Abstract
Background: As many SARS-CoV-2 infections are asymptomatic, it could be useful to be able to determine how much time has passed since infection. We explored the changes in the temporal levels of T cell-related proteins (including perforin and granzymes) in the sera of patients with SARS-CoV-2 infection using a commercially available assay. Methods: This study enrolled 36 patients infected with SARS-CoV-2 and 20 healthy control participants. Blood samples were collected at three different times based on the number of days since symptom onset (early phase: 1–5 days, mid-phase: 6–10 days, late phase: 11–18 days). We assessed the temporal changes in the serum levels of perforin and granzymes in patients with SARS-CoV-2 infection by comparing the results with those obtained in the healthy control group. Results: We identified a significantly low level of perforin in the early phase of SARS-CoV-2 infection (p < 0.01), which was restored to normal during the mid- and late phases of the infection. However, there was no difference in the temporal change in the level of granzymes in SARS-CoV-2-infected patients compared to the healthy control group. Conclusions: This finding suggests that SARS-CoV-2 infection paralyzed the perforin expression in the early period immediately after infection. Thus, serum perforin is a potential marker for identifying the acute phase of SARS-CoV-2 infection.
1. Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is well demonstrated that many individuals with the SARS-CoV-2 infection remain asymptomatic [1,2]. Since active surveillance and control measures have been relaxed in many countries [3,4,5], people infected with SARS-CoV-2 who are asymptomatic or have only mild illness often do not get tested. Therefore, it is becoming more challenging to identify the clinical progress of COVID-19 during the period immediately following SARS-CoV-2 infection.
Measuring the cytotoxic T cell response after viral infection may help to assess the viral activation and predict clinical progress. Therefore, it can provide useful information about the timing of the infection. However, the levels of virus-specific T cells are patient-specific, which means that their measurement and analysis require abundant resources [6].
Perforin and granzyme, a pore-forming cytolytic molecule, are released by CD8+ T cells to disrupt the membranes of the target cells [7,8]. The levels of perforin and granzymes often increase in various infectious disease conditions, including viral and bacterial diseases [9,10]. These proteins can be obtained from a venous sample and are easily evaluated using a commercially available assay. Therefore, we assumed that the serum perforin and granzyme levels could be used as a proxy measure to assess the cytotoxic T cell response dynamics and estimate the time that elapsed since infection [6,11].
To identify the temporal marker of the period after infection, we investigated the serum levels of perforin and granzymes A and B at different times after SARS-CoV-2 infection among patients.
2. Materials and Methods
2.1. Patients and Data Collection
In total, 36 patients infected with SARS-CoV-2 were enrolled following admission to a tertiary hospital in South Korea between April 2020 and March 2021. To identify the SARS-CoV-2 infection, we obtained nasopharyngeal swab samples from the patients. Then, the infection was confirmed using a real-time reverse polymerase chain reaction. Since the infection process is often unobserved, the time after infection is often calculated using a clinical measure, such as symptom onset [12]. We collected blood samples at three different times based on the number of days that elapsed since symptom onset (early phase: 1–5 days, mid-phase: 6–10 days, late phase: 11–18 days). Furthermore, to identify the effect of the severity of the disease on the immune response, the severity was graded at the time of the first sample collection according to the World Health Organization 10-point clinical progression score, which is often used to assess the severity of patients with emerging infectious diseases, including COVID-19 [13]. Based on the clinical severity score, we identified 28 patients in the mild group (score 1–5) and eight patients were placed in the moderate–severe group (score of six to nine). To compare the level of perforin and granzyme A and B with the healthy control group, twenty healthy control participants (aged 24–58 years) were enlisted from the local population and were screened to confirm the absence of any infection (including SARS-CoV-2 using real-time reverse polymerase chain reaction) and/or other underlying diseases. All the study participants were unvaccinated against SARS-CoV-2 at the time of the study.
2.2. Patient Consent
All the study participants provided written informed consent, and all the study procedures were approved by the Institutional Review Board (IRB) of Jeonbuk National University Hospital (IRB registration number 2020-02-050).
