Safety of Immunization for Children with Immune Thrombocytopenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Data Collection
2.3. Confirmation of Immunization Records
2.4. Immunization Recommendation and Follow-Up
2.5. Statistical Analyses
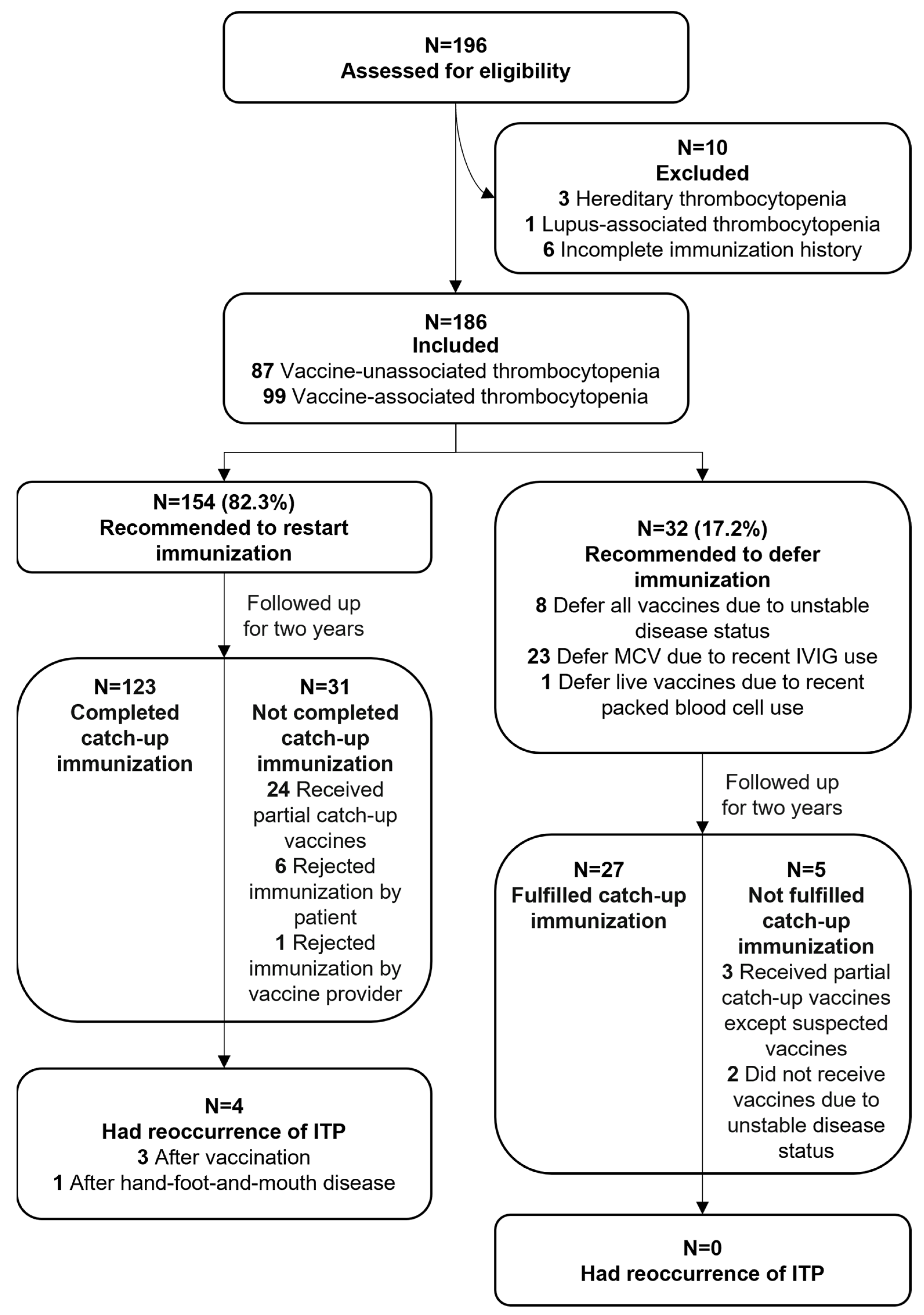
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 2009, 113, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- Gan, G.; Liu, H.; Liang, Z.; Zhang, G.; Liu, X.; Ma, L. Vaccine-associated thrombocytopenia. Thromb. Res. 2022, 220, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, K.; Xu, D.; Ye, J.; Liu, D.; Zheng, J.; Cao, L.; Wang, H.; Xiao, Q. Surveillance of Thrombocytopenic Purpura as an Adverse Event Following Immunization in China, 2010-2015. Chin. J. Vaccines Immun. 2018, 24, 186–192+216. [Google Scholar]
- Woo, E.J.; Wise, R.P.; Menschik, D.; Shadomy, S.V.; Iskander, J.; Beeler, J.; Varricchio, F.; Ball, R. Thrombocytopenia after vaccination: Case reports to the US Vaccine Adverse Event Reporting System, 1990–2008. Vaccine 2011, 29, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Jadavji, T.; Scheifele, D.; Halperin, S. Thrombocytopenia after immunization of Canadian children, 1992 to 2001. Pediatr. Infect. Dis. J. 2003, 22, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, K.; Li, Y.; Fan, C.; Li, Y.; Ren, M.; Yu, W.; Cao, L.; Cao, L.; Yin, Z. Surveillance of adverse events following immunization in China, 2020. Chin. J. Vaccines Immun. 2020, 28, 208–218. [Google Scholar]
- France, E.K.; Glanz, J.; Xu, S.; Hambidge, S.; Yamasaki, K.; Black, S.B.; Marcy, M.; Mullooly, J.P.; Jackson, L.A.; Nordin, J.; et al. Risk of immune thrombocytopenic purpura after measles-mumps-rubella immunization in children. Pediatrics 2008, 121, e687–e692. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.F.; Clothier, H.J.; Morgan, H.; Buttery, J.P.; Phuong, L.K.; Monagle, P.; Chunilal, S.; Wood, E.M.; Tran, H.; Szer, J.; et al. Immune thrombocytopenia following immunisation with Vaxzevria ChadOx1-S (AstraZeneca) vaccine, Victoria, Australia. Vaccine 2021, 39, 7052–7057. [Google Scholar] [CrossRef]
- Terrell, D.R.; Beebe, L.A.; Vesely, S.K.; Neas, B.R.; Segal, J.B.; George, J.N. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am. J. Hematol. 2010, 85, 174–180. [Google Scholar] [CrossRef]
