Predictors of Breakthrough SARS-CoV-2 Infection after Vaccination
Abstract
:1. Introduction
2. Methods and Materials
2.1. Recruitment
2.2. Inclusion Criteria
2.3. Consent
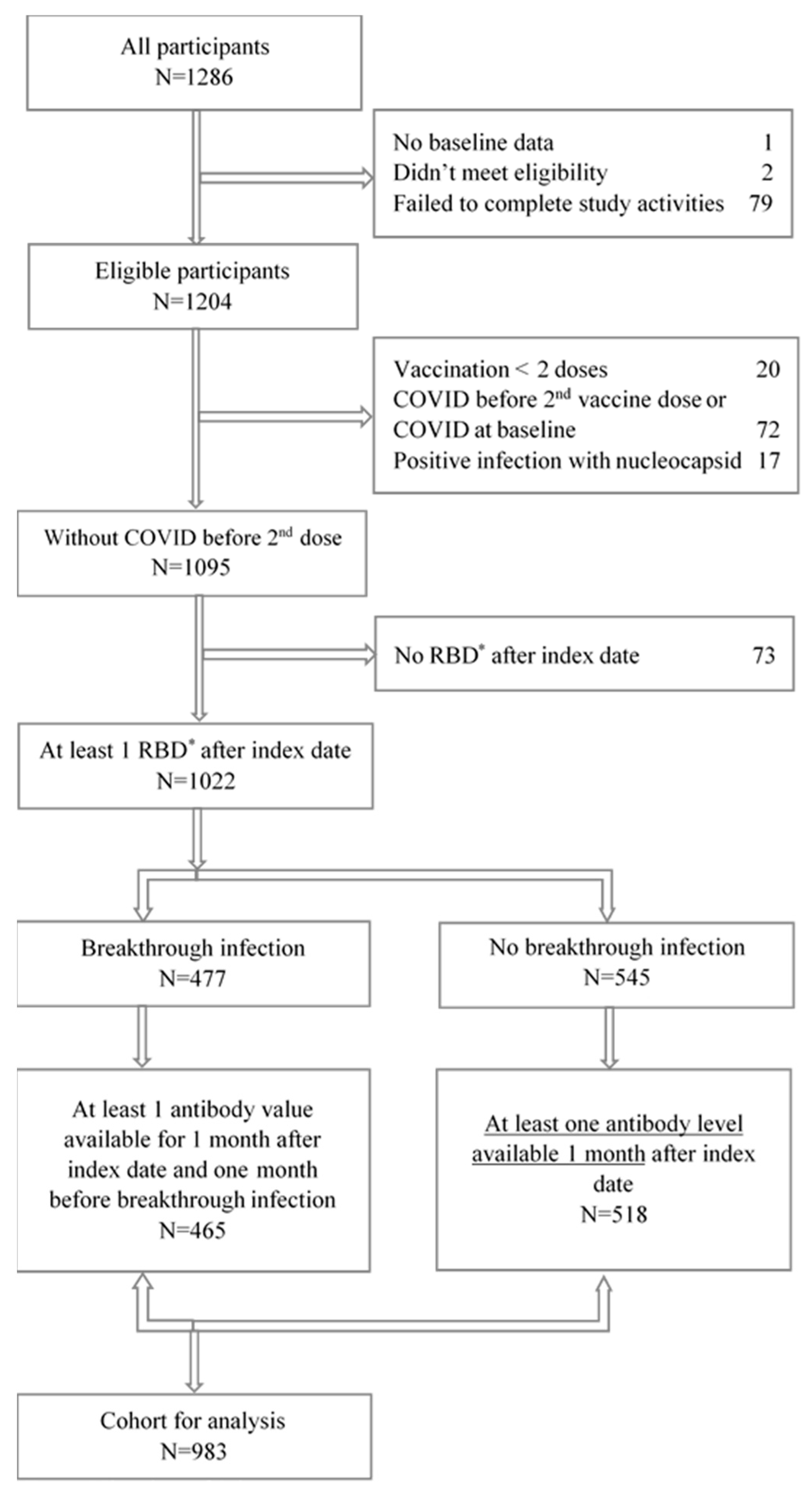
2.4. Study Population
2.5. Outcome of Breakthrough Infection
2.6. Exposures
2.7. Demographic Characteristics and Questionnaires
2.8. Bivalent Vaccination
2.9. Sample Collection for Antibody Testing
2.10. Serological Assays, Interpretation and Recording
2.11. Statistical Analysis
2.12. Data Access
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Stenehjem, E.; Grannis, S.; Ball, S.W.; Naleway, A.L.; Ong, T.C.; DeSilva, M.B.; Natarajan, K.; Bozio, C.H.; Lewis, N.; et al. Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings. N. Engl. J. Med. 2021, 385, 1355–1371. [Google Scholar] [CrossRef] [PubMed]
- Mayr, F.B.; Talisa, V.B.; Shaikh, O.; Yende, S.; Butt, A.A. Effectiveness of Homologous or Heterologous Covid-19 Boosters in Veterans. N. Engl. J. Med. 2022, 386, 1375–1377. [Google Scholar] [CrossRef]
- Hansen, C.H.; Michlmayr, D.; Gubbels, S.M.; Molbak, K.; Ethelberg, S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: A population-level observational study. Lancet 2021, 397, 1204–1212. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N. Engl. J. Med. 2022, 387, 21–34. [Google Scholar] [CrossRef]
- Nordstrom, P.; Ballin, M.; Nordstrom, A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: A retrospective, total population cohort study in Sweden. Lancet Infect. Dis. 2022, 22, 781–790. [Google Scholar] [CrossRef]
- Chi, W.Y.; Li, Y.D.; Huang, H.C.; Chan, T.E.H.; Chow, S.Y.; Su, J.H.; Ferrall, L.; Hung, C.F.; Wu, T.C. COVID-19 vaccine update: Vaccine effectiveness, SARS-CoV-2 variants, boosters, adverse effects, and immune correlates of protection. J. Biomed. Sci. 2022, 29, 82. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O'Connell, A.M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Smoot, K.; Yang, J.; Tacker, D.H.; Welch, S.; Khodaverdi, M.; Kimble, W.; Wen, S.; Amjad, A.; Marsh, C.; Perrotta, P.L.; et al. Persistence and Protective Potential of SARS-CoV-2 Antibody Levels After COVID-19 Vaccination in a West Virginia Nursing Home Cohort. JAMA Netw. Open 2022, 5, e2231334. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Stoesser, N.; Matthews, P.C.; Ayoubkhani, D.; Studley, R.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat. Microbiol. 2021, 6, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, J. The Flawed Science of Antibody Testing for SARS-CoV-2 Immunity. JAMA 2021, 326, 1781–1782. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, N.J.; Kibble, M.; Wong, A.; Silverwood, R.J.; Knuppel, A.; Williams, D.M.; Hamilton, O.K.L.; Lee, P.H.; Bridger Staatz, C.; Di Gessa, G.; et al. Antibody levels following vaccination against SARS-CoV-2: Associations with post-vaccination infection and risk factors in two UK longitudinal studies. eLife 2023, 12, e80428. [Google Scholar] [CrossRef]
- Khoury, D.S.; Schlub, T.E.; Cromer, D.; Steain, M.; Fong, Y.; Gilbert, P.B.; Subbarao, K.; Triccas, J.A.; Kent, S.J.; Davenport, M.P. Correlates of Protection, Thresholds of Protection, and Immunobridging among Persons with SARS-CoV-2 Infection. Emerg. Infect. Dis. 2023, 29, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Capri, A.; Petrone, D.; Colavita, F.; Meschi, S.; Matusali, G.; Mizzoni, K.; Notari, S.; Agrati, C.; Goletti, D.; et al. SARS-CoV-2 Breakthrough Infections According to the Immune Response Elicited after mRNA Third Dose Vaccination in COVID-19-Naïve Hospital Personnel. Biomedicines 2023, 11, 1247. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Keehner, J.; Horton, L.E.; Binkin, N.J.; Laurent, L.C.; Pride, D.; Longhurst, C.A.; Abeles, S.R.; Torriani, F.J. Resurgence of SARS-CoV-2 Infection in a Highly Vaccinated Health System Workforce. N. Engl. J. Med. 2021, 385, 1330–1332. [Google Scholar] [CrossRef]
