Impact of Maternal Antibodies on Infectious Bronchitis Virus (IBV) Infection in Primary and Secondary Lymphoid Organs of Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and the Animals
2.2. Experimental Design
2.3. Techniques
2.3.1. Enzyme-Linked Immunosorbent Assay (ELISA)
2.3.2. RNA Extraction and Complimentary Deoxy Ribonucleic Acid (cDNA) Synthesis
2.3.3. IBV Genome Load Quantification by Quantitative Polymerase Reaction (qPCR) assay
2.3.4. Immunohistochemistry Technique
2.3.5. Histopathology
2.4. Statistical Analyses
3. Results
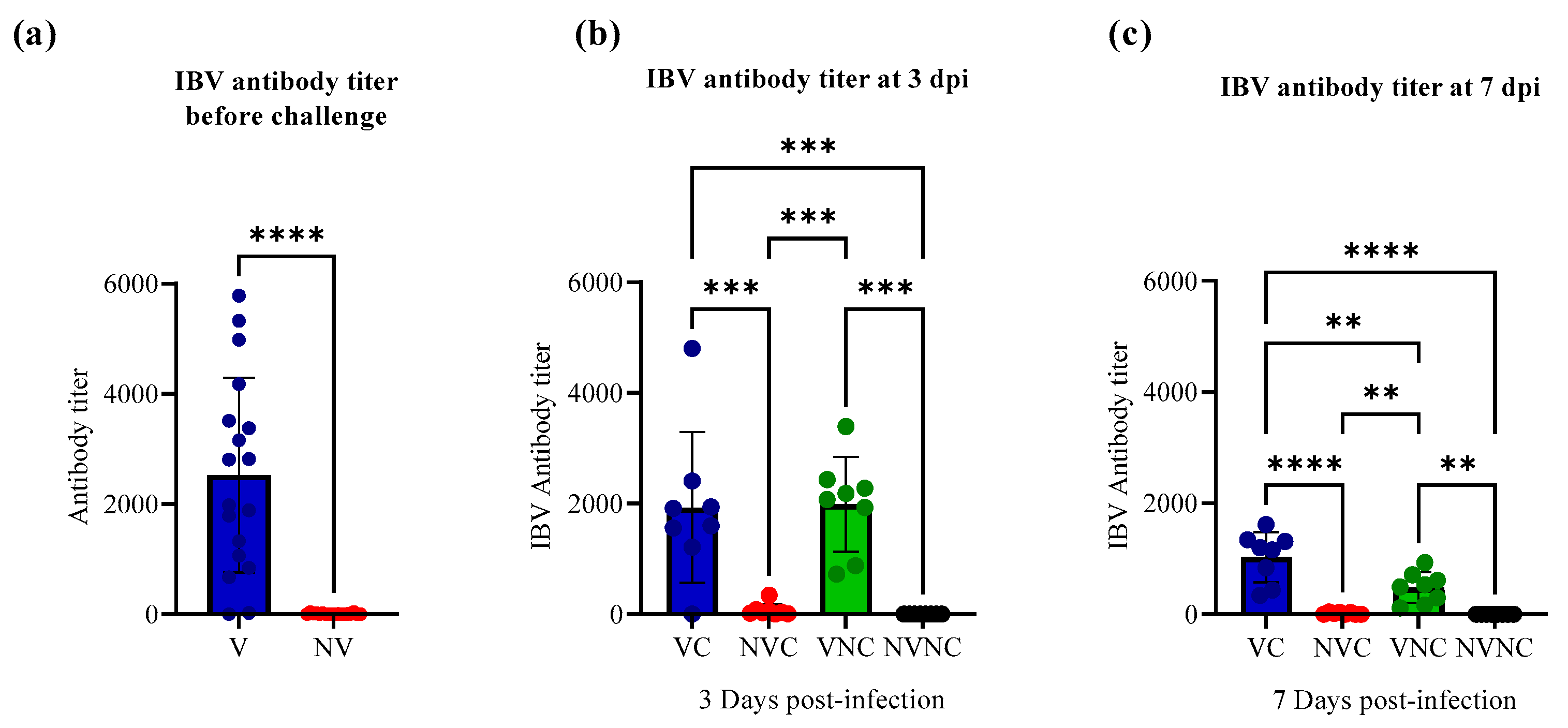
3.1. Serology
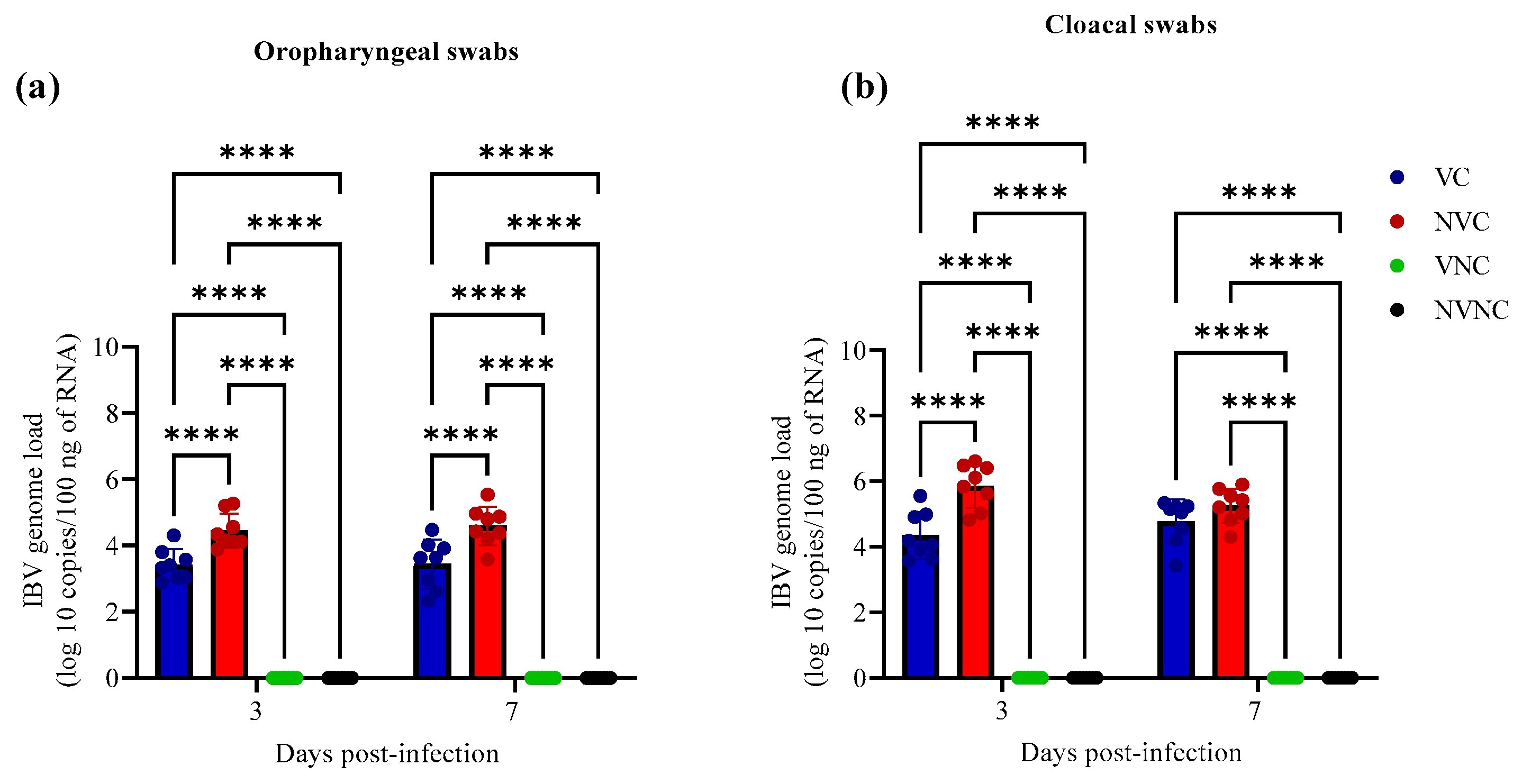
3.2. IBV Shedding
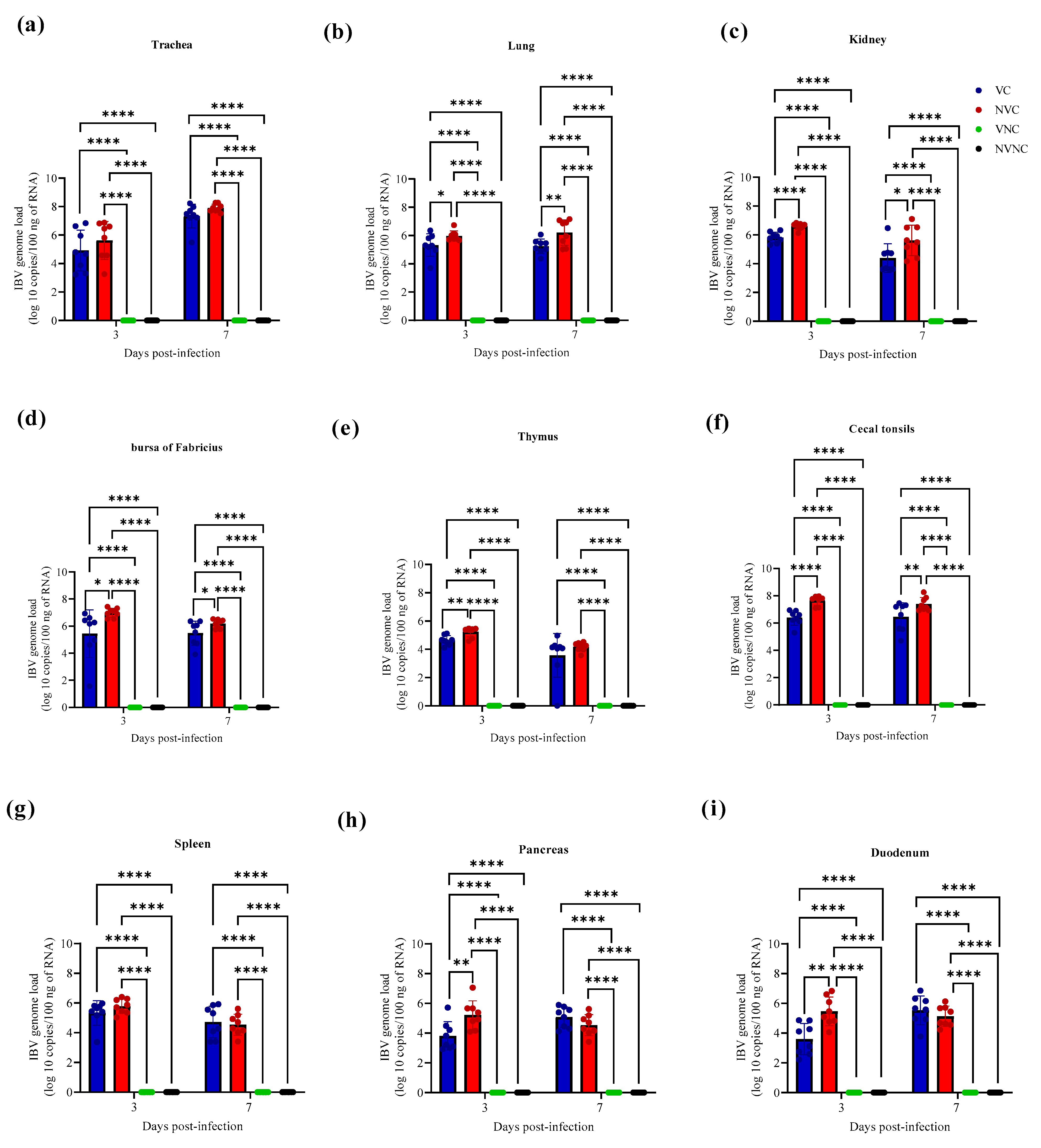
3.3. IBV Genome Loads in Tissues
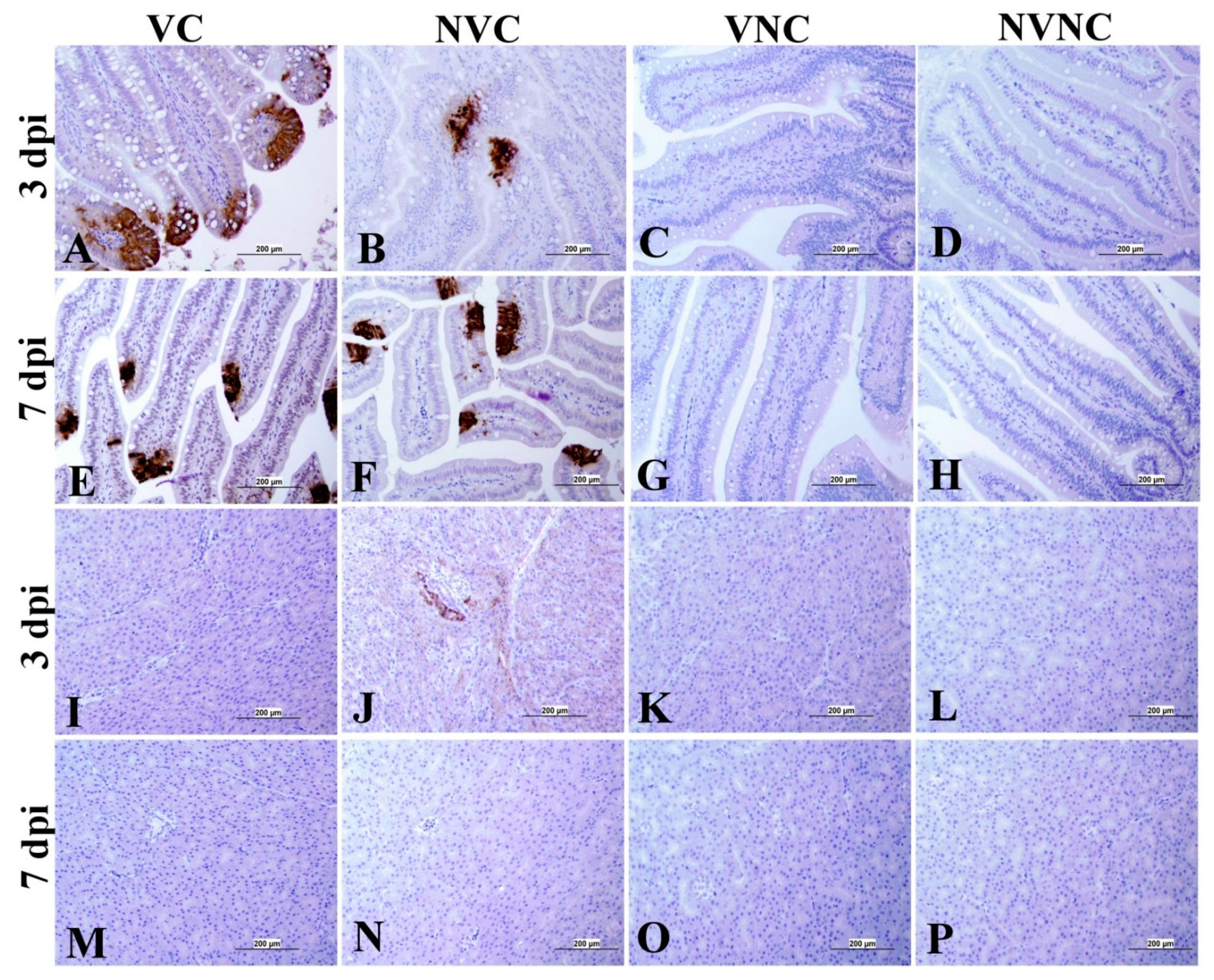
3.4. IBV Nuclear Antigen in Tissues
3.5. Histopathology
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miłek, J.; Blicharz-Domańska, K. Coronaviruses in Avian Species—Review with Focus on Epidemiology and Diagnosis in Wild Birds. J. Vet. Res. 2018, 62, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, A.; Abdul-Cader, M.S.; Nazir, S.; De Silva Senapathi, U.; van der Meer, F.; Cork, S.C.; Gomis, S.; Abdul-Careem, M.F. Infectious bronchitis corona virus establishes productive infection in avian macrophages interfering with selected antimicrobial functions. PLoS ONE 2017, 12, e0181801. [Google Scholar] [CrossRef] [PubMed]