- Kitazawa, J.; Nakadate, H.; Matsubara, K.; Takahashi, Y.; Ishiguro, A.; Inoue, E.; Sasahara, Y.; Fujisawa, K.; Maeda, N.; Oka, T.; et al. Favorable prognosis of vaccine-associated immune thrombocytopenia in children is correlated with young age at vaccination: Retrospective survey of a nationwide disease registry. Int. J. Hematol. 2022, 115, 114–122. [Google Scholar] [CrossRef]
- Cecinati, V.; Principi, N.; Brescia, L.; Giordano, P.; Esposito, S. Vaccine administration and the development of immune thrombocytopenic purpura in children. Hum. Vaccines Immunother. 2013, 9, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Provan, D.; Arnold, D.M.; Bussel, J.B.; Chong, B.H.; Cooper, N.; Gernsheimer, T.; Ghanima, W.; Godeau, B.; González-López, T.J.; Grainger, J.; et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019, 3, 3780–3817. [Google Scholar] [CrossRef] [PubMed]
- Kroger, A.B.L.; Hunter, P. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). 2021. Available online: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html (accessed on 11 August 2023).
- Committee on Infectious Diseases, American Academy of Pediatrics; Kimberlin, D.W.; Barnett, E.D.; Lynfield, R.; Sawyer, M.H. Red Book: 2021–2024 Report of the Committee on Infectious Diseases; American Academy of Pediatrics: Itasca, IL, USA, 2021. [Google Scholar]
- National Health Commission of the People’s Republic of China. National immunization program vaccine children’s immunization procedures and instructions 2021. Chin. J. Viral Dis. 2021, 11, 241–245. [Google Scholar]
- McLean, H.Q.; Fiebelkorn, A.P.; Temte, J.L.; Wallace, G.S. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2013, 62, 1–34. [Google Scholar] [PubMed]
- Wise, R.P.; Bonhoeffer, J.; Beeler, J.; Donato, H.; Downie, P.; Matthews, D.; Pool, V.; Riise-Bergsaker, M.; Tapiainen, T.; Varricchio, F. Thrombocytopenia: Case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2007, 25, 5717–5724. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Global Manual on Surveillance of Adverse Events following Immunization. Available online: https://www.who.int/publications/i/item/10665206144 (accessed on 10 May 2023).