2.3. Blood Serology
The early, mid- and late phase serum levels of perforin and granzymes A and B were assessed using a double antibody-based sandwich enzyme-linked immunosorbent assay (ELISA). Human perforin and granzymes A and B were analyzed in the ELISA Kit (Novus and Ray Biotech, Peachtree Corners, GA, USA) available from the Seoul Clinical Laboratories (SCL Healthcare, Seoul, Korea). A more detailed description of the assay has been described elsewhere [14,15,16].
2.4. Statistical Analyses
We compared the levels of perforin and granzymes A and B during different periods of infection and in different severities of disease with the healthy control group using a two-sided t-test or Mann–Whitney U-test where appropriate for the normal distribution or non-normal distribution of the data, respectively. In all the analyses, the perforin and granzyme A and B levels were log 10 transformed to reduce the variability of the data, including outlying observations, and a p-value < 0.05 was considered to indicate a statistical significance. All the statistical analyses and generating graphs were performed in R (version 4.0.1 for Windows, R Foundation for Statistical Computing).
3. Results
The mean age of the 36 patients was 50 years, and 45% and 56% of the study population were males and females, respectively. The most common symptoms were fever (56%), myalgia (44%) and cough (33%) (Table 1). The level of perforin was significantly reduced (p < 0.001) during the early phase of SARS-CoV-2 infection (mean: 2.81 ng/mL, standard deviation (SD): 2.51 ng/mL) compared to the healthy control group (mean: 5.03 ng/mL, SD: 2.78 ng/mL). After the mid-phase of the infection, the perforin level was not significantly different from that of the healthy control group (p = 0.85 for mid-phase and p = 0.45 for late phase, respectively). However, the granzyme A and B levels during all the phases of infection showed no significant difference compared to the healthy control group (Figure 1 and Table 2).

Table 1.
The demographic and clinical characteristics of the patients with SARS-CoV-2 infection.
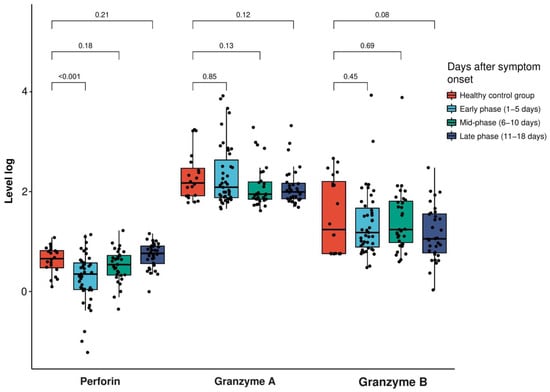
Figure 1.
Extracellular levels of perforin in log(ng/mL) and granzyme A and B in log(pg/mL) in the patients with SARS-CoV-2 infection and the healthy control participants at the early, mid and late phases (early phase: 1–5 days, mid-phase: 6–10 days, late phase: 11–18 days after symptom onset). The box plot indicates the position of the interquartile ranges and median values. The significance was determined via a t-test or Mann–Whitney U-tests where appropriate, and a p < 0.05 was considered statistically significant.

Table 2.
Temporal changes of the serum levels of perforin, granzyme A and granzyme B in the SARS-CoV-2 patients and healthy control participants and their differences by the severity of illness.
Furthermore, the level of perforin was significantly lower in the mild group compared to the healthy control group (p-value = 0.002), while the moderate–severe groups did not show a significant decrease (p-value = 0.05) (Figure 2, Table 2 and Supplementary Materials).
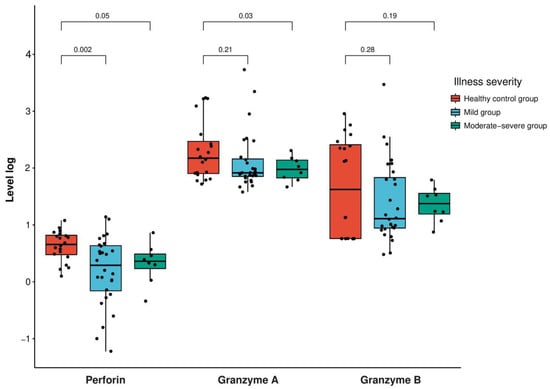
Figure 2.