- Kelly, J.D.; Leonard, S.; Hoggatt, K.J.; Boscardin, W.J.; Lum, E.N.; Moss-Vazquez, T.A.; Andino, R.; Wong, J.K.; Byers, A.; Bravata, D.M.; et al. Incidence of Severe COVID-19 Illness Following Vaccination and Booster With BNT162b2, mRNA-1273, and Ad26.COV2.S Vaccines. JAMA 2022, 328, 1427–1437. [Google Scholar] [CrossRef]
- Lang, R.; Humes, E.; Coburn, S.B.; Horberg, M.A.; Fathi, L.F.; Watson, E.; Jefferson, C.R.; Park, L.S.; Gordon, K.S.; Akgun, K.M.; et al. Analysis of Severe Illness After Postvaccination COVID-19 Breakthrough Among Adults with and without HIV in the US. JAMA Netw. Open 2022, 5, e2236397. [Google Scholar] [CrossRef] [PubMed]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.J.; Swail, H.; Jain, J.; Anderson, M.; Awadalla, P.; Behl, L.; Brown, P.E.; Charlton, C.L.; Colwill, K.; Drews, S.J.; et al. The evolution of SARS-CoV-2 seroprevalence in Canada: A time-series study, 2020–2023. CMAJ 2023, 195, E1030–E1037. [Google Scholar] [CrossRef] [PubMed]
- Moline, H.L.; Whitaker, M.; Deng, L.; Rhodes, J.C.; Milucky, J.; Pham, H.; Patel, K.; Anglin, O.; Reingold, A.; Chai, S.J.; et al. Effectiveness of COVID-19 Vaccines in Preventing Hospitalization Among Adults Aged ≥65 Years—COVID-NET, 13 States, February-April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Andersson, N.W.; Thiesson, E.M.; Baum, U.; Pihlström, N.; Starrfelt, J.; Faksová, K.; Poukka, E.; Meijerink, H.; Ljung, R.; Hviid, A. Comparative effectiveness of bivalent BA.4-5 and BA.1 mRNA booster vaccines among adults aged ≥50 years in Nordic countries: Nationwide cohort study. BMJ 2023, 382, e075286. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, S.; Harper, C.; Vrbicky, K.; Walsh, S.R.; Essink, B.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; Chang, Y.; et al. A Bivalent Omicron-Containing Booster Vaccine against Covid-19. N. Engl. J. Med. 2022, 387, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Winokur, P.; Gayed, J.; Fitz-Patrick, D.; Thomas, S.J.; Diya, O.; Lockhart, S.; Xu, X.; Zhang, Y.; Bangad, V.; Schwartz, H.I.; et al. Bivalent Omicron BA.1-Adapted BNT162b2 Booster in Adults Older than 55 Years. N. Engl. J. Med. 2023, 388, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bowen, A.; Valdez, R.; Gherasim, C.; Gordon, A.; Liu, L.; Ho, D.D. Antibody Response to Omicron BA.4-BA.5 Bivalent Booster. N. Engl. J. Med. 2023, 388, 567–569. [Google Scholar] [CrossRef]
- Kurhade, C.; Zou, J.; Xia, H.; Liu, M.; Chang, H.C.; Ren, P.; Xie, X.; Shi, P.Y. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat. Med. 2023, 29, 344–347. [Google Scholar] [CrossRef]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286.e8. [Google Scholar] [CrossRef]
- Chae, C.; Kim, R.K.; Jang, E.J.; Shim, J.A.; Park, E.; Lee, K.H.; Hong, S.L.; Aziz, A.B.; Tadesse, B.T.; Marks, F.; et al. Comparing the Effectiveness of Bivalent and Monovalent COVID-19 Vaccines against COVID-19 Infection during the Winter Season of 2022–2023: A Real-World Retrospective Observational Matched Cohort Study in the Republic of Korea. Int. J. Infect. Dis. 2023, 135, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kirsebom, F.C.M.; Andrews, N.; Stowe, J.; Ramsay, M.; Lopez Bernal, J. Duration of protection of ancestral-strain monovalent vaccines and effectiveness of bivalent BA.1 boosters against COVID-19 hospitalisation in England: A test-negative case-control study. Lancet Infect. Dis. 2023, 23, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Peretz, A.; Sergienko, R.; Friger, M.; Beckenstein, T.; Duskin-Bitan, H.; Yaron, S.; Hammerman, A.; Bilenko, N.; Netzer, D. Effectiveness of a bivalent mRNA vaccine booster dose to prevent severe COVID-19 outcomes: A retrospective cohort study. Lancet Infect. Dis. 2023, 23, 914–921. [Google Scholar] [CrossRef]