- National Health Commission of the People’s Republic of China. National immunization program vaccine children’s immunization procedures and instructions 2016. Chin. J. Viral Dis. 2017, 7, 81–86. [Google Scholar]
- Greinacher, A.; Eichler, P.; Lubenow, N.; Kiefel, V. Drug-induced and drug-dependent immune thrombocytopenias. Rev. Clin. Exp. Hematol. 2001, 5, 166–200, discussion 311–312. [Google Scholar] [CrossRef]
- Okazaki, N.; Takeguchi, M.; Sonoda, K.; Handa, Y.; Kakiuchi, T.; Miyahara, H.; Akiyoshi, K.; Korematsu, S.; Suenobu, S.; Izumi, T. Detection of platelet-binding anti-measles and anti-rubella virus IgG antibodies in infants with vaccine-induced thrombocytopenic purpura. Vaccine 2011, 29, 4878–4880. [Google Scholar] [CrossRef]
- Zhang, L.; Li, K.; Du, W.; Li, Y.; Fan, C.; Shi, L.; Yu, W.; Cao, L.; Cao, L.; Yin, Z. Surveillance of adverse events following immunization in China, 2018. Chin. J. Vaccines Immun. 2020, 26, 363–371. [Google Scholar]
- Zhang, L.; Li, K.; Du, W.; Li, Y.; Fan, C.; Yu, W.; Cao, L.; Cao, L.; Yin, Z. Surveillance of adverse events following immunization in China, 2019. Chin. J. Vaccines Immun. 2021, 27, 438–445. [Google Scholar]
- O’Leary, S.T.; Glanz, J.M.; McClure, D.L.; Akhtar, A.; Daley, M.F.; Nakasato, C.; Baxter, R.; Davis, R.L.; Izurieta, H.S.; Lieu, T.A.; et al. The risk of immune thrombocytopenic purpura after vaccination in children and adolescents. Pediatrics 2012, 129, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Zufferey, A.; Kapur, R.; Semple, J.W. Pathogenesis and Therapeutic Mechanisms in Immune Thrombocytopenia (ITP). J. Clin. Med. 2017, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Stasi, R.; Cooper, N.; Del Poeta, G.; Stipa, E.; Laura Evangelista, M.; Abruzzese, E.; Amadori, S. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood 2008, 112, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Najaoui, A.; Bakchoul, T.; Stoy, J.; Bein, G.; Rummel, M.J.; Santoso, S.; Sachsm, U.J. Autoantibody-mediated complement activation on platelets is a common finding in patients with immune thrombocytopenic purpura (ITP). Eur. J. Haematol. 2012, 88, 167–174. [Google Scholar] [CrossRef] [PubMed]
- McMillan, R.; Wang, L.; Tomer, A.; Nichol, J.; Pistillo, J. Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood 2004, 103, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.M.; Souza, C.; Rocha, G.A.; de Melo, F.F.; Clementino, N.C.; Marino, M.C.; Bozzi, A.; Silva, M.L.; Martins Filho, O.A.; Queiroz, D.M. The levels of IL-17A and of the cytokines involved in Th17 cell commitment are increased in patients with chronic immune thrombocytopenia. Haematologica 2011, 96, 1560–1564. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Bussel, J.B.; Heck, S.; He, W.; Karpoff, M.; Boulad, N.; Yazdanbakhsh, K. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood 2010, 116, 4639–4645. [Google Scholar] [CrossRef]
- Hamiel, U.; Kventsel, I.; Youngster, I. Recurrent Immune Thrombocytopenia After Influenza Vaccination: A Case Report. Pediatrics 2016, 138, e20160124. [Google Scholar] [CrossRef]
