Abstract
Cyprinid herpesvirus 2 (CyHV-2) is a pathogen that causes significant losses to the global aquaculture industry due to mass mortality in crucian carp and goldfish. This study demonstrates that the ORF55/ORF57 deletion mutants CyHV-2-Δ55-CP and CyHV-2-Δ57-CP obtained through homologous recombination replicate effectively within the caudal fin of Carassius auratus gibelio (GiCF) cells and exhibit morphologies similar to the CyHV-2 wild-type strain. Both mutants demonstrated a decrease in virulence, with CyHV-2-Δ57-CP exhibiting a more significant reduction. This serves as a reference for the subsequent development of recombinant attenuated vaccines against CyHV-2. Additionally, both mutants expressed the inserted RGNNV-CP (capsid protein of Redspotted grouper nervous necrosis virus) fusion protein gene, and inoculation with CyHV-2-Δ57-CP-infected GiCF cell lysates elicited an antibody response in the grouper. These results indicate that, while ORF55 and ORF57 genes of CyHV-2 are not required for viral replication in vitro, they do play a role in virulence in vivo. Additionally, expression of foreign protein in CyHV-2 suggests that the fully attenuated mutant of CyHV-2 could potentially function as a viral vector for developing subunit vaccines or multivalent recombinant attenuated vaccines.
1. Introduction
Cyprinid herpesvirus 2 (CyHV-2), also referred to as Cyvirus cyprinidallo 2 and herpesviral hematopoietic necrosis virus (HVHNV), is a virus with an icosahedral capsid containing a dsDNA genome and is surrounded by a lipid envelope containing viral glycoproteins. CyHV-2 causes acute mass mortality (up to 100%) in populations of crucian carp (Carassius auratus) and its variants such as goldfish (C. auratus L.) and gibel carp (C. auratus gibelio), resulting in significant economic losses in the aquaculture industry [1]. For environmentally friendly aquaculture, vaccination strategies have demonstrated high effectiveness and cost-effectiveness in protecting fish against various viruses [2]. Previous studies have reported the development of vaccines for CyHV-2 in various forms, including conventional live attenuated [3,4], inactivated [5,6,7,8], DNA [9,10], subunit [11], and live vector vaccines [12,13,14,15,16,17]. However, currently, there is no commercially available licensed vaccine against CyHV-2. Additionally, immersion and oral vaccines are better suited for large-scale operations in fish farms when immunizing relatively affordable juvenile carp and goldfish [18]. Live attenuated vaccines, on the other hand, tend to be highly immunogenic and closely resemble natural pathogen infections due to their ability to replicate within the host and stimulate robust cellular responses related to both innate and adaptive immune systems [19]. Hence, attenuated live vaccines can elicit long-lasting immunity within the host through oral or immersion routes. However, a major concern with conventional live attenuated vaccines is the risk of reversion to virulence [20]. Recombinant attenuated vaccines, involving the deletion of virulence-related genes, are a type of live vaccine that cannot be easily reversed under natural conditions. Furthermore, recombinant attenuated vaccines represent a crucial avenue for the advancement of aquaculture vaccines, given their high stability, potent immunogenicity, and guaranteed safety [21]. To date, no studies have reported the creation of recombinant attenuated vaccines against CyHV-2.
CyHV-2 is a member of the genus Cyvirus in the family Alloherpesviridae, to which Cyvirus cyprinidallo 1 (Cyprinid herpesvirus 1, CyHV-1; carp pox virus), Cyvirus cyprinidallo 3 (Cyprinid herpesvirus 3, CyHV-3; koi herpesvirus, KHV), and Cyvirus anguillidallo 1 (Anguillid herpesvirus 1, AngHV-1) also belong [22]. Phylogenetically, all three strains of cyprinid herpesviruses (CyHVs) are closely related, with CyHV-2 and CyHV-3 being slightly more closely related to one another than to CyHV-1 [23]. In 2018, a patent was granted in the United States for a recombinant attenuated CyHV-3 vaccine with mutations in ORF56 and ORF57 genes [21]. This vaccine has demonstrated exceptional immunoprotective potential against CyHV-3 in both common carp and koi [24]. Indeed, subsequent research has identified the ORF57 gene as the essential virulence factor in the double deletion of ORF56 and ORF57 genes [25]. However, the function of the ORF57 protein in CyHV-2 and CyHV-3 remains to be determined. The identification of ORF57 protein as a critical virulence factor of CyHV-3 has provided a crucial target for further development of recombinant attenuated vaccines against CyHV-2 [26]. Additionally, homology analysis and RNA interference (RNAi) experiments were performed to investigate the ORF57 and thymidine kinase (TK) genes of CyHVs [27]. The findings suggest that the ORF57 and TK genes are conserved in CyHVs and may have an impact on the virulence of CyHV-2. Furthermore, the TK gene was revealed to be unnecessary for replication in vitro but pertinent to virulence in vivo as shown in numerous herpesviruses including CyHV-3 [28,29,30,31]. Therefore, this study selected the ORF55 (TK) and ORF57 genes as targets for constructing CyHV-2 recombinant mutants.
The Redspotted grouper nervous necrosis virus (RGNNV) is a non-enveloped, small icosahedral virus (25–30 nm) whose genome contains two positive-sense, single-stranded RNA molecules: RNA1 and RNA2 [32]. RGNNV belongs to the genus Betanodavirus in the family Nodaviridae, along with Barfin flounder nervous necrosis virus (BFNNV), Striped jack nervous necrosis virus (SJNNV), and Tiger puffer nervous necrosis virus (TPNNV) [22]. Viral nervous necrosis (VNN) disease caused by Betanodavirus, also known as viral encephalopathy and retinopathy (VER), and viral encephalopathy, is a highly destructive disease that negatively impacts at least 57 species of marine fish and 13 species of freshwater fish (including goldfish) worldwide, resulting in financial losses to the aquaculture industry [33]. The only structural protein of Betanodavirus, capsid protein (CP), encoded by RNA2, is a promising candidate for future vaccine development because of its ability to elicit effective immune responses [33]. A recently reported, recombinant bivalent live viral vectored vaccine candidate expresses the major protective antigen domain of NNV-CP in attenuated Viral hemorrhagic septicemia virus (VHSV) and has been shown to protect against lethal VHSV and NNV challenge [34].
Viral vector vaccines, derived from non-pathogenic virions whose genomes have been modified by inserting one or more genes encoding for the heterologous antigens, can express several heterologous antigens to elicit strong immune responses and increase cellular immunity in hosts [35]. These types of vaccines have been widely utilized in both human and veterinary medicine [36,37]. Among these, herpesviruses have become significant vectors because of their ability to carry large exogenous genes, infect only a limited range of hosts, express envelope glycoproteins on, and elicit both cellular and humoral immune responses [36,38]. However, only a handful of viruses, including baculovirus [14,15], adenovirus [39,40], Semliki Forest virus (SFV) [41], Salmon pancreas disease virus (Salmonid alphavirus, SAV) [42,43], Viral hemorrhagic septicemia virus (VHSV) [34], Infectious hematopoietic necrosis virus (IHNV) [44], and Ictalurid herpesvirus 1 (IcHV-1; channel catfish herpesvirus, CCV) [45], have been utilized as viral vectors for the creation of aquaculture vaccines or expression systems. In this study, we inserted the RGNNV-CP gene into the genome of CyHV-2 and conducted an initial assessment of its potential as a viral vector for expressing heterologous proteins.
2. Materials and Methods
2.1. Animals, Cells and Virus
Gibel carps (C. auratus gibelio) var. CAS V weighing 8 ± 2 g and 200 ± 20 g, gibel carps (C. auratus gibelio) var. CAS III weighing 200 ± 20 g, Fang Zheng crucian carps (C. auratus gibelio) weighing 200 ± 20 g, and white crucian carps (C. auratus cuvieri) weighing 200 ± 20 g were obtained from a fishery located in the Nanhai District of Foshan, Guangdong Province, China. Additionally, goldfish (C. auratus L.) weighing 8 ± 2 g (one year old) and 100 ± 10 g (over two years old) were obtained from a fishery in Dianshan Lake Town, Kunshan, Jiangsu Province, China. All of the fish mentioned above, confirmed as CyHV-2-negative through PCR testing, were maintained in recirculating aquaculture systems at a temperature of 25 °C until the start of the experiments.
Orange-spotted groupers (Epinephelus coioides) weighing 200 ± 20 g were acquired from a fishery in the Hailing District of Yangjiang, Guangdong Province, China. The Groupers were confirmed to be NNV-negative by PCR testing and housed in a seawater recirculating aquaculture system at a temperature of 28 °C until the experiments.
The C. auratus gibelio caudal fin (GiCF) cell line, established and maintained in our laboratory [46], was cultured in medium 199 (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco, NY, USA) at 27 °C.