- Tseng, H.F.; Ackerson, B.K.; Bruxvoort, K.J.; Sy, L.S.; Tubert, J.E.; Lee, G.S.; Ku, J.H.; Florea, A.; Luo, Y.; Qiu, S.; et al. Effectiveness of mRNA-1273 vaccination against SARS-CoV-2 omicron subvariants BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. Nat. Commun. 2023, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Arashiro, T.; Arima, Y.; Kuramochi, J.; Muraoka, H.; Sato, A.; Chubachi, K.; Yanai, A.; Arioka, H.; Uehara, Y.; Ihara, G.; et al. Effectiveness of BA.1- and BA.4/BA. 5-Containing Bivalent COVID-19 mRNA Vaccines Against Symptomatic SARS-CoV-2 Infection During the BA.5-Dominant Period in Japan. Open Forum Infect. Dis. 2023, 10, ofad240. [Google Scholar] [CrossRef] [PubMed]
- Solera, J.T.; Ierullo, M.; Arbol, B.G.; Mavandadnejad, F.; Kurtesi, A.; Qi, F.; Hu, Q.; Gingras, A.C.; Ferreira, V.H.; Humar, A.; et al. Bivalent COVID-19 mRNA vaccine against omicron subvariants in immunocompromised patients. Lancet Infect. Dis. 2023, 23, e266–e267. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, S.L.; Szadkowski, L.; Wouters, B.; Clarke, R.; Colwill, K.; Rochon, P.; Brudno, M.; Ravindran, R.; Raboud, J.; McGeer, A.; et al. COVID-19 vaccine antibody responses in community-dwelling adults to 48 weeks post primary vaccine series. iScience 2023, 26, 106506. [Google Scholar] [CrossRef]
- Colwill, K.; Galipeau, Y.; Stuible, M.; Gervais, C.; Arnold, C.; Rathod, B.; Abe, K.T.; Wang, J.H.; Pasculescu, A.; Maltseva, M.; et al. A scalable serology solution for profiling humoral immune responses to SARS-CoV-2 infection and vaccination. Clin. Transl. Immunol. 2022, 11, e1380. [Google Scholar] [CrossRef]
- Abubakar, H.; Valdez, C.; Lovblum, E.; Ravindran, R.; Clarke, R.; Colwill, K.; Dayam, R.; Gingras, A.; Walmsley, S.; on behalf of the STOPCoV Research Team. Feasibility and Acceptability of Self-Collected Dried Blood Spots for SARS-CoV-2 Vaccine Response in Community-Dwelling Elderly: A Large Decentralized Prospective Study. J. Community Med. Public Health 2023, 7, 309. [Google Scholar] [CrossRef]
- Abe, K.T.; Li, Z.; Samson, R.; Samavarchi-Tehrani, P.; Valcourt, E.J.; Wood, H.; Budylowski, P.; Dupuis, A.P., 2nd; Girardin, R.C.; Rathod, B.; et al. A simple protein-based surrogate neutralization assay for SARS-CoV-2. JCI Insight 2020, 5, e142362. [Google Scholar] [CrossRef]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2021, 22, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.M.; Simmons, R.; Wellington, E.; Cole, M.J.; Saei, A.; Oguti, B.; et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN). Lancet 2021, 397, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, M.; Gonen, T.; Barda, N.; Cohn, S.; Indenbaum, V.; Weiss-Ottolenghi, Y.; Amit, S.; Asraf, K.; Joseph, G.; Levin, T.; et al. Factors Associated With Protection From SARS-CoV-2 Omicron Variant Infection and Disease Among Vaccinated Health Care Workers in Israel. JAMA Netw. Open 2023, 6, e2314757. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, D.; Fiore-Gartland, A.; Johnson, M.; Hunt, A.; Bengt, C.; Zavadska, D.; Snipe, H.D.; Brown, J.S.; Workman, L.; Zar, H.J.; et al. Towards a population-based threshold of protection for COVID-19 vaccines. Vaccine 2022, 40, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Dimeglio, C.; Migueres, M.; Bouzid, N.; Chapuy-Regaud, S.; Gernigon, C.; Da-Silva, I.; Porcheron, M.; Martin-Blondel, G.; Herin, F.; Izopet, J. Antibody Titers and Protection against Omicron (BA.1 and BA.2) SARS-CoV-2 Infection. Vaccines 2022, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef]
- White, E.M.; Yang, X.; Blackman, C.; Feifer, R.A.; Gravenstein, S.; Mor, V. Incident SARS-CoV-2 Infection among mRNA-Vaccinated and Unvaccinated Nursing Home Residents. N. Engl. J. Med. 2021, 385, 474–476. [Google Scholar] [CrossRef]