- Morin, E.; Sadarangani, M. Recurrent immune thrombocytopenia following different vaccines. BMJ Case Rep. 2019, 12, e231260. [Google Scholar] [CrossRef]
- Hsieh, Y.L.; Lin, L.H. Thrombocytopenic purpura following vaccination in early childhood: Experience of a medical center in the past 2 decades. J. Chin. Med. Assoc. 2010, 73, 634–637. [Google Scholar] [CrossRef]
- Vianelli, N.; Auteri, G.; Buccisano, F.; Carrai, V.; Baldacci, E.; Clissa, C.; Bartoletti, D.; Giuffrida, G.; Magro, D.; Rivolti, E.; et al. Refractory primary immune thrombocytopenia (ITP): Current clinical challenges and therapeutic perspectives. Ann. Hematol. 2022, 101, 963–978. [Google Scholar] [CrossRef] [PubMed]
| Birth | 1 mos | 2 mos | 3 mos | 4 mos | 5 mos | 6 mos | 8 mos | 9 mos | 18 mos | 2 yrs | 3 yrs | 4 yrs | 6 yrs | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacillus Calmette–Guérin vaccine | 1st dose | |||||||||||||
| Hepatitis B vaccine | 1st dose | 2nd dose | 3rd dose | |||||||||||
| Inactive poliomyelitis vaccine | 1st dose | |||||||||||||
| Bivalent oral poliomyelitis attenuated live vaccine | 1st dose | 2nd dose | 3rd dose | |||||||||||
| Diphtheria, tetanus, and acellular pertussis combined vaccine | 1st dose | 2nd dose | 3rd dose | 4th dose | ||||||||||
| Diphtheria and tetanus combined vaccine | 1st dose | |||||||||||||
| Measles and rubella combined attenuated live vaccine | 1st dose | |||||||||||||
| Measles, mumps, and rubella combined attenuated live vaccine | 1st dose | |||||||||||||
| Group A meningococcal polysaccharide vaccine | 1st dose | 2nd dose | ||||||||||||
| Group A and C meningococcal polysaccharide vaccine | 1st dose | 2nd dose | ||||||||||||
| Japanese encephalitis attenuated live vaccine | 1st dose | 2nd dose | ||||||||||||
| Hepatitis A inactivated vaccine | 1st dose | 2nd dose |
| Total n = 186 | Vaccine-Unassociated Thrombocytopenia n = 87 | Vaccine-Associated Thrombocytopenia n = 99 | p Value | |
|---|---|---|---|---|
| Male | 127 (68.3) | 58 (66.7) | 69 (70.0) | 0.658 |
| Age at first visit, month | 21 (12, 36) | 23 (12, 45) | 19 (12, 32) | 0.288 |
| Age at disease onset, month | 4 (2, 10) | 6 (2, 14) | 3 (2, 8) | 0.205 |
| 0–5 mo | 100 (53.8) | 40 (46.0) | 60 (60.6) | |
| 6–11 mo | 44 (23.6) | 17 (19.5) | 27 (27.3) | |
| 12–23 mo | 27 (14.5) | 17 (19.5) | 10 (10.1) | |
| ≥24 mo | 15 (8.1) | 13 (14.9) | 2 (2.0) | |
| Interval from disease onset to first visit, month | 12 (7, 23.8) | 12 (7, 24) | 12 (6, 26) | 0.910 |
| Platelet count at diagnosis, per μL | 13.5 (7, 25.5) | 17.5 (10, 40) | 10 (5.5, 20) | 0.006 |
| Treatment after diagnosis | 0.407 | |||
| Observation | 20 (10.8) | 12 (13.8) | 8 (8.1) | |
| IVIG alone | 105 (56.4) | 44 (50.6) | 61 (61.6) | |
| Steroid alone | 9 (4.8) | 5 (5.7) | 4 (4.0) | |
| IVIG + steroid | 52 (28.0) | 26 (29.9) | 26 (26.3) | |
| Diagnosis type a | 0.124 | |||
| Newly diagnosed | 158 (84.9) | 69 (79.3) | 89 (89.9) | |
| Persistent | 18 (9.7) | 12 (13.8) | 6 (6.1) | |