The CyHV-2 wild-type (WT) strain YC01 (Genebank: MN593216.1), isolated from diseased gibel carp in our previous study [47], was propagated in GiCF cells (MOI = 1). The infected GiCF cell lysate was harvested at 5 days after infection (dpi) and stored at −80 °C.
The virus-like particles of NNV (NNV VLP) were generously provided by Dr. Junfeng Xie, State Key Laboratory of Biocontrol/School of Life Sciences, Sun Yat-sen University, Guangzhou, Guangdong, China.
2.2. Construction of Transfer Vectors and Virus Recombinants
The pUC18 vector was employed for the construction of recombinant transfer vectors, namely pUC18-Δ55-CP and pUC18-Δ57-CP. These vectors were designed to incorporate the CMV promoter, the full-length RGNNV capsid protein gene (Genebank: AF534998.3), puromycin resistance gene (Puror), enhanced green fluorescent protein (EGFP) gene, as well as 1 kb upstream and downstream arms of the ORF55 or ORF57 gene. In brief, the corresponding segments were initially obtained using the primers listed in Table 1 and KOD DNA Polymerase (TOYOBO, Osaka, Japan). Subsequently, the CP-Puro fusion protein segment was generated through overlap extension PCR, along with the 55UD and 57UD segments which contain double restriction enzyme sites between the upstream and downstream arms. Using the ClonExpress® II One Step Cloning Kit (Vazyme, Nanjing, China), the CP-Puro fusion protein segment was ligated into the EcoRI/BamHI-digested plasmid vector pEGFP-N3 to obtain pEGFP-N3-CP-Puror. Additionally, the 55UD and 57UD segments were separately ligated into the BamHI/EcoRI-digested plasmid vector pUC-18, resulting in pUC-18-55UD and pUC-18-57UD. Finally, the NNV-CP fusion protein expression cassette was obtained from pEGFP-N3-CP-Puror and subsequently ligated into the KpnI/EcoRI-digested plasmid vectors pUC-18-55UD and the BamHI/EcoRI-digested plasmid vectors pUC-18-57UD to obtain the recombinant transfer vectors, pUC18-Δ55-CP and pUC18-Δ57-CP, respectively.

Table 1.
Primers used for constructing recombinant plasmids in this study.
GiCF cells were individually transfected with the recombinant transfer vectors pUC18-Δ55-CP and pUC18-Δ57-CP using the EZ 3000 Plus transfection reagent (ELGBIO, Guangzhou, China) and maintained in 10% FBS medium 199 at 27 °C for 24 h. Subsequently, GiCF cells were infected with the CyHV-2-WT strain (MOI = 1) and kept in 2% FBS medium 199 at 27 °C, to generate the CyHV-2 recombinant mutants by homologous recombination.
Two rounds of puromycin selection (Sigma-Aldrich, St. Louis, MO, USA) were performed using a final concentration of 1 μg/mL to enrich the CyHV-2 recombinant mutants by eliminating CyHV-2-WT-infected cells lacking puromycin resistance. Subsequently, recombinant mutants CyHV-2-Δ55-CP and CyHV-2-Δ57-CP were purified to homogeneity by multiple rounds of fluorescent plaque purification. In brief, green fluorescent cell foci were picked by aspiration, and subsequently used for infecting new cells through the limited dilution method, repeating this process iteratively to obtain homogeneous recombinant strains. The purity of obtained CyHV-2 recombinant mutants was confirmed via the nested-PCR amplification technique along with sequencing analysis.
2.3. DNA Extraction and Sequence Analysis
Genomic DNA was extracted using the FastPure Cell/Tissue DNA Isolation Mini Kit (Vazyme, Nanjing, China). Whole-genomic sequences of CyHV-2-WT, CyHV-2-55-CP, and CyHV-2-57-CP strains were sequenced utilizing the Illumina NovaSeq PE150 platform provided by Beijing Novogene Bioinformatics Technology Co., Ltd. (Beijing, China). Trinity software (version 2.14.0) was used to assemble whole-genomic sequences against reference sequence YC01 (GenBank: MN593216.1) if CyHV-2 wild-type strain, and unmapped reads were retrieved employing Bowtie2 tool (version 2.4.5). Using the assembled sequence of CyHV-2-WT as the reference, the final assembly results were aligned by DNAMAN (version 9.0.1) and SnapGene (version 2.3.2) software.
2.4. Western Blotting Analysis
The expression of the NNV-CP fusion protein derived from the CyHV-2 recombinant mutants within GiCF cells was assessed using Western blotting (WB). Briefly, GiCF cells were collected at 48 h post-infection with CyHV-2-WT, CyHV-2-Δ55-CP, or CyHV-2-Δ57-CP (MOI = 1) and lysed using IP lysis buffer (Pierce, Rockford, IL, USA). Total proteins in the lysates were separated by SDS polyacrylamide gel electrophoresis, transferred to PVDF (polyvinylidene fluoride) membranes (Merck, Boston, MA, USA), and blocked with 5% fat-free milk in PBS-T (0.05% Tween 20). The membranes were then incubated with primary antibody (1:2000 dilution), including rabbit anti-NNV VLP polyclonal antibody (provided by Dr. Junfeng Xie) or mouse anti-GFP monoclonal antibody (Abmart, Shanghai, China). After washing the membranes with PBS-T at room temperature, secondary antibodies (1:5000 dilution), HRP-conjugated goat anti-rabbit or anti-mouse IgG (Promega, Madison, WI, USA), were added. The reactive bands were visualized using Tanon High-sig ECL Western Blotting Substrate (Tanon, Shanghai, China).
2.5. Indirect Immunofluorescence Analysis
GiCF cells infected with CyHV-2-WT, CyHV-2-Δ55-CP, or CyHV-2-Δ57-CP (MOI = 1) were incubated at 27 °C for 48 h. Subsequently, infected cells were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.5% Triton X-100 for 20 min followed by blocking with 10% goat serum for 30 min. Incubation with rabbit anti-NNV VLP polyclonal antibody as the primary antibody (1:1000 dilution) and Alexa Fluor 555-conjugated (red fluorescence) goat anti-rabbit IgG (Abcam, Cambridge, UK) as the secondary antibody (1:1000 dilution) was performed. Finally, cell nuclei were stained using DAPI (Abcam, Cambridge, UK). The cell samples were observed under a TCS SP8 confocal microscope (Leica, Wetzlar, Germany).
2.6. Virus Purification and Transmission Electron Microscope (TEM)
The infected GiCF cell lysates were prepared as described in Section 2.1, followed by three freeze–thaw cycles. Cell debris was removed by centrifugation at 5000× g for 30 min at 4 °C, and the supernatants were further centrifuged at 80,000× g for 2 h at 4 °C to pellet virus. The viral pellets were then resuspended in sterile phosphate-buffered saline (PBS, pH 7.4) after being washed with PBS. Subsequently, the resulting suspensions were subjected to centrifugation through discontinuous sucrose gradients (20%, 30%, 40%, 50%, and 66%) at 150,000× g for 1 h at 4 °C. Finally, the viral bands were carefully extracted and pelleted by centrifugation in sterile PBS at 150,000× g for 2 h at 4 °C. To observe the basic morphological structure of viral particles, the purified viruses were resuspended in PBS and examined using TEM (JEOL JEM-1400 electron microscope, Tokyo, Japan) after negative staining with 3% phosphotungstic acid (pH 7.2–7.4).
To observe the intracellular structure of viral particles, GiCF cells were infected with CyHV-2-WT, CyHV-2-Δ55-CP, or CyHV-2-Δ57-CP (MOI = 1), and infected cells collected at 2 dpi underwent TEM assay following staining with uranyl acetate–lead citrate.
2.7. TCID50 Assay
The TCID50 assay was performed to determine the viral replication kinetics in GiCF cells. GiCF cells were infected with CyHV-2-WT, CyHV-2-Δ55-CP, or CyHV-2-Δ57-CP (MOI = 1) and incubated at 27 °C. The virus-infected cells were collected daily from day one to seven post-infection and lysed by two freeze–thaw cycles. Subsequently, the viral titers of the lysates were analyzed using TCID50 assays based on the Reed–Muench method [48].
2.8. In Vivo Virulence Assay
Due to the partial attenuation after serial passages in cell cultures, it is necessary to screen suitable susceptible hosts for CyHV-2-WT. Twenty individuals of each commonly cultured crucian carp variety, including gibel carp var. CAS V (8 ± 2 and 200 ± 20 g), gibel carp var. CAS III (200 ± 20 g), Fang Zheng crucian carp (200 ± 20 g), white crucian carp (200 ± 20 g), and goldfish (8 ± 2 and 100 ± 10 g), were separately challenged using both intraperitoneal injection (at a dose of 106 TCID50) and immersion (in water with a final virus concentration of 103 TCID50/mL for 2 h or 2 days) methods. Subsequently, the fish were maintained in recirculating aquaculture systems at a temperature of 25 °C. To activate latent CyHV-2 infection, temperature stress was applied from the 14th day post-infection by gradually reducing the water temperature from 25 °C to 15 °C at a rate of 1 °C per day followed by an equal rate of temperature increase back to 25 °C. The fish were observed daily for 2 months and the survival rates were recorded for each group.