- Qin, C.X.; Moore, L.W.; Anjan, S.; Rahamimov, R.; Sifri, C.D.; Ali, N.M.; Morales, M.K.; Tsapepas, D.S.; Basic-Jukic, N.; Miller, R.A.; et al. Risk of Breakthrough SARS-CoV-2 Infections in Adult Transplant Recipients. Transplantation 2021, 105, e265–e266. [Google Scholar] [CrossRef]
- Deng, G.; Zhou, Q.; Meng, Y.; Sun, H.; Du, S.; Liu, Y.; Zeng, F. Risk and outcomes of breakthrough COVID-19 infections in vaccinated immunocompromised patients: A meta-analysis. MedComm 2023, 4, e307. [Google Scholar] [CrossRef]
- Liu, C.; Lee, J.; Ta, C.; Soroush, A.; Rogers, J.R.; Kim, J.H.; Natarajan, K.; Zucker, J.; Perl, Y.; Weng, C. Risk Factors Associated With SARS-CoV-2 Breakthrough Infections in Fully mRNA-Vaccinated Individuals: Retrospective Analysis. JMIR Public Health Surveill. 2022, 8, e35311. [Google Scholar] [CrossRef]
- Smits, P.D.; Gratzl, S.; Simonov, M.; Nachimuthu, S.K.; Goodwin Cartwright, B.M.; Wang, M.D.; Baker, C.; Rodriguez, P.; Bogiages, M.; Althouse, B.M.; et al. Risk of COVID-19 breakthrough infection and hospitalization in individuals with comorbidities. Vaccine 2023, 41, 2447–2455. [Google Scholar] [CrossRef]
- Abe, K.T.; Queenie, H.; Mozafarihashjin, M.; Samson, R.; Manguiat, K.; Robinson, A.; Rathod, B.; Wang, J.H.; Iskilova, M.; Pasculescu, A.; et al. Neutralizing antibody responses to SARS-CoV-2 variants in vaccinated Ontario long-term care home residents and workers. medRxiv 2021, 1–31. [Google Scholar] [CrossRef]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef]
- Yau, K.; Abe, K.T.; Naimark, D.; Oliver, M.J.; Perl, J.; Leis, J.A.; Bolotin, S.; Tran, V.; Mullin, S.I.; Shadowitz, E.; et al. Evaluation of the SARS-CoV-2 Antibody Response to the BNT162b2 Vaccine in Patients Undergoing Hemodialysis. JAMA Netw. Open 2021, 4, e2123622. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Kaweski, S.E.; Irvine, M.A.; Kim, S.; Chuang, E.S.Y.; Sabaiduc, S.; Fraser, M.; Reyes, R.C.; Henry, B.; Levett, P.N.; et al. Serial cross-sectional estimation of vaccine-and infection-induced SARS-CoV-2 seroprevalence in British Columbia, Canada. Can. Med. Assoc. J. 2022, 194, E1599. [Google Scholar] [CrossRef]
- Eliakim-Raz, N.; Stemmer, A.; Ghantous, N.; Ness, A.; Awwad, M.; Leibovici-Weisman, Y.; Stemmer, S.M. Antibody Titers After a Third and Fourth SARS-CoV-2 BNT162b2 Vaccine Dose in Older Adults. JAMA Netw. Open 2022, 5, e2223090. [Google Scholar] [CrossRef]
- Goh, Y.S.; Rouers, A.; Fong, S.W.; Zhuo, N.Z.; Hor, P.X.; Loh, C.Y.; Huang, Y.; Neo, V.K.; Kam, I.K.J.; Wang, B.; et al. Waning of specific antibodies against Delta and Omicron variants five months after a third dose of BNT162b2 SARS-CoV-2 vaccine in elderly individuals. Front. Immunol. 2022, 13, 1031852. [Google Scholar] [CrossRef] [PubMed]
- Springer, D.N.; Bauer, M.; Medits, I.; Camp, J.V.; Aberle, S.W.; Burtscher, C.; Höltl, E.; Weseslindtner, L.; Stiasny, K.; Aberle, J.H. Bivalent COVID-19 mRNA booster vaccination (BA.1 or BA.4/BA.5) increases neutralization of matched Omicron variants. NPJ Vaccines 2023, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Carreño, J.M.; Singh, G.; Simon, V.; Krammer, F.; PVI Study Group. Bivalent COVID-19 booster vaccines and the absence of BA.5-specific antibodies. Lancet Microbe 2023, 4, e569. [Google Scholar] [CrossRef]
- Gupta, S.L.; Jaiswal, R.K. An Assessment of the Bivalent Vaccine as a Second Booster for COVID-19. Vaccines 2022, 11, 79. [Google Scholar] [CrossRef]
- Hoffmann, M.; Behrens, G.M.N.; Arora, P.; Kempf, A.; Nehlmeier, I.; Cossmann, A.; Manthey, L.; Dopfer-Jablonka, A.; Pöhlmann, S. Effect of hybrid immunity and bivalent booster vaccination on omicron sublineage neutralisation. Lancet Infect. Dis. 2023, 23, 25–28. [Google Scholar] [CrossRef]
- Auvigne, V.; Tamandjou Tchuem, C.R.; Schaeffer, J.; Vaux, S.; Parent Du Chatelet, I. Protection against symptomatic SARS-CoV-2 infection conferred by the Pfizer-BioNTech Original/BA.4-5 bivalent vaccine compared to the mRNA Original monovalent vaccines—A matched cohort study in France. Vaccine 2023, 41, 5490–5493. [Google Scholar] [CrossRef]