| Chronic | 10 (5.4) | 6 (6.9) | 4 (4.0) | |
| Serious ITP case b | 3 (1.6) | 3 (3.4) | 0 (0) | 0.100 |
| Disease in remission c | 0.002 | |||
| Yes | 164 (88.2) | 70 (80.5) | 94 (94.9) | |
| No | 22 (11.8) | 17 (19.5) | 5 (5.1) |
| Vaccine Type | Number of Involving Dose | Days from Vaccination to Disease Onset, Median (Range) |
|---|---|---|
| IPV | 32 | 7 (0–30) |
| DTaP | 16 | 7 (0–20) |
| HepB | 16 | 13 (1–28) |
| MR | 16 | 19.5 (4–34) |
| JEV-L | 13 | 19.5 (2–34) |
| MPV-A | 8 | 24 (1–34) |
| bOPV | 6 | 7 (0–20) |
| BCG | 4 | 14.5 (5–21) |
| EV71 | 4 | 6.5 (1–14) |
| HepA-I | 4 | 18.5 (3–30) |
| VarV | 4 | 17 (1–25) |
| PPCV13 | 3 | 7 (4–21) |
| ORV | 2 | 10.5 (0–21) |
| MMR | 2 | 10 (3–17) |
| Vaccine-Unassociated Thrombocytopenia n = 87 | Vaccine-Associated Thrombocytopenia n = 99 | p Value | |
|---|---|---|---|
| No. of children who stopped receiving all vaccines | 71 (81.6) | 66 (66.7) | 0.021 |
| No. of children who only stopped receiving live vaccines a | 3 (3.4) | 17 (17.2) | 0.003 |
| No. of children who stopped receiving incident vaccines b | 0 (0) | 9 (9.1) | 0.004 |
| No. of children who received irregular immunization | 8 (9.2) | 3 (3.0) | 0.117 |
| No. of children who received all types of immunization b | 5 (5.7) | 4 (4.0) | 0.736 |
| Completing All Catch-Up Immunizations n = 150 | Completing Partial Catch-Up Immunizations n = 36 | p Value | |
|---|---|---|---|
| Male | 102 (68.0) | 25 (69.4) | 0.876 |
| Age at first visit, month | 18.5 (10, 32) | 26.5 (19.5, 43.5) | 0.003 |
| Age at disease onset, month | 4 (2, 11) | 5 (2, 8) | 0.962 |
| Interval from disease onset to first visit, month | 11 (6, 18.5) | 18.5 (12, 34.8) | <0.001 |
| Platelet count at diagnosis, per μL | 15 (7, 32) | 10 (6, 18) | 0.082 |
| Treatment of IVIG + steroid | 34 (22.7) | 18 (50.0) | 0.001 |
| Chronic ITP case a | 2 (1.3) | 8 (22.2) | <0.001 |
| Serious ITP case b,c | 5 (3.3) | 1 (2.8) | 1.000 |
| Case of disease status in treatment c | 17 (11.3) | 5 (13.9) | 0.773 |
| Vaccine-associated thrombocytopenia case | 73 (48.7) | 26 (72.2) | 0.011 |
| Relapse of ITP c | 0 (0) | 2 (5.6) | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Feng, T.; Wang, C.; Li, J.; Ge, Y.; Zhai, X.; Wang, H.; Zeng, M. Safety of Immunization for Children with Immune Thrombocytopenia. Vaccines 2024, 12, 66. https://doi.org/10.3390/vaccines12010066
Wang X, Feng T, Wang C, Li J, Ge Y, Zhai X, Wang H, Zeng M. Safety of Immunization for Children with Immune Thrombocytopenia. Vaccines. 2024; 12(1):66. https://doi.org/10.3390/vaccines12010066
Chicago/Turabian StyleWang, Xiangshi, Tianxing Feng, Chuning Wang, Jingjing Li, Yanling Ge, Xiaowen Zhai, Hongsheng Wang, and Mei Zeng. 2024. "Safety of Immunization for Children with Immune Thrombocytopenia" Vaccines 12, no. 1: 66. https://doi.org/10.3390/vaccines12010066
APA StyleWang, X., Feng, T., Wang, C., Li, J., Ge, Y., Zhai, X., Wang, H., & Zeng, M. (2024). Safety of Immunization for Children with Immune Thrombocytopenia. Vaccines, 12(1), 66. https://doi.org/10.3390/vaccines12010066






