Pulmonary Infection with Both Species of Coccidioides. Vaccines, 12(1), 67. https://doi.org/10.3390/vaccines12010067