- Link-Gelles, R.; Ciesla, A.A.; Fleming-Dutra, K.E.; Smith, Z.R.; Britton, A.; Wiegand, R.E.; Miller, J.D.; Accorsi, E.K.; Schrag, S.J.; Verani, J.R.; et al. Effectiveness of Bivalent mRNA Vaccines in Preventing Symptomatic SARS-CoV-2 Infection—Increasing Community Access to Testing Program, United States, September-November 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1526–1530. [Google Scholar] [CrossRef]
- Mateo-Urdiales, A.; Sacco, C.; Fotakis, E.A.; Del Manso, M.; Bella, A.; Riccardo, F.; Bressi, M.; Rota, M.C.; Petrone, D.; Siddu, A.; et al. Relative effectiveness of monovalent and bivalent mRNA boosters in preventing severe COVID-19 due to omicron BA.5 infection up to 4 months post-administration in people aged 60 years or older in Italy: A retrospective matched cohort study. Lancet Infect. Dis. 2023, 23, 1349–1359. [Google Scholar] [CrossRef]
- Uraki, R.; Ito, M.; Kiso, M.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Furusawa, Y.; Sakai-Tagawa, Y.; Imai, M.; Koga, M.; Yamamoto, S.; et al. Antiviral and bivalent vaccine efficacy against an omicron XBB.1.5 isolate. Lancet Infect. Dis. 2023, 23, 402–403. [Google Scholar] [CrossRef]
- Vasin, A.V.; Stukova, M.A. Bivalent omicron (BA.1) booster vaccination against SARS-CoV-2. Lancet Infect. Dis. 2023, 23, 880–881. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effect of prior infection, vaccination, and hybrid immunity against symptomatic BA.1 and BA.2 Omicron infections and severe COVID-19 in Qatar. medRxiv 2022. [Google Scholar] [CrossRef]
- Cerqueira-Silva, T.; de Araujo Oliveira, V.; Paixao, E.S.; Florentino, P.T.V.; Penna, G.O.; Pearce, N.; Werneck, G.L.; Barreto, M.L.; Boaventura, V.S.; Barral-Netto, M. Vaccination plus previous infection: Protection during the omicron wave in Brazil. Lancet Infect. Dis. 2022, 22, 945–946. [Google Scholar] [CrossRef] [PubMed]
- Suarez Castillo, M.; Khaoua, H.; Courtejoie, N. Vaccine-induced and naturally-acquired protection against Omicron and Delta symptomatic infection and severe COVID-19 outcomes, France, December 2021 to January 2022. Euro Surveill. 2022, 27, 2200250. [Google Scholar] [CrossRef] [PubMed]

| COVID-19 Variant Infection Wave | Calendar Time Interval |
|---|---|
| Delta | 1 August 2021–14 December 2021 |
| Omicron BA.1 | 15 December 2021–28 February 2022 |
| Omicron BA.2 | 22 March 2022–18 June 2022 |
| Omicron BA.4/5 | 19 June 2022–25 March 2023 |
| Omicron XBB | 26 March 2023–present |
| Components | Cox Regression after Index Date | Cox Regression after 26 September 2022 |
|---|---|---|
| Outcome | The first breakthrough after index date during 96-week follow-up period | The first breakthrough infection after 26 September 2022 up to 96 weeks after the index date. |
| Follow-up date | Infected: the first breakthrough infection date during the 96-week follow-up after the index date. Non-infected: Three weeks after the last RBD date up to 96 weeks after the index date. | Infected: the first breakthrough infection date after 26 September 2022 up to 96 weeks after the index date. Non-infected: three weeks after the last RBD date up to 96 weeks after the index date. |
| Risk set | 983 participants. | 776 participants; participants with follow-up dates before 26 September 2022 are excluded. |
| Exposures | RBD values during the 96-week follow-up and the first two vaccine brands. | RBD values during the 96-week follow-up, the first two vaccine brands, receipt of bivalent booster after 26 September 2022 and hybrid infection before 26 September 2022. |
| Characteristics | No Breakthrough SARS-CoV-2 Infection | Breakthrough SARS-CoV-2 Infection | p-Value |
|---|---|---|---|
| n = 518 | n = 465 | ||
| Age group | |||
| 30–50-year cohort | 98 (18.9%) | 150 (32.3%) | <0.001 |