- Markowski-Grimsrud, C.J.; Schat, K.A. Infection with chicken anaemia virus impairs the generation of pathogen-specific cytotoxic T lymphocytes. Immunology 2003, 109, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, C.A.; Bournival, V.; Koszegi, M.; Nantel-Fortier, N.; St-Sauveur, V.G.; Provost, C.; Lair, S. Quebec: Avian pathogens identification and genomic characterization: 2021 annual review of the Molecular Diagnostic Laboratory, Université de Montréal. Can. Vet. J. 2022, 63, 486–490. [Google Scholar]
- Hassan, M.S.H.; Ojkic, D.; Coffin, C.S.; Cork, S.C.; van der Meer, F.; Abdul-Careem, M.F. Delmarva (DMV/1639) Infectious Bronchitis Virus (IBV) Variants Isolated in Eastern Canada Show Evidence of Recombination. Viruses 2019, 11, 1054. [Google Scholar] [CrossRef]
- Ojkić, D.; Martin, E. Infectious bronchitis virus and its variants in Canada. In Proceedings of the 12th International Congress for Veterinary Virology, Ghent, Belgium, 20–23 September 2022. [Google Scholar]
- Hassan, M.S.H.; Ali, A.; Buharideen, S.M.; Goldsmith, D.; Coffin, C.S.; Cork, S.C.; van der Meer, F.; Boulianne, M.; Abdul-Careem, M.F. Pathogenicity of the Canadian Delmarva (DMV/1639) Infectious Bronchitis Virus (IBV) on Female Reproductive Tract of Chickens. Viruses 2021, 13, 2488. [Google Scholar] [CrossRef]
- Hassan, M.S.H.; Buharideen, S.M.; Ali, A.; Najimudeen, S.M.; Goldsmith, D.; Coffin, C.S.; Cork, S.C.; van der Meer, F.; Abdul-Careem, M.F. Efficacy of Commercial Infectious Bronchitis Vaccines against Canadian Delmarva (DMV/1639) Infectious Bronchitis Virus Infection in Layers. Vaccines 2022, 10, 1194. [Google Scholar] [CrossRef]
- Ambali, A.G.; Jones, R.C. Early pathogenesis in chicks of infection with an enterotropic strain of infectious bronchitis virus. Avian Dis. 1990, 34, 809–817. [Google Scholar] [CrossRef]
- Cook, J.K.; Orbell, S.J.; Woods, M.A.; Huggins, M.B. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 1999, 28, 477–485. [Google Scholar] [CrossRef]
- Jackwood, M.W.; Lee, D.H. Different evolutionary trajectories of vaccine-controlled and non-controlled avian infectious bronchitis viruses in commercial poultry. PLoS ONE 2017, 12, e0176709. [Google Scholar] [CrossRef]