The virulence assays for CyHV-2-Δ55-CP and CyHV-2-Δ57-CP strains were conducted on goldfish weighing 8 ± 2 g. Goldfish were divided into three groups with 40 fish per group, which were immersed in continuously aerated water with final virus concentrations of 102 TCID50/mL, 103 TCID50/mL, and 104 TCID50/mL, respectively, for 2 days, ensuring comprehensive exposure to the virus. Moreover, the same volume of M199 was used as in the negative control group. Subsequently, the goldfish were maintained in recirculating aquaculture systems at a constant temperature of 25 °C throughout the entire assay period. Goldfish symptoms and survival rates within each group were monitored daily while PCR verification was performed on three randomly selected dead goldfish from each group. In each CyHV-2 strain, these assays were repeated three times.
To assess the in vivo replication capabilities of CyHV-2-Δ55-CP and CyHV-2-Δ57-CP, gibel carps (var. CAS V) weighing 200 ± 20 g were randomly divided into three groups with 70 fish per group and subsequently infected with CyHV-2-WT, CyHV-2-Δ55-CP and CyHV-2-Δ57-CP via intraperitoneal injection at a dose of 4 × 106 TCID50, respectively. The water temperature was maintained at 25 °C throughout the entire experiment. At 24 h post-infection and every 2 days thereafter, liver, spleen, and kidney samples were collected from nine randomly selected fish in each group. Tissues from every three fish were homogenized to generate one sample for viral titer determination using qPCR.
2.9. Absolute Quantitative PCR (qPCR)
Viral replication in gibel carps was evaluated by selecting the ORF72 gene encoding the major capsid protein of CyHV-2. The copy number of the ORF72 gene was quantified using a quantitative Real-Time PCR assay with primers 72qF (5′-GCGGATACGTTGGACGATCT-3′) and 72qR (5′-CTCGGCTCTGATGGTGTTGT-3′). A standard curve generated through gradient dilution of plasmid pGEX-4T-3-ORF72 was used for normalization purposes. Absolute qPCR was performed using Polarsignal qPCR mix (MIKX, Shenzhen, China) in the Roche LightCycler 480 system (Roche Diagnostics, Basel, Switzerland) under the following conditions: 94 °C for 20 s, followed by 40 cycles consisting of 94 °C for 10 s, 56 °C for 10 s and 72 °C for 10 s. Three independent biological replicates were conducted for each CyHV-2 strain, and three technical replicates were conducted for each qPCR assay.
2.10. NNV-Specific IgM Determined via ELISA
There was a more significant decrease in virulence observed in CyHV-2-Δ57-CP, which was selected for the preliminary assessment of its ability to induce antibody response in groupers. Orange-spotted groupers weighing 200 ± 20 g were intraperitoneally injected with 200 μL of infected GiCF cell lysates (109 copies/mL) of CyHV-2-WT, CyHV-2-Δ57-CP, and 0.1% formalin-inactivated GiCF cell lysates of CyHV-2-Δ57-CP, respectively. Serum samples (n = 6) were collected on the 21st day post-injection. The levels of anti-NNV antibodies in sera were evaluated using indirect enzyme-linked immunosorbent assay (ELISA). ELISA plates (Nunc Maxisorp, Fisher Scientific, MA, USA) were coated overnight at 4 °C with NNV-VLP in coating buffer (100 mM bicarbonate/carbonate, pH 9.6), followed by blocking with 5% goat serum in PBST for one hour at room temperature. After the washing steps, serum samples diluted at a ratio of 1:10 in PBST were added to the plate and incubated for one hour at room temperature. Subsequently, the plate was incubated with mouse anti-grouper IgM-specific monoclonal antibodies (1:5000 dilution) (provided by Prof. Hui Gong, Biotechnology Institute, Fujian Academy of Agricultural Sciences, Fuzhou, Fujian, China) for one hour at room temperature, which was detected by HRP-conjugated goat anti-mouse IgG (1:5000 dilution) (Promega, WI, USA) following the same procedure. Finally, the color reaction was developed by adding 100 μL per well of a single solution of 3,3′,5,5′-tetramethylbenzidine (Tiangen, Beijing, China) for 20 min and stopped by adding 50 µL of 2 M sulfuric acid. Absorbance (optical density) was measured at a wavelength of 450 nm.
2.11. Statistics Analysis
Statistical analysis was carried out using GraphPad Prism 9.5.1. Two-way analysis of variance (ANOVA) was employed to analyze viral replications both in vitro and in vivo. Survival curves of goldfish infected with CyHV-2 were analyzed using the Kaplan–Meier method. ELISA data were analyzed using one-way ANOVA. Results are presented as mean ± standard error of the mean (SEM). Statistical significance was represented as follows: significant differences (* p < 0.05), very significant differences (** p < 0.01), and highly significant differences (*** p < 0.001).
3. Results
3.1. Construction of the CyHV-2 Recombinant Mutants
Recombinant transfer vectors pUC18-Δ55-CP and pUC18-Δ57-CP were constructed as shown in Figure 1. GiCF cells were transfected with pUC18-Δ55-CP and pUC18-Δ57-CP, respectively, and then infected with CyHV-2-WT for recombination. Plaques exhibiting green fluorescence activity were selected after two rounds of puromycin selection to enrich recombinant CyHV-2 mutants. These mutants were further purified through eighteen rounds of fluorescent plaque purification (Figure 2a). After 1 and 10 passages (P21 and P30) in GiCF cells, the absence of ORF55 and ORF57 genes was verified in recombinant mutants CyHV-2-Δ55-CP and CyHV-2-Δ57-CP, respectively, using nested-PCR (Figure 2b). Furthermore, the whole-genome sequencing confirmed that only the target gene (ORF55 or ORF57) had been replaced by the NNV-CP fusion protein expression cassette (Figure 3). These results indicate that CyHV-2 recombinant mutants CyHV-2-Δ55-CP and CyHV-2-Δ57-CP have been successfully generated, and their purity meets the requirements for subsequent experiments.
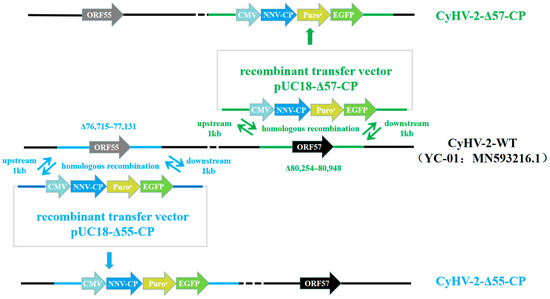
Figure 1.
Construction of CyHV-2-Δ55-CP and CyHV-2-Δ57-CP. The ORF55 or ORF57 of the CyHV-2 genome was replaced by homologous recombination with transfer vectors containing the CMV promoter, NNV-CP gene, puromycin resistance gene, and EGFP gene.
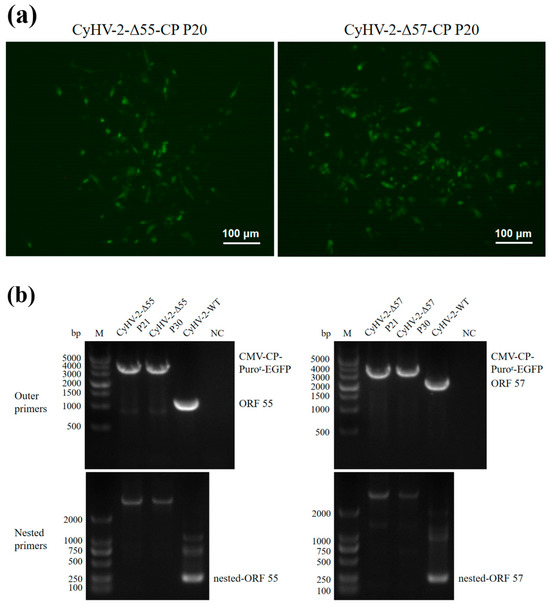
Figure 2.
Purity assessment of CyHV-2-Δ55-CP and CyHV-2-Δ57-CP. (a) Fluorescence plaques of the CyHV-2 recombinant mutants in GiCF. (b) Nested-PCR targeting ORF55 or ORF57. No ORF55 or ORF57 amplicons were detected in both the 21st and 30th passage CyHV-2 recombinant mutants.