Expression levels of perforin in log(ng/mL) and granzyme A and B in log(pg/mL) in the patients with SARS-CoV-2 infection according to the severity (mild group, scoring 1–5; moderate–severe group, scoring 6–9 according to the World Health Organization’s 10-point clinical progression score) compared with the healthy control participants. The box plot indicates the position of the interquartile ranges and median values. The significance was determined via a t-test or Mann–Whitney U-tests where appropriate, and a p < 0.05 was considered statistically significant.
4. Discussion
Our findings showed a decreased level of perforin during the early phase after SARS-CoV-2 infection. The trend line for perforin from each individual also indicated a low level of perforin during the early period of infection (Figure 3). One of the possible explanations for a decreased level of serum perforin during the early infection phase was high perforin consumption at the intracellular level [17]. Another possible reason for decreased serum perforin was that the SARS-CoV-2 infection paralyzed the CD8+T cytotoxic functions and decreased the expression of perforin in the early phase of infection [18]. The low level of perforin was likely a result of defective cell-mediated immunity, which is a crucial defense against viral infection, and led to persistent SARS-CoV-2 (i.e., long COVID) [19]. We identified that the median level of perforin returned to normal after the mid-phase of the infection. This finding was similar to a previous Korean study that reported that increased perforin levels were observed during the third week of SARS-CoV-2 infection compared to the first week of the infection [8]. Another study also reported an increased level of serum perforin compared to the healthy control group during the second and third weeks of SARS-CoV-2 infection [17]. This finding was likely due to the disruption of the acid–base homeostasis in the acute phase of SARS-CoV-2 infection (i.e., acidosis in the patients with SARS-CoV-2 infection likely inhibited the release of perforin and blocked its activity) [20].
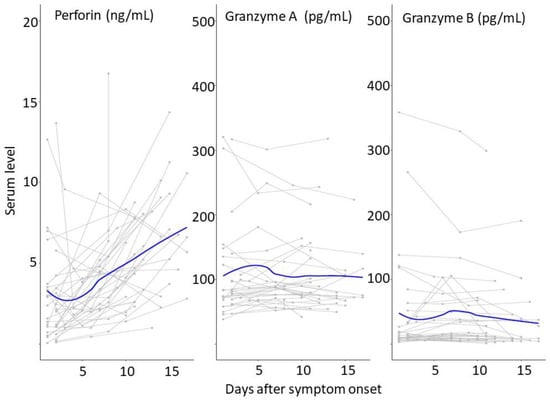
Figure 3.
Temporal patterns of serum perforin and granzymes. Serum perforin and granzyme A and B were measured from the patients infected with SARS-CoV-2. The serums were obtained from the different days after their symptom onset. The thick blue lines indicate the trend of the serum perforin and granzyme A and B levels using smoothing splines.
We identified that there was no significant difference in the levels of the granzymes during the different periods of infection compared to the healthy control groups. This finding was comparable to a previous study where granzymes increased during the third week (more than 21 days) of SARS-CoV-2 infection compared to the first week [8]. Therefore, the level of granzymes is unlikely to provide helpful information for the timing of SARS-CoV-2 infection during the acute phase after the infection.
A study demonstrated a tendency for decreased levels of intracellular perforin after the infection due to cytotoxic functional inactivity [18]. However, this study did not include information about the timing of infections and did not demonstrate whether the findings were statistically significant. Another study described statistically increased levels of intracellular perforin compared to the healthy control [21]. However, the timing of the extraction of the specimens was not demonstrated. Previous studies also demonstrated that the cell-mediated immune function, including the perforin expression, was statistically reduced for the elderly (above 70-year-old age) [22,23] and age-matched males. However, we did not include the elderly in the patients or the heathy control group. Furthermore, we did not identify a statistically significant difference in the number of males between the two groups. Therefore, the strength of our study was that it provided the temporal kinetics of perforin and granzymes A and B in the acute phase of SARS-CoV-2 infection.