| 70+ year cohort | 420 (81.1%) | 315 (67.7%) | |
| Age, mean (SD) | 67.7 (14.2) | 63.5 (16.2) | <0.001 |
| Sex | |||
| Male | 186 (35.9%) | 166 (35.7%) | 0.999 |
| Female | 332 (64.1%) | 299 (64.3%) | |
| Caucasian Race | |||
| No | 48 (9.27%) | 63 (13.5%) | 0.044 |
| Yes | 470 (90.7%) | 402 (86.5%) | |
| Diabetes | |||
| No | 458 (88.4%) | 419 (90.1%) | 0.453 |
| Yes | 60 (11.6%) | 46 (9.89%) | |
| Cardiovascular diseases | |||
| No | 307 (59.3%) | 319 (68.6%) | 0.003 |
| Yes | 211 (40.7%) | 146 (31.4%) | |
| Respiratory diseases | |||
| No | 460 (88.8%) | 408 (87.7%) | 0.676 |
| Yes | 58 (11.2%) | 57 (12.3%) | |
| Cancer | |||
| No | 437 (84.4%) | 387 (83.2%) | 0.692 |
| Yes | 81 (15.6%) | 78 (16.8%) | |
| Transplant | |||
| No | 493 (95.2%) | 448 (96.3%) | 0.455 |
| Yes | 25 (4.83%) | 17 (3.66%) | |
| Body mass index, mean (SD) | 2.71 (0.55) | 2.71 (0.55) | 0.941 |
| Obesity (BMI ≥ 30) | |||
| No | 381 (74.6%) | 353 (76.7%) | 0.475 |
| Yes | 130 (25.4%) | 107 (23.3%) | |
| Smoking | |||
| Never | 282 (54.4%) | 277 (59.6%) | 0.037 |
| Prior | 209 (40.3%) | 177 (38.1%) | |
| Current | 27 (5.21%) | 11 (2.37%) | |
| First two vaccine brand combination | |||
| mRNA-1273–mRNA-1273 | 59 (11.4%) | 42 (9%) | 0.535 |
| Other–mRNA-1273 | 104 (20.1%) | 105 (22.6%) | |
| Pfizer–Pfizer | 329 (63.5%) | 297 (63.9%) | |
| Other–Other | 26 (5%) | 21 (4.5%) | |
| COVID-19 Vaccine Booster doses | |||
| None | 47 (9.07%) | 20 (4.3%) | <0.001 |
| One | 111 (21.43%) | 66 (14.19%) | |
| Two | 92 (17.76%) | 141 (30.32%) | |
| Three | 215 (41.51%) | 203 (43.66%) | |
| Four | 53 (10.23%) | 35 (7.53%) | |
| Original/Omicron Bivalent vaccination (after 26 September 2022) | |||
| No | 276 (53.3%) | 389 (83.7%) | <0.001 |
| Yes | 242 (46.7%) | 76 (16.3%) | |
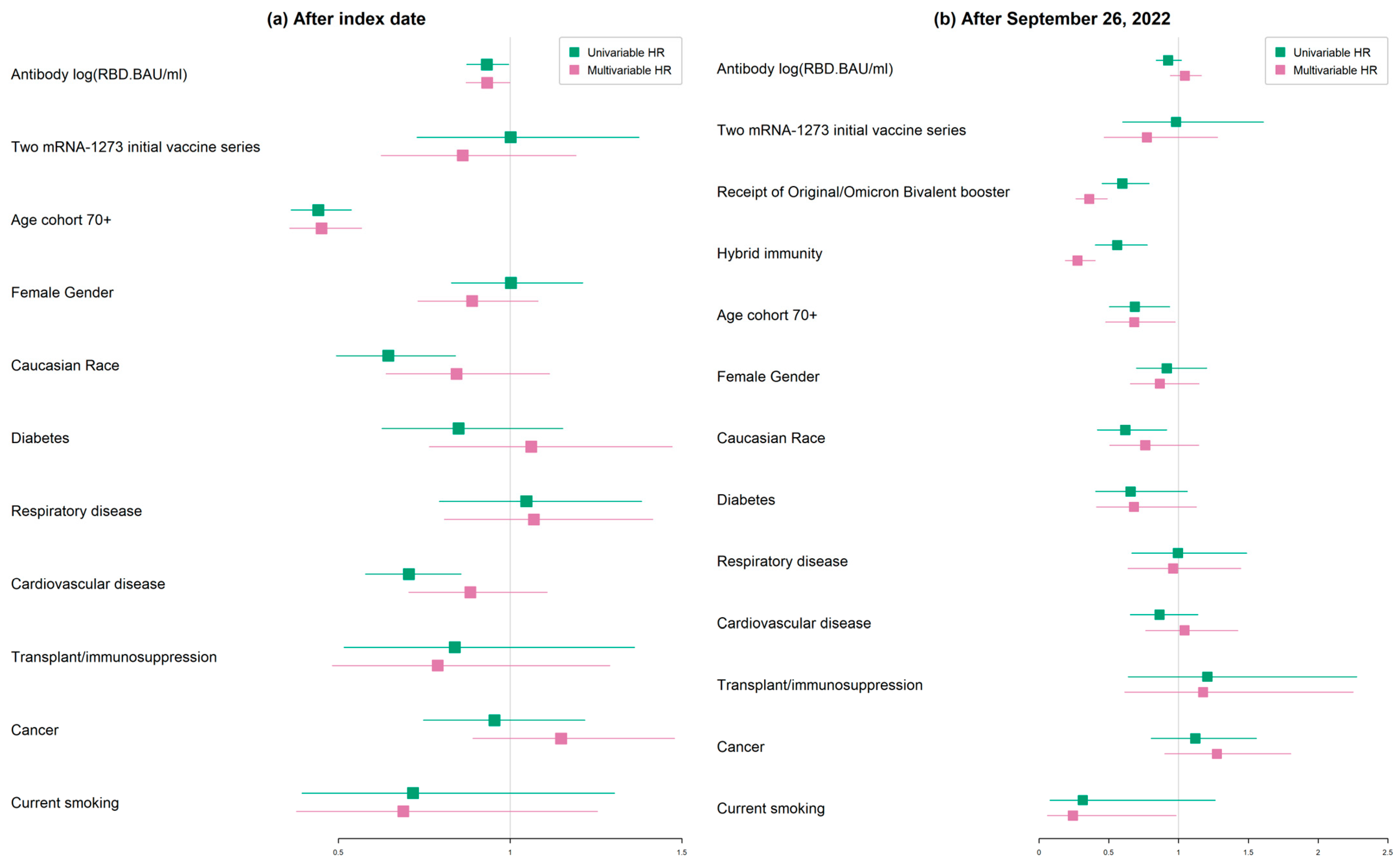
| Characteristics | Univariable Hazard Ratio [95% CI] | Multivariable Hazard Ratio [95% CI] |
|---|---|---|
| 96 weeks after index date | ||
| Antibody log (RBD.BAU/mL) | 0.932 [0.873, 0.996] | 0.933 [0.872, 0.999] |
| Two mRNA-1273 initial vaccine series | 1.001 [0.729, 1.375] | 0.862 [0.624, 1.192] |
| Age cohort 70+ | 0.442 [0.363, 0.538] | 0.451 [0.358, 0.568] |
| Female gender | 1.002 [0.829, 1.211] | 0.889 [0.731, 1.081] |
| Caucasian race | 0.645 [0.494, 0.841] | 0.844 [0.639, 1.115] |
| Diabetes | 0.85 [0.627, 1.153] | 1.061 [0.765, 1.471] |
| Respiratory disease | 1.047 [0.794, 1.382] | 1.069 [0.808, 1.415] |
| Cardiovascular disease | 0.705 [0.579, 0.857] | 0.884 [0.705, 1.108] |
| Transplant/immunosuppression | 0.839 [0.517, 1.362] | 0.789 [0.482, 1.29] |
| Cancer | 0.954 [0.748, 1.217] | 1.148 [0.891, 1.478] |
| Currently smoking | 0.717 [0.394, 1.304] | 0.689 [0.378, 1.254] |
| Delta + Omicron BA.1/.2 waves | ||
| Antibody log (RBD.BAU/mL) | 0.899 [0.829, 0.975] | 0.884 [0.813, 0.963] |
| Two mRNA-1273 initial vaccine series | 1.244 [0.799, 1.939] | 1.057 [0.669, 1.67] |
| Age 70+ cohort | 0.348 [0.261, 0.463] | 0.299 [0.211, 0.424] |
| Female gender | 0.967 [0.724, 1.291] | 0.799 [0.59, 1.081] |
| Caucasian race | 0.785 [0.516, 1.194] | 1.227 [0.785, 1.918] |
| Diabetes | 0.966 [0.614, 1.518] | 1.267 [0.777, 2.065] |
| Respiratory disease | 1.096 [0.721, 1.667] | 1.047 [0.68, 1.611] |
| Cardiovascular disease | 0.694 [0.514, 0.936] | 0.96 [0.672, 1.371] |
| Transplant/immunosuppression | 0.926 [0.436, 1.97] | 0.836 [0.389, 1.798] |
| Cancer | 1.013 [0.701, 1.466] | 1.312 [0.888, 1.938] |
| Currently Smoking | 1.325 [0.653, 2.688] | 1.252 [0.612, 2.563] |
| Omicron BA.4/5 + XBB waves | ||
| Antibody log (RBD.BAU/mL) | 0.99 [0.904, 1.084] | 1.01 [0.92, 1.109] |
| Two mRNA-1273 initial vaccine series | 0.857 [0.543, 1.351] | 0.692 [0.435, 1.101] |
| Age 70+ cohort | 0.464 [0.353, 0.61] | 0.512 [0.373, 0.702] |
| Female gender | 1.02 [0.793, 1.312] | 0.9 [0.695, 1.165] |