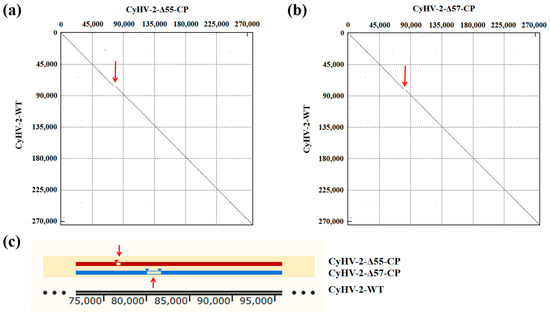
Figure 3.
Resequencing of the whole genomes of the CyHV-2 recombinant mutants. (a) The dot matrix alignment graph between CyHV-2-Δ55-CP and CyHV-2-WT. (b) The dot matrix alignment graph between CyHV-2-Δ57-CP and CyHV-2-WT. (c) Resequencing of the whole genomes of the CyHV-2 recombinant mutants. Multiple sequence alignment of CyHV-2-Δ55-CP, CyHV-2-Δ57-CP, and CyHV-2-WT. The gaps indicated by the red arrows in the figure represent the replacement of CyHV-2 ORF55 or ORF57 with the NNV-CP fusion protein expression cassette.
3.2. Characteristics of the CyHV-2 Recombinant Mutants
To analyze the morphology of CyHV-2-Δ55-CP and CyHV-2-Δ57-CP virions, purified virus particles were negatively stained with phosphotungstic acid before being observed under TEM. Imaging examination revealed that both CyHV-2-Δ55-CP and CyHV-2-Δ57-CP virions were enveloped, icosahedral particles, and closely resembled CyHV-2-WT virions (Figure 4a). Transmission electron microscopy (TEM) observations of GiCF cells infected with any one of these CyHV-2 strains revealed a similar and orderly arrangement of numerous capsomers within the cell nucleus (Figure 4b). This suggests that the deletion of either the ORF55 or ORF57 gene from CyHV-2 did not have any discernible influence on viral morphology or structure. Additionally, the replication abilities of CyHV-2-Δ55-CP and CyHV-2-Δ57-CP were evaluated in GiCF cells through in vitro experiments (Figure 4c). Cells were infected with CyHV-2-Δ55-CP, CyHV-2-Δ57-CP, or CyHV-2-WT strains, and viral titers were quantified at the designated time points post-infection. The replication abilities of CyHV-2-Δ55-CP, CyHV-2-Δ57-CP, and CyHV-2-WT in GiCF cells were found to be comparable, as depicted in Figure 4c (p > 0.05). Furthermore, all three variants reached their peak titers at 5 dpi with measurements of 6.50, 6.23, and 6.41 TCID50/mL, respectively. These results suggest that the replacement of the ORF55 or ORF57 gene with an NNV-CP fusion protein expression cassette does not have any discernible impact on the replication ability of CyHV-2 in GiCF cells.
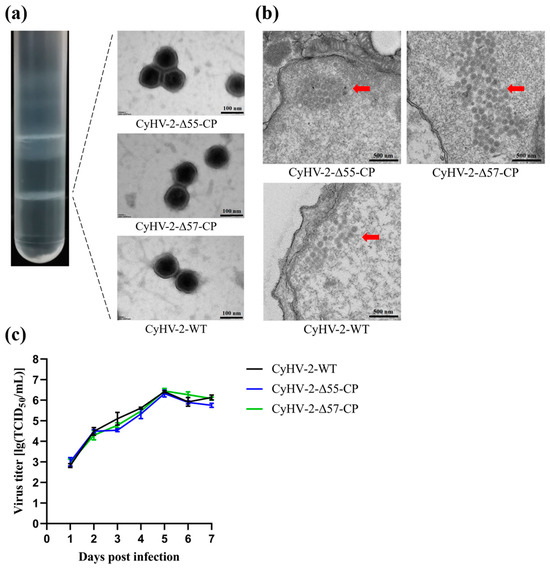
Figure 4.
Characteristics of CyHV-2-Δ55-CP and CyHV-2-Δ57-CP. (a) Virion images photographed by a transmission electron microscope. Purified recombinant and wild-type CyHV-2 virions treated by negative staining methods. Scale bar = 100 nm. (b) Transmission electron micrograph of infected GiCF cells. Red arrows indicate the arrangement of recombinant and wild-type CyHV-2 nucleocapsids in the nucleus. Scale bar = 500 nm. (c) Replication kinetics of CyHV-2-Δ55-CP and CyHV-2-Δ57-CP were evaluated in GiCF cells. At the indicated time points, cell samples were collected, and viral titers were determined using TCID50 assays.
3.3. The Expression of the NNV-CP Fusion Protein
The expression of the NNV-CP fusion protein by CyHV-2-Δ55-CP and CyHV-2-Δ57-CP was evaluated in GiCF cells. At 2 dpi, Western blotting assays confirmed the presence of the expected 86 kDa NNV-CP fusion protein in infected GiCF cell lysates of both CyHV-2 recombinant mutants (Figure 5a). Furthermore, indirect immunofluorescence assays conducted on fixed cells at 2 dpi revealed co-localization between enhanced green fluorescent protein (EGFP) and the rabbit polyclonal antibody against NNV VLP, confirming expression of the NNV-CP fusion protein within GiCF cells. Fluorescence signals were observed in both the cytoplasm and the nucleus (Figure 5b), suggesting that the NNV-CP fusion protein still maintains the nuclear localization function of NNV-CP [49].
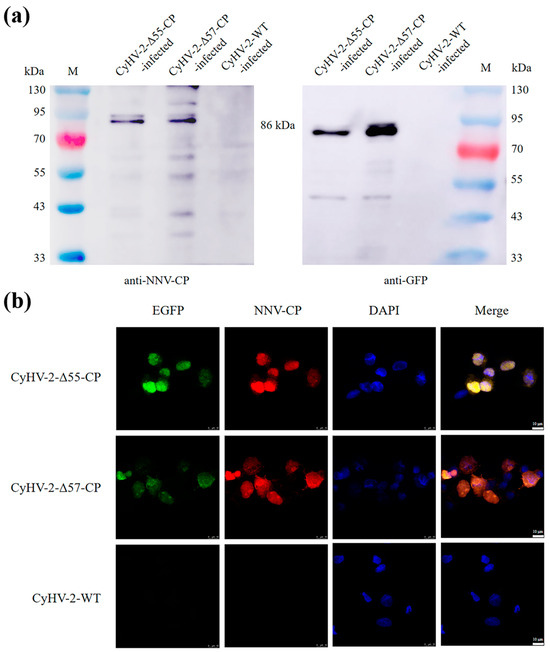
Figure 5.
Validation of the NNV-CP fusion protein expression in CyHV-2-Δ55-CP and CyHV-2-Δ57-CP by Western blotting (a) and indirect immunofluorescence assay (b). (a) At 48 h post-infection, the NNV-CP fusion protein was detected with the rabbit polyclonal antibody against NNV VLP and the mouse monoclonal antibody against GFP, respectively. (b) At 48 h post-infection, the NNV-CP fusion protein was detected using the rabbit polyclonal antibody against NNV VLP, followed by Alexa Fluor 555-conjugated goat anti-rabbit IgG (red). Cell nuclei were stained using DAPI (blue). Scale bar = 10 μm.
3.4. Virulence Attenuation in the CyHV-2 Recombinant Mutants
To identify suitable animal models for further experiments, a challenge experiment using CyHV-2-WT was conducted on various common crucian carp varieties as shown in Table 2. Results showed that only juvenile goldfish weighing 8 ± 2 g (one year old) exhibited mortality, possibly due to other varieties’ stronger disease resistance resulting from selective breeding. A recent study indicates that, although CyHV-2 showed a higher susceptibility in adult goldfish, it was more permissive to replication in larvae, resulting in rapid systemic infection and high mortality in juvenile goldfish [50]. Moreover, prolonged immersion in virus-contaminated water is more likely to induce acute mortality in goldfish. Therefore, challenge experiments were performed using juvenile goldfish to evaluate the virulence of CyHV-2-Δ55-CP and CyHV-2-Δ57-CP (Figure 6). Fish infected with either mutant or wild-type strain displayed typical clinical symptoms such as hemorrhaging, anorexia, lethargy, and bottom-dwelling behavior. The mean survival rates for each group are presented in Table 3.

Table 2.
Survival rates of common cultured crucian carp varieties challenged by CyHV-2-WT.
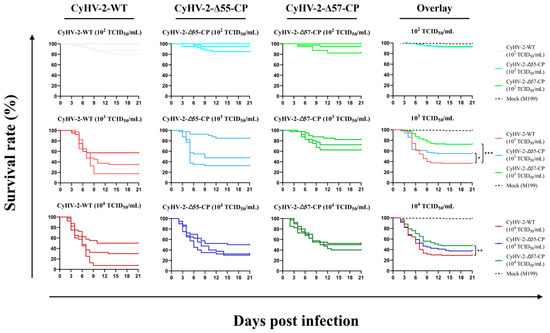
Figure 6.