There were some limitations to our study. First, this was a single-center study with a small sample size, which limited support for the generalization of our findings. Second, we did not perform flow cytometry to demonstrate whether the activated T cells were expressing perforin or granzyme A and B. However, perforin and granzymes are easy-to-measure serological markers of T cell viral activation [4]. Third, due to the limited specimens, we did not explore the correlation of perforin and granzymes with the SARS-CoV-2 RNA viral loads and immune cell numbers. An additional study that can disentangle and examine the correlation is warranted. Fourth, asymptomatic and severe patients with SARS-CoV-2 infection were not included in the study. Last, the time of symptom onset was used as a proxy for the time of infection. Therefore, the difference between the two may have affected our classification of the infection phases.
In conclusion, the serum level of perforin decreased during the early phase of SARS-CoV-2 infection. This finding indicates that the serum perforin level can provide useful clinical information as a temporal kinetic marker of the early period of SARS-CoV-2 infection. Further study that includes the cytokine associated with the T cell activation and the impact of COVID-19 vaccinations on the dynamics of perforin and granzymes using a large sample size is warranted.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11081314/s1, Table S1: Temporal changes of serum levels of perforin, granzyme A, and granzyme B in the mild group of SARS-CoV-2 patients and healthy control participants; Table S2: Temporal changes of serum levels of perforin, granzyme A, and granzyme B in the moderate-severe group of SARS-CoV-2 patients, and healthy control participants.
Author Contributions
C.-S.L. and S.R. contributed to the study design. M.T.R., S.R., C.A., J.-H.H. (Joo-Hee Hwang), J.-H.H. (Jeong-Hwan Hwang) and C.-S.L. contributed to the analysis, plan and implementation of the research and manuscript writing. M.T.R. and C.-S.L. had access to and verified all the data. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Biomedical Research Institute of Jeonbuk National University Hospital and by the Basic Science Research Programs (NRF-2015R1D1A1A01060251 and 2018R1D1A3B07049557) of the National Research Foundation of Korea, which is funded by the Ministry of Education.
Institutional Review Board Statement
All the study procedures were approved by the Institutional Review Board (IRB) of Jeonbuk National University Hospital (IRB registration number 2020-02-050).
Informed Consent Statement
Written informed consent has been obtained from the study participants to publish this paper.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors report no potential conflict of interest.
References
- Ryu, S.; Ali, S.T.; Noh, E.; Kim, D.; Lau, E.H.Y.; Cowling, B.J. Transmission dynamics and control of two epidemic waves of SARS-CoV-2 in South Korea. BMC Infect. Dis. 2021, 21, 485. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Lim, J.S.; Song, S.A.; Achangwa, C.; Sim, W.; Kim, G.; Ryu, S. Transmission Dynamics of the Delta Variant of SARS-CoV-2 Infections in South Korea. J. Infect. Dis. 2022, 225, 793–799. [Google Scholar] [PubMed]
- Ryu, S.; Han, C.; Kim, D.; Tsang, T.K.; Cowling, B.J.; Lee, S. Association Between the Relaxation of Public Health and Social Measures and Transmission of the SARS-CoV-2 Omicron Variant in South Korea. JAMA Netw. Open 2022, 5, e2225665. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Ryu, S.; Shin, H.; Kim, D.; Modchang, C. Impact of national lockdown on the suspected SARS-CoV-2 epidemic in terms of the number of fever cases in North Korea. J. Travel. Med. 2022, 29, taac090. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Seo, H.; Cho, S.; Chiara, A.; Ryu, S. Impact of travel restrictions for travelers from China on the internal spread of SARS-CoV-2 in South Korea. J. Travel. Med. 2023, 30, taad047. [Google Scholar] [CrossRef] [PubMed]