- Fan, W.Q.; Wang, H.N.; Zhang, Y.; Guan, Z.B.; Wang, T.; Xu, C.W.; Zhang, A.Y.; Yang, X. Comparative dynamic distribution of avian infectious bronchitis virus M41, H120, and SAIBK strains by quantitative real-time RT-PCR in SPF chickens. Biosci. Biotechnol. Biochem. 2012, 76, 2255–2260. [Google Scholar] [CrossRef]
- Goryo, M.; Umemura, T.; Itakura, C. Concurrence of nephrosis-nephritis due to infectious bronchitis virus and infectious bursal disease in broiler chickens. Avian Pathol. 1984, 13, 191–200. [Google Scholar] [CrossRef]
- Cook, J.K. Duration of experimental infectious bronchitis in chickens. Res. Vet. Sci. 1968, 9, 506–514. [Google Scholar] [CrossRef]
- El-Houadfi, M.; Jones, R.C.; Cook, J.K.; Ambali, A.G. The isolation and characterisation of six avian infectious bronchitis viruses isolated in Morocco. Avian Pathol. 1986, 15, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.D.; Peterson, R.D.A.; Good, R.A. Delineation of the Thymic and Bursal Lymphoid Systems in the Chicken. Nature 1965, 205, 143–146. [Google Scholar] [CrossRef]
- Cooper, M.D.; Raymond, D.A.; Peterson, R.D.; South, M.A.; Good, R.A. The functions of the thymus system and the bursa system in the chicken. J. Exp. Med. 1966, 123, 75–102. [Google Scholar] [CrossRef] [PubMed]