The virulence of recombinant and wild-type CyHV-2 strains in goldfish. On day 0, goldfish were infected for 2 days by immersion in water with virus concentrations of 102 TCID50/mL, 103 TCID50/mL, and 104 TCID50/mL, respectively. The nine panels at left show the survival curves observed for three replicates. The three overlay panels at right show the cumulative survival curves based on the three replicates. Statistical significance was represented as follows: * p < 0.05, ** p < 0.01, and *** p < 0.001.

Table 3.
Mean survival rates of goldfish challenged by recombinant or wild-type CyHV-2 strains.
In the challenge experiment with a virus concentration of 102 TCID50/mL, all CyHV-2 strains only induced mortality in a few weak individuals without any significant statistical differences observed among the groups (p > 0.05). However, in the challenge experiment with a virus concentration of 103 TCID50/mL, compared to the CyHV-2-WT-challenged group, both the CyHV-2-Δ55-CP- and CyHV-2-Δ57-CP-challenged groups showed higher survival rates with significant (* p < 0.05) and highly significant (*** p < 0.001) differences, respectively. Moreover, in the challenge experiment with a virus concentration of 104 TCID50/mL, only the CyHV-2-Δ57-CP-challenged group demonstrated a very significant difference in survival rate compared to the CyHV-2-WT-challenged group (** p < 0.01).
Furthermore, in vivo replication capabilities of CyHV-2-Δ55-CP and CyHV-2-Δ57-CP have been evaluated in gibel carps (var. CAS V). As shown in Figure 7, the copy numbers of CyHV-2-WT in liver, spleen, and kidney reached their peak at 9 days post-infection, while the CyHV-2 recombinant mutants, although exhibiting higher copies in some samples, remained relatively stable overall and were gradually reduced. In the liver, compared to the CyHV-2-WT-challenged group, only the copy numbers in the CyHV-2-Δ57-CP-challenged group showed significant (* p < 0.05) and very significant (** p < 0.01) differences at 9 and 11 days post-infection, respectively (Figure 7a). In the spleen, copy numbers of CyHV-2-Δ55-CP and CyHV-2-Δ57-CP demonstrated very significant (** p < 0.01) and highly significant (*** p < 0.001) differences at 13 dpi, respectively, compared with CyHV-2-WT (Figure 7b). Similarly, in the kidney, copy numbers of CyHV-2-Δ55-CP and CyHV-2-Δ57-CP showed significant (* p < 0.05) and very significant (** p < 0.01) differences at 9 dpi, respectively, compared with CyHV-2-WT (Figure 7c).
Figure 7.
The replication of recombinant or wild-type CyHV-2 strains in gibel carp. On day 0, gibel carps were infected by intraperitoneal injection at a viral dose of 4 × 106 TCID50. Liver (a), spleen (b) and kidney (c) tissues were collected for absolute qPCR targeting CyHV-2 ORF72. Statistical significance was represented as follows: * p < 0.05, ** p < 0.01, and *** p < 0.001.
In conclusion, these results suggest that both CyHV-2-Δ55-CP and CyHV-2-Δ57-CP showed partial attenuation of virulence which was more obvious in CyHV-2-Δ57-CP.
3.5. Infected GiCF Cell Lysates of CyHV-2-Δ57-CP Induced Antibody Response in Grouper
On the 21st day post-vaccination, blood samples were collected from six groupers in each group for subsequent anti-NNV specific IgM level determination using indirect ELISA assay. The results revealed that the presence of specific anti-NNV IgM antibodies in the serum of groupers injected with either cell culture suspension of CyHV-2-Δ57-CP or 0.1% formalin-inactivated suspension of CyHV-2-Δ57-CP (Figure 8). Moreover, compared to the control group injected with cell culture suspension of CyHV-2-WT, injection of cell culture suspension of CyHV-2-Δ57-CP significantly induced a production of specific anti-NNV IgM antibodies with very significant differences (** p < 0.01), while injection of 0.1% formalin-inactivated suspension of CyHV-2-Δ57-CP only resulted in significant differences (* p < 0.05). These results suggested that live CyHV-2-Δ57-CP suspension may elicit higher titers of anti-NNV IgM antibodies compared to formalin-inactivated suspension.
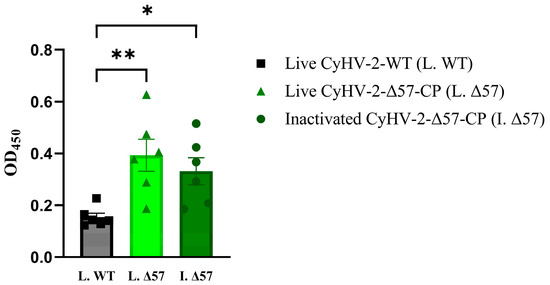
Figure 8.
NNV-specific IgM determined by ELISA. Twenty-one days post-vaccination, serum samples were collected from vaccinated grouper, and the levels of NNV-specific IgM were determined by ELSA. Statistical significance was represented as follows: * p < 0.05 and ** p < 0.01.
4. Discussion
The global crucian carp and goldfish industries have suffered significant economic losses due to the epidemic of herpesviral hematopoietic necrosis (HVHN) disease [1]. Therefore, there is an urgent need for an effective vaccine that allows mass immunization of cost-effective juveniles, such as crucian carp fry. Attenuated vaccines have shown the most promising overall performance for CyHV-3 vaccines so far [26]. Identifying key virulence genes that are non-essential for in vitro amplification is crucial for developing recombinant attenuated vaccines. In this study, we constructed two CyHV-2 recombinant mutants, namely CyHV-2-Δ55-CP and CyHV-2-Δ57-CP, and evaluated their morphology, replication capability, pathogenicity, and ability to express heterologous proteins.
In the Alloherpesviridae family, several gene-deleted strains have been reported; however, current research mainly focuses on CCV and CyHV-3. In 1995, Zhang et al. reported a TK-deleted recombinant CCV strain that exhibited unaltered replicative capacity in channel catfish ovary (CCO) cells but showed significantly reduced virulence while inducing immune protection against CCV [28]. The infection kinetics of this TK-deleted CCV were similar to those of the wild-type CCV; however, the infection duration was shorter and shedding ability weaker [51]. Another study by Kunec et al. demonstrated that ORF12 gene deletion does affect in vitro replication of CCV [52], although its in vivo virulence was not determined. Vanderheijden et al., on the other hand, reported an attenuated CCV strain V60 with a large deletion observed in ORF50 gene (which may encode a secreted glycoprotein) [53], although it was not conclusively proven whether attenuation of virulence directly resulted from ORF50 segment deletion.
In CyHV-3, the TK gene initially garnered attention. Costes et al. generated ORF16 (putative G protein-coupled receptor)-deleted and ORF55 (TK)-excised CyHV-3 mutants; however, only the ORF55-excised mutant exhibited partially attenuated virulence [54]. Fuchs et al. constructed a series of CyHV-3 recombinant mutants with deletions in ORF55, ORF123 (deoxyuridine triphosphate pyrophosphatase), ORF141 (large subunit of ribonucleotide reductase), and both ORF55 and ORF123 genes [29]. The results indicated that all mutants can replicate in vitro, with only the ORF141-deleted CyHV-3 mutant showing reduced replication capability in vitro, while single-gene deletions of either ORF55 or ORF123 resulted in partial attenuation of CyHV-3 virulence [29]. Subsequent studies have revealed that double deletions of both ORF55 and ORF123 genes significantly attenuate virulence and induce significant immune protection in CyHV-3, making them a potential candidate for a recombinant attenuated vaccine [30,55]. Vancsok et al. created gene deletion mutants for 16 predicted virion transmembrane proteins (VTPs) in CyHV-3, including ORFs 25, 32, 59, 64, 65, 81, 83, 99, 106, 108, 115, 131, 132, 136, 148, and 149 [56]. However, only the deletion of the ORF25 gene resulted in substantial attenuation but showed poor immune protection [57]. In another study, the extent of virulence attenuation in the double-gene deletion mutants of ORF148 and ORF149 remains insufficient to meet the requirements for recombinant attenuated vaccine candidates [57]. Although deleting members of the glycoprotein family may lead to virulence attenuation, their suitability as vaccine candidates is limited due to potential effects on immunogenicity and relatively high sensitivity to mutations in paralogous genes. A recent study has demonstrated that the deletion of the ORF150 gene lead to significant virulence attenuation and effective immune protection in CyHv-3 mutants [58]. However, this vaccine candidate still requires extensive field trials to assess its effectiveness in real aquaculture environments. As previously mentioned in the introduction, ORF57 has been identified as a dispensable key virulence gene in CyHV-3 and it is conserved within the CyHVs [25,27]. Furthermore, the safety and efficacy of ORF57-deleted CyHV-3 mutants as candidates for recombinant attenuated vaccines have also been validated [24,25,56].