| Caucasian race | 0.526 [0.373, 0.742] | 0.645 [0.45, 0.926] |
| Diabetes | 0.77 [0.51, 1.163] | 0.951 [0.611, 1.48] |
| Respiratory disease | 1.023 [0.707, 1.48] | 1.054 [0.725, 1.533] |
| Cardiovascular disease | 0.695 [0.537, 0.901] | 0.828 [0.616, 1.111] |
| Transplant/immunosuppression | 0.832 [0.442, 1.565] | 0.835 [0.44, 1.584] |
| Cancer | 0.899 [0.65, 1.242] | 1.055 [0.754, 1.476] |
| Currently smoking | 0.338 [0.108, 1.056] | 0.321 [0.103, 1.004] |
| Characteristics | COVID-19 Breakthrough Infected Participants | p-Value | ||||
|---|---|---|---|---|---|---|
| Delta (n = 20) | BA.1 (n = 53) | BA.2 (n = 127) | BA.4/5 (n = 238) | XBB (n = 27) | ||
| Age cohort | ||||||
| 30–50 years | 5 (25.0%) | 29 (54.7%) | 45 (35.4%) | 66 (27.7%) | 5 (18.5%) | <0.001 |
| 70+ years | 15 (75.0%) | 24 (45.3%) | 82 (64.6%) | 172 (72.3%) | 22 (81.5%) | |
| Age, mean (SD) | 64.4 (18.4) | 57.1 (18.4) | 61.8 (16.4) | 65.1 (15.5) | 68.7 (11.4) | 0.005 |
| Sex | ||||||
| Male | 8 (40.0%) | 21 (39.6%) | 43 (33.9%) | 83 (34.9%) | 11 (40.7%) | 0.898 |
| Female | 12 (60.0%) | 32 (60.4%) | 84 (66.1%) | 155 (65.1%) | 16 (59.3%) | |
| Caucasian Race | ||||||
| No | 2 (10.0%) | 6 (11.3%) | 17 (13.4%) | 36 (15.1%) | 2 (7.41%) | 0.86 |
| Yes | 18 (90.0%) | 47 (88.7%) | 110 (86.6%) | 202 (84.9%) | 25 (92.6%) | |
| Diabetes | ||||||
| No | 19 (95.0%) | 51 (96.2%) | 109 (85.8%) | 221 (92.9%) | 19 (70.4%) | 0.002 |
| Yes | 1 (5.00%) | 2 (3.77%) | 18 (14.2%) | 17 (7.14%) | 8 (29.6%) | |
| Cardiovascular disease | ||||||
| No | 19 (95.0%) | 40 (75.5%) | 79 (62.2%) | 165 (69.3%) | 16 (59.3%) | 0.024 |
| Yes | 1 (5.00%) | 13 (24.5%) | 48 (37.8%) | 73 (30.7%) | 11 (40.7%) | |
| Respiratory diseases | ||||||
| No | 17 (85.0%) | 47 (88.7%) | 111 (87.4%) | 209 (87.8%) | 24 (88.9%) | 0.991 |
| Yes | 3 (15.0%) | 6 (11.3%) | 16 (12.6%) | 29 (12.2%) | 3 (11.1%) | |
| Cancer | ||||||
| No | 17 (85.0%) | 43 (81.1%) | 106 (83.5%) | 199 (83.6%) | 22 (81.5%) | 0.984 |
| Yes | 3 (15.0%) | 10 (18.9%) | 21 (16.5%) | 39 (16.4%) | 5 (18.5%) | |
| Transplant/immunosuppression | ||||||
| No | 19 (95.0%) | 50 (94.3%) | 124 (97.6%) | 229 (96.2%) | 26 (96.3%) | 0.646 |
| Yes | 1 (5.00%) | 3 (5.66%) | 3 (2.36%) | 9 (3.78%) | 1 (3.70%) | |
| Obesity | ||||||
| No | 13 (68.4%) | 38 (73.1%) | 93 (74.4%) | 189 (79.7%) | 20 (74.1%) | 0.527 |
| Yes | 6 (31.6%) | 14 (26.9%) | 32 (25.6%) | 48 (20.3%) | 7 (25.9%) | |
| BMI, mean (SD) | 2.71 (0.65) | 2.80 (0.57) | 2.78 (0.59) | 2.65 (0.53) | 2.77 (0.44) | 0.171 |
| Smoking | ||||||
| No | 8 (40.0%) | 30 (56.6%) | 77 (60.6%) | 150 (63.0%) | 12 (44.4%) | 0.002 |
| Previous | 11 (55.0%) | 17 (32.1%) | 49 (38.6%) | 86 (36.1%) | 14 (51.9%) | |
| Current | 1 (5.00%) | 6 (11.3%) | 1 (0.79%) | 2 (0.84%) | 1 (3.70%) | |
| First two vaccine combination | ||||||
| Other–Other | 15 (75.0%) | 33 (62.3%) | 86 (67.7%) | 167 (70.2%) | 17 (63.0%) | 0.453 |
| Other–mRNA-1273 | 5 (25.0%) | 15 (28.3%) | 24 (18.9%) | 53 (22.3%) | 8 (29.6%) | |
| mRNA-1273–mRNA-1273 | 0 (0.00%) | 5 (9.43%) | 17 (13.4%) | 18 (7.56%) | 2 (7.41%) | |
| Original/Omicron Bivalent vaccination (after 26 September 2022) | ||||||
| No | 20 (100%) | 53 (100%) | 127 (100%) | 178 (74.8%) | 11 (40.7%) | <0.001 |
| Yes | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 60 (25.2%) | 16 (59.3%) | |
| Median time to infection, days (week) | 122 (17) | 214 (31) | 304 (43) | 487 (70) | 669 (96) | - |
| Antibody Quintiles of Log (RBD.BAU/mL) | Univariable HR [95% CI] | Multivariable HR [95% CI] |
|---|---|---|
| All SARS-CoV-2 waves | ||
| <4.92 | Ref. | |
| 4.92–6.03 | 0.851 [0.576, 1.256] | 0.823 [0.557, 1.216] |
| 6.03–7.13 | 0.795 [0.549, 1.152] | 0.77 [0.53, 1.118] |
| 7.13–8.13 | 0.861 [0.602, 1.233] | 0.872 [0.608, 1.252] |
| >8.13 | 0.688 [0.475, 0.996] | 0.689 [0.474, 1] |
| Delta + Omicron BA.1 | ||
| <4.92 | Ref. | |
| 4.92–6.03 | 0.974 [0.521, 1.818] | 0.885 [0.47, 1.666] |