- Pietersma, F.L.; van Dorp, S.; Jacobi, R.; Ran, L.; Nanlohy, N.M.; Schuurman, R.; Minnema, M.C.; Meijer, E.; van Baarle, D. High level of perforin expression in T cells: An early prognostic marker of the severity of herpesvirus reactivation after allogeneic stem cell transplantation in adults. Clin. Infect. Dis. 2010, 50, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.A. Perforin forms transient pores on the target cell plasma membrane to facilitate rapid access of granzymes during killer cell attack. Immunobiology 2013, 121, 2659–2668. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.K.; Han, G.-C.; Kim, M.; Kim, G.; Shin, H.M.; Song, K.-H.; Choe, P.G.; Park, W.B.; Kim, E.S.; Kim, H.B.; et al. Aberrant hyperactivation of cytotoxic T-cell as a potential determinant of COVID-19 severity. Int. J. Infect. Dis. 2020, 97, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Prager, I. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Choi, J.K.; Achangwa, C.; Cho, S.; Hwang, J.H.; Hwang, J.H.; Bovenschen, N.; Lee, C.S. Temporal Dynamics of Serum Perforin and Granzymes in Three Different Clinical Stages of Virus-Induced Severe Fever with Thrombocytopenia Syndrome. Am. J. Trop. Med. Hyg. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Hwang, J.H.; Lee, C.S. Elevated Extracellular Levels of Granzymes in Patients with Scrub Typhus. Am. J. Trop. Med. Hyg. 2021, 105, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Kim, D.; Lim, J.S.; Ali, S.T.; Cowling, B.J. Serial Interval and Transmission Dynamics during SARS-CoV-2 Delta Variant Predominance, South Korea. Emerg. Infect. Dis. 2022, 28, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.K.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; Blackwood, B.; et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef] [PubMed]
- Novus Biologicals. Human Perforin ELISA Kit. Available online: https://www.novusbio.com/products/perforin-elisa-kit_nbp3-11726 (accessed on 1 May 2023).
- Novus Biologicals. Human Granzyme A ELISA Kit. Available online: https://www.novusbio.com/products/granzyme-a-elisa-kit_nbp3-11790 (accessed on 1 May 2023).
- Novus Biologicals. Human Granzyme B Quantikine ELISA Kit. Available online: https://www.novusbio.com/products/human-granzyme-b-quantikine-elisa-kit_dgzb00 (accessed on 1 May 2023).
- Li, M.; Guo, W.; Dong, Y.; Wang, X.; Dai, D.; Liu, X.; Wu, Y.; Li, M.; Zhang, W.; Zhou, H.; et al. Elevated Exhaustion Levels of NK and CD8(+) T Cells as Indicators for Progression and Prognosis of COVID-19 Disease. Front. Immunol. 2020, 11, 580237. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Trautwein, C.; Fendel, R.; Krickeberg, N.; Berezhnoy, G.; Bissinger, R.; Ossowski, S.; Salker, M.S.; Casadei, N.; Riess, O. SARS-CoV-2 infection paralyzes cytotoxic and metabolic functions of the immune cells. Heliyon 2021, 7, e07147. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Kieninger, M. Lower blood pH as a strong prognostic factor for fatal outcomes in critically ill COVID-19 patients at an intensive care unit: A multivariable analysis. PLoS ONE 2021, 16, e0258018. [Google Scholar] [CrossRef] [PubMed]
- Kundura, L.; Cezar, R.; Andre, S.; Campos-Mora, M.; Lozano, C.; Vincent, T.; Muller, L.; Lefrant, J.-Y.; Roger, C.; Claret, P.-G.; et al. Low perforin expression in CD8+ T lymphocytes during the acute phase of severe SARS-CoV-2 infection predicts long COVID. Front. Immunol. 2022, 13, 1029006. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, L.; Kimber, I.; Basketter, D.; Simmonds, P.; McSweeney, S.; Tziotzios, C.; McFadden, J.P. Perforin, COVID-19 and a possible pathogenic auto-inflammatory feedback loop. Scand. J. Immunol. 2021, 94, e13102. [Google Scholar] [CrossRef] [PubMed]
- Rukavina, D.; Laskarin, G.; Rubesa, G.; Strbo, N.; Bedenicki, I.; Manestar, D.; Glavas, M.; Christmas, S.E.; Podack, E.R. Age-related decline of perforin expression in human cytotoxic T lymphocytes and natural killer cells. Blood 1998, 92, 2410–2420. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).