In our study, there was no significant alterations in the morphology and in vitro replication capabilities of CyHV-2-Δ55-CP and CyHV-2-Δ57-CP, indicating that both ORF55 and ORF57 genes are dispensable in CyHV-2. In the in vivo virulence assay, partial attenuation of virulence was observed for both CyHV-2-Δ55-CP and CyHV-2-Δ57-CP, with a more pronounced reduction seen for CyHV-2-Δ57-CP. Although the function of orthologues of pORF57 in genus Cyvirus remains unknown, previous research has demonstrated their abundant presence in viral particles for both orthologues in CyHV-3 [59] and AngHV-1 [60], while pORF57 has been identified as a major immunogenic protein in CyHV-2 [61]. The deletion of the TK gene primarily affects viral replication in non-replicating cells lacking cellular TK [26]. However, this negative impact on TK-deleted viruses can be partially offset by a higher proportion of dividing cells found in juvenile fish [26]. This may contribute to the incomplete virulence attenuation of TK-deleted viruses. It should be noted that the potential impact of NNV-CP fusion protein on the replication capabilities of CyHV-2 remains unknown, but research has shown that the precursor protein α of RGNNV-CP can induce apoptosis in host cells [62]. Overall, although neither CyHV-2 mutants CyHV-2-Δ55-CP and CyHV-2-Δ57-CP achieved sufficient levels of virulence attenuation to meet the requirements for recombinant attenuated vaccine candidates, ORF55 and ORF57 genes still serve as reference targets for constructing multi-gene-deleted CyHV-2 mutants.
Furthermore, capsid protein (CP) is known as the sole structural protein of NNV and has been extensively studied for vaccine development through recombinant expression using various vectors and systems, such as bacteria [63], yeast [64], insect cells [65], avian cells [66], and plant cells [67]. In our study using CyHV-2 as viral vector, successful expression of the NNV-CP fusion protein was achieved in GiCF cells. Some NNV-CP fusion proteins can self-assemble into VLPs, with foreign proteins fused at their C-terminal displayed on their surface [68]. However, in this study, the assembly of NNV VLPs within GiCF cells was difficult due to strong interference caused by C-terminus fusion with large protein [68]. In some studies, NNV-CP fusion proteins without a VLP formation have also demonstrated strong immunogenicity and provided effective protection for grouper [63,69]. Groupers that received intraperitoneal injections of GiCF cell lysates containing live or inactivated CyHV-2-Δ57-CP also produced specific anti-NNV IgM antibodies. However, further research is needed to determine its capacity for inducing effective immune protection and the ability of CyHV-2 recombinant mutants to replicate in unnatural hosts. In summary, this study demonstrates that CyHV-2 has the ability to express foreign proteins, which elicit the production of specific IgM antibodies in vaccinated fish, suggesting its potential as a viral vector. This provides insights into the development of replicative vaccines expressing foreign proteins within hosts and expression systems for foreign proteins in sensitive cell lines. For instance, by replacing the key virulence gene with the glycoprotein gene of SVCV (spring viremia of carp virus, Sprivivirus cyprinus), it would be possible to construct bivalent recombinant attenuated vaccine candidates against the two main viruses affecting crucian carp: CyHV-2 and SVCV.
5. Conclusions
In conclusion, we successfully generated two CyHV-2 recombinant mutants, namely CyHV-2-Δ55-CP and CyHV-2-Δ57-CP, by inserting the NNV-CP fusion protein expression cassette while deleting the ORF55 or ORF57 gene. Although dispensable for viral replication in vitro, both ORF55 and ORF57 genes of CyHV-2 contribute to virulence in vivo. Additionally, our findings demonstrate that CyHV-2 can effectively express foreign proteins capable of inducing an antibody response in vaccinated fish. These preliminary research results highlight the potential of fully attenuated CyHV-2 as a viral vector for developing subunit vaccines or multivalent recombinant attenuated vaccines.
Author Contributions
Conceptualization, D.X., C.D. and L.L.; methodology, C.D., D.X. and Z.F.; software, M.M.; validation, Z.F., W.C. and C.D.; formal analysis, Z.F., C.Y. and Y.Z.; investigation, Z.F., C.Y. and H.W.; resources, C.D., D.X., L.L. and L.G.; data curation, D.X. and Z.F.; writing—original draft preparation, Z.F.; writing—review and editing, C.D., D.X. and Y.Z.; visualization, Z.F., Y.Z. and M.M.; supervision, C.D., D.X. and H.W.; project administration, D.X. and L.L.; funding acquisition, D.X., C.D., H.W. and L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the General Fund Project of Shanghai Natural Science Foundation (Program No. 22ZR1427200), the National Key Research and Development Program of China (2022YFD2401600), and the Guangdong Provincial Special Fund for MAITIT (2019KJ141).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Experimental Animal Management Law of China and was approved by the Animal Ethics Review Committee at Shanghai Ocean University (Number: SHOW-DW-2023-078, SHOW-DW-2023-079, SHOW-DW-2023-080 and SHOW-DW-2023-081).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank Junfeng Xie, State Key Laboratory of Biocontrol/School of Life Sciences, Sun Yat-sen University, Guangzhou, Guangdong, China, for providing us with the NNV VLP and rabbit anti-NNV VLP polyclonal antibody. The authors would also like to thank Hui Gong, Biotechnology Institute, Fujian Academy of Agricultural Sciences, Fuzhou, Fujian, China, for providing us with the mouse anti-grouper IgM-specific monoclonal antibody.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thangaraj, R.S.; Nithianantham, S.R.; Dharmaratnam, A.; Kumar, R.; Pradhan, P.K.; Thangalazhy Gopakumar, S.; Sood, N. Cyprinid herpesvirus-2 (CyHV-2): A comprehensive review. Rev. Fish. Sci. 2020, 13, 796–821. [Google Scholar] [CrossRef]
- Dadar, M.; Dhama, K.; Vakharia, V.N.; Hoseinifar, S.H.; Karthik, K.; Tiwari, R.; Khandia, R.; Munjal, A.; Salgado-Miranda, C.; Joshi, S.K. Advances in Aquaculture Vaccines Against Fish Pathogens: Global Status and Current Trends. Rev. Fish. Sci. 2016, 25, 184–217. [Google Scholar] [CrossRef]
- Saito, H.; Okamura, T.; Shibata, T.; Kato, G.; Sano, M. Development of a live attenuated vaccine candidate against herpesviral hematopoietic necrosis of goldfish. Aquaculture 2022, 552, 737974. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, C.; Wang, H.; Qiao, G.; Wang, Z.; Li, Z.; Li, Q.; Wei, C. An attenuated strain of cyprinid herpesvirus 2 as a vaccine candidate against herpesviral hematopoietic necrosis disease in gibel carp, Carassius auratus gibelio. Fish Shellfish Immunol. 2023, 138, 108826. [Google Scholar] [CrossRef] [PubMed]
- Ototake, M.; Ito, T. Vaccination against cyprinid herpesvirus 2 (CyHV-2) infection in goldfish Carassius auratus. Bull. Eur. Assoc. Fish Pathol. 2013, 33, 158–164. [Google Scholar]
- Zhang, L.L.; Ma, J.; Fan, Y.D.; Zhou, Y.; Xu, J.; Liu, W.Z.; Gu, Z.M.; Zeng, L.B. Immune response and protection in gibel carp, Carassius gibelio, after vaccination with β-propiolactone inactivated cyprinid herpesvirus 2. Fish Shellfish Immunol. 2016, 49, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Huo, X.; Ai, T.; Su, J. β-glucan and anisodamine can enhance the immersion immune efficacy of inactivated cyprinid herpesvirus 2 vaccine in Carassius auratus gibelio. Fish Shellfish Immunol. 2020, 98, 285–295. [Google Scholar] [CrossRef]