| 6.03–7.13 | 0.955 [0.487, 1.875] | 0.88 [0.443, 1.748] |
| 7.13–8.13 | 0.292 [0.114, 0.747] | 0.302 [0.117, 0.776] |
| >8.13 | 0.198 [0.08, 0.486] | 0.205 [0.082, 0.508] |
| Omicron BA.2 | ||
| <4.92 | Ref. | |
| 4.92–6.03 | 0.846 [0.35, 2.044] | 0.674 [0.276, 1.646] |
| 6.03–7.13 | 0.755 [0.332, 1.718] | 0.645 [0.282, 1.476] |
| 7.13–8.13 | 0.96 [0.448, 2.058] | 0.746 [0.344, 1.616] |
| >8.13 | 1.192 [0.563, 2.523] | 0.894 [0.417, 1.918] |
| Omicron BA.4/5 | ||
| <4.92 | Ref. | |
| 4.92–6.03 | 1.243 [0.615, 2.511] | 1.226 [0.603, 2.489] |
| 6.03–7.13 | 1.291 [0.661, 2.522] | 1.256 [0.638, 2.472] |
| 7.13–8.13 | 1.508 [0.782, 2.908] | 1.562 [0.805, 3.033] |
| >8.13 | 0.977 [0.495, 1.929] | 1.012 [0.509, 2.012] |
| XBB | ||
| <0.92 | Ref. | |
| 4.92–6.03 | --- | --- |
| 6.03–7.13 | 0.349 [0.102, 1.192] | 0.414 [0.101, 1.7] |
| 7.13–8.13 | 0.475 [0.151, 1.492] | 0.618 [0.163, 2.35] |
| >8.13 | 0.404 [0.108, 1.503] | 0.57 [0.132, 2.464] |
| Waves and Age Cohorts | Univariable HR [95% CI] | Multivariable HR [95% CI] |
|---|---|---|
| All SARS-CoV-2 waves | ||
| 30–50 years | Ref. | |
| 70+ years | 0.442 [0.363, 0.538] | 0.448 [0.355, 0.564] |
| Delta + Omicron BA.1 | ||
| 30–50 years | Ref. | |
| 70+ years | 0.277 [0.175, 0.439] | 0.312 [0.179, 0.542] |
| Omicron BA.2 | ||
| 30–50 years | Ref. | |
| 70+ years | 0.361 [0.251, 0.52] | 0.326 [0.21, 0.506] |
| Omicron BA.4/5 | ||
| 30–50 years | Ref. | |
| 70+ years | 0.464 [0.349, 0.617] | 0.519 [0.372, 0.723] |
| Omicron XBB | ||
| 30–50 years | Ref. | |
| 70+ years | 0.361 [0.137, 0.955] | 0.232 [0.074, 0.73] |
| Characteristic | Univariable HR [95% CI] | Multivariable HR [95% CI] |
|---|---|---|
| Antibody level log (RBD.BAU/mL) | 0.925 [0.839, 1.02] | 1.045 [0.94, 1.163] |
| Two mRNA-1273 doses in initial vaccine series | 0.981 [0.598, 1.609] | 0.773 [0.467, 1.28] |
| Receipt of original/Omicron bivalent booster | 0.597 [0.452, 0.788] | 0.36 [0.264, 0.49] |
| Hybrid immunity | 0.56 [0.403, 0.777] | 0.276 [0.189, 0.404] |
| Age cohort 70+ | 0.687 [0.504, 0.936] | 0.682 [0.477, 0.976] |
| Female gender | 0.915 [0.697, 1.202] | 0.865 [0.653, 1.147] |
| Caucasian race | 0.618 [0.418, 0.914] | 0.761 [0.506, 1.145] |
| Diabetes | 0.656 [0.405, 1.062] | 0.68 [0.411, 1.128] |
| Respiratory disease | 0.995 [0.665, 1.488] | 0.96 [0.638, 1.446] |
| Cardiovascular disease | 0.863 [0.654, 1.139] | 1.043 [0.763, 1.424] |
| Transplant/immunosuppression | 1.206 [0.64, 2.275] | 1.175 [0.613, 2.25] |
| Cancer | 1.119 [0.804, 1.557] | 1.274 [0.901, 1.803] |
| Current smoking | 0.314 [0.078, 1.263] | 0.243 [0.06, 0.982] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walmsley, S.; Nabipoor, M.; Lovblom, L.E.; Ravindran, R.; Colwill, K.; McGeer, A.; Dayam, R.M.; Manase, D.; Gingras, A.-C.; on behalf of the STOPCoV Team. Predictors of Breakthrough SARS-CoV-2 Infection after Vaccination. Vaccines 2024, 12, 36. https://doi.org/10.3390/vaccines12010036
Walmsley S, Nabipoor M, Lovblom LE, Ravindran R, Colwill K, McGeer A, Dayam RM, Manase D, Gingras A-C, on behalf of the STOPCoV Team. Predictors of Breakthrough SARS-CoV-2 Infection after Vaccination. Vaccines. 2024; 12(1):36. https://doi.org/10.3390/vaccines12010036
Chicago/Turabian StyleWalmsley, Sharon, Majid Nabipoor, Leif Erik Lovblom, Rizani Ravindran, Karen Colwill, Alison McGeer, Roya Monica Dayam, Dorin Manase, Anne-Claude Gingras, and on behalf of the STOPCoV Team. 2024. "Predictors of Breakthrough SARS-CoV-2 Infection after Vaccination" Vaccines 12, no. 1: 36. https://doi.org/10.3390/vaccines12010036
APA StyleWalmsley, S., Nabipoor, M., Lovblom, L. E., Ravindran, R., Colwill, K., McGeer, A., Dayam, R. M., Manase, D., Gingras, A.-C., & on behalf of the STOPCoV Team. (2024). Predictors of Breakthrough SARS-CoV-2 Infection after Vaccination. Vaccines, 12(1), 36. https://doi.org/10.3390/vaccines12010036