- Dharmaratnam, A.; Sudhagar, A.; Das, S.; Nair, R.R.; Nithianantham, S.R.; Preena, P.G.; Lekshmi, N.; Swaminathan, T.R. Immune gene expression and protective effects in goldfish (Carassius auratus L.) immunized with formalin-inactivated cyprinid herpesvirus-2 (CyHV-2) vaccine. Microb. Pathog. 2022, 164, 105452. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Fan, C.; Ai, T.; Su, J. The Combination of Molecular Adjuvant CCL35.2 and DNA Vaccine Significantly Enhances the Immune Protection of Carassius auratus gibelio against CyHV-2 Infection. Vaccines 2020, 8, 567. [Google Scholar] [CrossRef]
- Yuan, X.; Shen, J.; Pan, X.; Yao, J.; Lyu, S.; Liu, L.; Zhang, H. Screening for protective antigens of Cyprinid herpesvirus 2 and construction of DNA vaccines. J. Virol. Methods 2020, 280, 113877. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, N.; Ma, J.; Fan, Y.D.; Zhang, L.; Xu, J.; Zeng, L.B. Protective immunity in gibel carp, Carassius gibelio of the truncated proteins of cyprinid herpesvirus 2 expressed in Pichia pastoris. Fish Shellfish Immunol. 2015, 47, 1024–1031. [Google Scholar] [CrossRef]
- Yan, N.; Xu, K.; Li, X.; Liu, Y.; Bai, Y.; Zhang, X.; Han, B.; Chen, Z.; Zhang, Z. Recombinant Saccharomyces cerevisiae serves as novel carrier for oral DNA vaccines in Carassius auratus. Fish Shellfish Immunol. 2015, 47, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.W.; Liu, S.J.; Nan, H.; Zhao, K.X.; Xu, X.D.; Wang, G.X.; Ji, H.; Chen, H.Y. Immersion immunization with recombinant baculoviruses displaying cyprinid herpesvirus 2 membrane proteins induced protective immunity in gibel carp. Fish Shellfish Immunol. 2019, 93, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yuan, R.; Zhang, M.T.; Zhang, T.T.; Gu, Y.C.; Zhou, Y.; Dai, Y.; Fang, P.; Feng, Y.; Hu, X.; et al. Recombinant baculovirus BacCarassius-D4ORFs has potential as a live vector vaccine against CyHV-2. Fish Shellfish Immunol. 2019, 92, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gu, Y.; Liu, X.; Yuan, R.; Zhou, Y.; Dai, Y.; Fang, P.; Feng, Y.; Cao, G.; Chen, H.; et al. Incidence of Carassius auratus Gibelio Gill Hemorrhagic Disease Caused by CyHV-2 Infection Can Be Reduced by Vaccination with Polyhedra Incorporating Antigens. Vaccines 2021, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.R.; Mu, Q.J.; Kong, W.G.; Qin, D.C.; Zhou, Y.; Wang, X.Y.; Cheng, G.F.; Luo, Y.Z.; Ai, T.S.; Xu, Z. Gut mucosal immune responses and protective efficacy of oral yeast Cyprinid herpesvirus 2 (CyHV-2) vaccine in Carassius auratus gibelio. Front. Immunol. 2022, 13, 932722. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, M.; Luo, S.; Yang, G.; Lu, X.; Lu, J.; Chen, J. Oral Vaccination of Recombinant Saccharomyces cerevisiae Expressing ORF132 Induces Protective Immunity against Cyprinid Herpesvirus-2. Vaccines 2023, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ji, W.; Xu, Z. Current use and development of fish vaccines in China. Fish Shellfish Immunol. 2020, 96, 223–234. [Google Scholar] [CrossRef]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef]
- Mondal, H.; Thomas, J. A review on the recent advances and application of vaccines against fish pathogens in aquaculture. Aquac. Int. 2022, 30, 1971–2000. [Google Scholar] [CrossRef]
- Su, H.; Su, J. Cyprinid viral diseases and vaccine development. Fish Shellfish Immunol. 2018, 83, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Current ICTV Taxonomy Release. Available online: https://ictv.global/taxonomy (accessed on 7 December 2023).
- Davison, A.J.; Kurobe, T.; Gatherer, D.; Cunningham, C.; Korf, I.; Fukuda, H.; Hedrick, R.P.; Waltzek, T.B. Comparative genomics of carp herpesviruses. J. Virol. 2013, 87, 2908–2922. [Google Scholar] [CrossRef] [PubMed]
- Boutier, M.; Ronsmans, M.; Ouyang, P.; Fournier, G.; Reschner, A.; Rakus, K.; Wilkie, G.S.; Farnir, F.; Bayrou, C.; Lieffrig, F.; et al. Rational development of an attenuated recombinant cyprinid herpesvirus 3 vaccine using prokaryotic mutagenesis and in vivo bioluminescent imaging. PLoS Pathog. 2015, 11, e1004690. [Google Scholar] [CrossRef] [PubMed]
- Boutier, M.; Gao, Y.; Vancsok, C.; Suarez, N.M.; Davison, A.J.; Vanderplasschen, A. Identification of an essential virulence gene of cyprinid herpesvirus 3. Antivir. Res. 2017, 145, 60–69. [Google Scholar] [CrossRef]
- Boutier, M.; Gao, Y.; Donohoe, O.; Vanderplasschen, A. Current knowledge and future prospects of vaccines against cyprinid herpesvirus 3 (CyHV-3). Fish Shellfish Immunol. 2019, 93, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.P. Study of Potential Virulence Genes of Cyprinid herpesvirus 2 (CyHV-2); Shanghai Ocean University: Shanghai, China, 2021. [Google Scholar]
- Zhang, H.G.; Hanson, L.A. Deletion of Thymidine Kinase Gene Attenuates Channel Catfish Herpesvirus While Maintaining Infectivity. Virology 1995, 209, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, W.; Fichtner, D.; Bergmann, S.M.; Mettenleiter, T.C. Generation and characterization of koi herpesvirus recombinants lacking viral enzymes of nucleotide metabolism. Arch. Virol. 2011, 156, 1059–1063. [Google Scholar] [CrossRef]
- Schroder, L.; Klafack, S.; Bergmann, S.M.; Fichtner, D.; Jin, Y.; Lee, P.Y.; Hoper, D.; Mettenleiter, T.C.; Fuchs, W. Generation of a potential koi herpesvirus live vaccine by simultaneous deletion of the viral thymidine kinase and dUTPase genes. J. Gen. Virol. 2019, 100, 642–655. [Google Scholar] [CrossRef]
- Qian, M.; Xiao, S.; Yang, Y.; Yu, F.; Wen, J.; Lu, L.; Wang, H. Screening and identification of cyprinid herpesvirus 2 (CyHV-2) ORF55-interacting proteins by phage display. Virol. J. 2023, 20, 66. [Google Scholar] [CrossRef]
- Yang, Z.; Yue, G.H.; Wong, S.-M. VNN disease and status of breeding for resistance to NNV in aquaculture. Aquac. Fish. 2022, 7, 147–157. [Google Scholar] [CrossRef]
- Bandin, I.; Souto, S. Betanodavirus and VER Disease: A 30-year Research Review. Pathogens 2020, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Souto, S.; Merour, E.; Le Coupanec, A.; Lamoureux, A.; Bernard, J.; Bremont, M.; Millet, J.K.; Biacchesi, S. Recombinant viral hemorrhagic septicemia virus with rearranged genomes as vaccine vectors to protect against lethal betanodavirus infection. Front. Immunol. 2023, 14, 1138961. [Google Scholar] [CrossRef] [PubMed]
- McCann, N.; O’Connor, D.; Lambe, T.; Pollard, A.J. Viral vector vaccines. Curr. Opin. Immunol. 2022, 77, 102210. [Google Scholar] [CrossRef] [PubMed]
- Sasso, E.; D’Alise, A.M.; Zambrano, N.; Scarselli, E.; Folgori, A.; Nicosia, A. New viral vectors for infectious diseases and cancer. Semin. Immunol. 2020, 50, 101430. [Google Scholar] [CrossRef]
- Allam, A.M.; Elbayoumy, M.K.; Ghazy, A.A. Perspective vaccines for emerging viral diseases in farm animals. Clin. Exp. Vaccine Res. 2023, 12, 179–192. [Google Scholar] [CrossRef]
- Kamel, M.; El-Sayed, A. Utilization of herpesviridae as recombinant viral vectors in vaccine development against animal pathogens. Virus Res. 2019, 270, 197648. [Google Scholar] [CrossRef]
- Li, S.; Xie, H.; Yan, Z.; Li, B.; Wu, P.; Qian, X.; Zhang, X.; Wu, J.; Liu, J.; Zhao, X. Development of a live vector vaccine against infectious hematopoietic necrosis virus in rainbow trout. Fish Shellfish Immunol. 2019, 89, 516–524. [Google Scholar] [CrossRef]
- Li, S.; Hu, Y.; Li, X.; Han, S.; Zhang, B.; Yan, Z.; Xue, R.; Gao, Q.; Wu, J.; Zhao, X.; et al. Development of a live vector vaccine against infectious pancreatic necrosis virus in rainbow trout. Aquaculture 2020, 524, 735275. [Google Scholar] [CrossRef]
- McKenna, B.M.; Fitzpatrick, R.M.; Phenix, K.V.; Todd, D.; Vaughan, L.M.; Atkins, G.J. Formation of infectious pancreatic necrosis virus-like particles following expression of segment A by recombinant semliki forest virus. Mar. Biotechnol. 2001, 3, 103–110. [Google Scholar] [CrossRef]
- Hikke, M.C.; Verest, M.; Vlak, J.M.; Pijlman, G.P. Salmonid alphavirus replication in mosquito cells: Towards a novel vaccine production system. Microb. Biotechnol. 2014, 7, 480–484. [Google Scholar] [CrossRef]
- Wolf, A.; Hodneland, K.; Frost, P.; Braaen, S.; Rimstad, E. A hemagglutinin-esterase-expressing salmonid alphavirus replicon protects Atlantic salmon (Salmo salar) against infectious salmon anemia (ISA). Vaccine 2021, 31, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Z.; Liu, M.; Xu, L.M.; Zhang, Z.Y.; Cao, Y.S.; Shao, Y.Z.; Yin, J.S.; Liu, H.B.; Lu, T.Y. A chimeric recombinant infectious hematopoietic necrosis virus induces protective immune responses against infectious hematopoietic necrosis and infectious pancreatic necrosis in rainbow trout. Mol. Immunol. 2019, 116, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.G.; Hanson, L.A. Recombinant channel catfish virus (Ictalurid herpesvirus 1) can express foreign genes and induce antibody production against the gene product. J. Fish Dis. 1996, 19, 121–128. [Google Scholar] [CrossRef]
- Lu, J.F.; Xu, D.; Lu, L.Q. A novel cell line established from caudal fin tissue of Carassius auratus gibelio is susceptible to cyprinid herpesvirus 2 infection with the induction of apoptosis. Virus Res. 2018, 258, 19–27. [Google Scholar] [CrossRef]
- Xu, L.; Podok, P.; Xie, J.; Lu, L. Comparative analysis of differential gene expression in kidney tissues of moribund and surviving crucian carp (Carassius auratus gibelio) in response to cyprinid herpesvirus 2 infection. Arch. Virol. 2014, 159, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Guo, Y.X.; Dallmann, K.; Kwang, J. Identification of nucleolus localization signal of betanodavirus GGNNV protein α. Virology 2003, 306, 225–235. [Google Scholar] [CrossRef]
- He, B.; Sridhar, A.; Streiff, C.; Deketelaere, C.; Zhang, H.; Gao, Y.; Hu, Y.; Pirotte, S.; Delrez, N.; Davison, A.J.; et al. In Vivo Imaging Sheds Light on the Susceptibility and Permissivity of Carassius auratus to Cyprinid Herpesvirus 2 According to Developmental Stage. Viruses 2023, 15, 1746. [Google Scholar] [CrossRef]
- Kancharla, S.; Kancharla, S. Production and shedding of channel catfish virus (CCV) and thymidine kinase negative CCV in immersion exposed channel catfish fingerlings. Dis. Aquat. Org. 1996, 27, 25–34. [Google Scholar] [CrossRef]
- Kunec, D.; Hanson, L.A.; van Haren, S.; Nieuwenhuizen, I.F.; Burgess, S.C. An overlapping bacterial artificial chromosome system that generates vectorless progeny for channel catfish herpesvirus. J. Virol. 2008, 82, 3872–3881. [Google Scholar] [CrossRef]
- Vanderheijden, N.; Alard, P.; Lecomte, C.; Martial, J.A. The Attenuated V60 Strain of Channel Catfish Virus Possesses a Deletion in ORF50 Coding for a Potentially Secreted Glycoprotein. Virology 1996, 218, 422–426. [Google Scholar] [CrossRef]
- Costes, B.; Fournier, G.; Michel, B.; Delforge, C.; Raj, V.S.; Dewals, B.; Gillet, L.; Drion, P.; Body, A.; Schynts, F.; et al. Cloning of the koi herpesvirus genome as an infectious bacterial artificial chromosome demonstrates that disruption of the thymidine kinase locus induces partial attenuation in Cyprinus carpio koi. J. Virol. 2008, 82, 4955–4964. [Google Scholar] [CrossRef] [PubMed]
- Adamek, M.; Matras, M.; Rebl, A.; Stachnik, M.; Falco, A.; Bauer, J.; Miebach, A.C.; Teitge, F.; Jung-Schroers, V.; Abdullah, M.; et al. Don’t Let It Get Under Your Skin!—Vaccination Protects the Skin Barrier of Common Carp From Disruption Caused by Cyprinid Herpesvirus 3. Front. Immunol. 2022, 13, 787021. [Google Scholar] [CrossRef]
- Vancsok, C.; Penaranda, M.M.D.; Raj, V.S.; Leroy, B.; Jazowiecka-Rakus, J.; Boutier, M.; Gao, Y.; Wilkie, G.S.; Suarez, N.M.; Wattiez, R.; et al. Proteomic and Functional Analyses of the Virion Transmembrane Proteome of Cyprinid Herpesvirus 3. J. Virol. 2017, 91, e01209-17. [Google Scholar] [CrossRef] [PubMed]
- Schroder, L.; Klafack, S.; Bergmann, S.M.; Lee, P.A.; Franzke, K.; Hoper, D.; Mettenleiter, T.C.; Fuchs, W. Characterization of gene deletion mutants of Cyprinid herpesvirus 3 (koi herpesvirus) lacking the immunogenic envelope glycoproteins pORF25, pORF65, pORF148 and pORF149. Virus Res. 2019, 261, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Klafack, S.; Schroder, L.; Jin, Y.; Lenk, M.; Lee, P.Y.; Fuchs, W.; Avarre, J.C.; Bergmann, S.M. Development of an attenuated vaccine against Koi Herpesvirus Disease (KHVD) suitable for oral administration and immersion. NPJ Vaccines 2022, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Michel, B.; Leroy, B.; Stalin Raj, V.; Lieffrig, F.; Mast, J.; Wattiez, R.; Vanderplasschen, A.F.; Costes, B. The genome of cyprinid herpesvirus 3 encodes 40 proteins incorporated in mature virions. J. Gen. Virol. 2010, 91, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Beurden, S.J.V.; Leroy, B.; Wattiez, R.; Haenen, O.L.; Boeren, S.; Vervoort, J.J.; Peeters, B.P.; Rottier, P.J.; Engelsma, M.Y.; Vanderplasschen, A.F. Identification and localization of the structural proteins of anguillid herpesvirus 1. Vet. Res. 2011, 42, 105. [Google Scholar] [CrossRef]
- Gao, W.; Wen, H.; Wang, H.; Lu, J.Q.; Lu, L.Q.; Jiang, Y.S. Identification of structure proteins of cyprinid herpesvirus 2. Aquaculture 2020, 523, 735184. [Google Scholar] [CrossRef]
- Wu, H.C.; Chiu, C.S.; Wu, J.L.; Gong, H.Y.; Chen, M.C.; Lu, M.W.; Hong, J.R. Zebrafish anti-apoptotic protein zfBcl-xL can block betanodavirus protein alpha-induced mitochondria-mediated secondary necrosis cell death. Fish Shellfish Immunol. 2008, 24, 436–449. [Google Scholar] [CrossRef]
- Lin, T.; Xing, J.; Tang, X.; Sheng, X.; Chi, H.; Zhan, W. Immune and protective effects of subunit vaccines from S-domain or P-domain in capsid protein against nervous necrosis virus in pearl gentian grouper. Aquaculture 2023, 566, 739177. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, H.J.; Lan, N.T.; Han, H.J.; Lee, D.C.; Hwang, J.Y.; Kwon, M.G.; Kang, B.K.; Han, S.Y.; Moon, H.; et al. Oral vaccination through voluntary consumption of the convict grouper Epinephelus septemfasciatus with yeast producing the capsid protein of red-spotted grouper nervous necrosis virus. Vet. Microbiol. 2017, 204, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Thiery, R.; Cozien, J.; Cabon, J.; Lamour, F.; Baud, M.; Schneemann, A. Induction of a protective immune response against viral nervous necrosis in the European sea bass Dicentrarchus labrax by using betanodavirus virus-like particles. J. Virol. 2006, 80, 10201–10207. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.N.; Lin, L.; Weng, S.P.; He, J.G. High expression of capsid protein of red-spotted grouper nervous necrosis virus in an avian cell line requires viral RNA2 non-coding regions. J. Fish Dis. 2007, 30, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Marsian, J.; Hurdiss, D.L.; Ranson, N.A.; Ritala, A.; Paley, R.; Cano, I.; Lomonossoff, G.P. Plant-Made Nervous Necrosis Virus-Like Particles Protect Fish Against Disease. Front. Plant. Sci. 2019, 10, 880. [Google Scholar] [CrossRef]
- Yang, J.I.; Kim, K.H. Display of Streptococcus iniae alpha-Enolase on the Surface of Virus-Like Particles (VLPs) of Nervous Necrosis Virus (NNV) Using SpyTag/SpyCatcher. Mar. Biotechnol. 2022, 24, 1066–1072. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, J.; Zhang, Z.; Liang, X.; Liu, S.; Pan, Y.; Wei, J.; Huang, Y.; Huang, X.; Qin, Q. An improved oral vaccine with molecular adjuvant beta-defensin protects grouper against nervous necrosis virus infection. Fish Shellfish Immunol. 2023, 136, 108709. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).