- Mockett, A.P.; Cook, J.K.; Huggins, M.B. Maternally-derived antibody to infectious bronchitis virus: Its detection in chick trachea and serum and its role in protection. Avian Pathol. 1987, 16, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.P.; Naqi, S.A. Maternal antibody to infectious bronchitis virus: Its role in protection against infection and development of active immunity to vaccine. Vet. Immunol. Immunopathol. 2001, 79, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Davelaar, F.G.; Kouwenhoven, B. Influence of maternal antibodies on vaccination of chicks of different ages against infectious bronchitis. Avian Pathol. 1977, 6, 41–50. [Google Scholar] [CrossRef]
- Meher, M.M.; Hossain, M.; Haider, G. Detection of MDA titer of ınfectious bronchitis virus and comparison of antibody titer produced by two different ınfectious bronchitis vaccine. J. Poult. Res. 2022, 19, 44–51. [Google Scholar] [CrossRef]
- Kameka, A.M.; Haddadi, S.; Kim, D.S.; Cork, S.C.; Abdul-Careem, M.F. Induction of innate immune response following infectious bronchitis corona virus infection in the respiratory tract of chickens. Virology 2014, 450–451, 114–121. [Google Scholar] [CrossRef]
- Hassan, M.S.H.; Abd-Elsalam, R.M.; Ratcliff, N.; Herath-Mudiyanselage, H.; Abdul-Careem, M.F. The impact of the experimental route of challenge on the host responses and pathogenesis of the Canadian Delmarva (DMV/1639) infectious bronchitis virus infection in laying chickens. Vet. Immunol. Immunopathol. 2023, 261, 110623. [Google Scholar] [CrossRef] [PubMed]
- Hussein, E.A.; Hair-Bejo, M.; Adamu, L.; Omar, A.R.; Arshad, S.S.; Awad, E.A.; Aini, I. Scoring System for Lesions Induced by Different Strains of Newcastle Disease Virus in Chicken. Vet. Med. Int. 2018, 2018, 9296520. [Google Scholar] [CrossRef] [PubMed]
- Darbyshire, J.H.; Peters, R.W. Humoral antibody response and assessment of protection following primary vaccination of chicks with maternally derived antibody against avian infectious bronchitis virus. Res. Vet. Sci. 1985, 38, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Manswr, B.; Ball, C.; Forrester, A.; Chantrey, J.; Ganapathy, K. Host immune response to infectious bronchitis virus Q1 in two commercial broiler chicken lines. Res. Vet. Sci. 2021, 136, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Klein, D. Quantification using real-time PCR technology: Applications and limitations. Trends Mol. Med. 2002, 8, 257–260. [Google Scholar] [CrossRef]




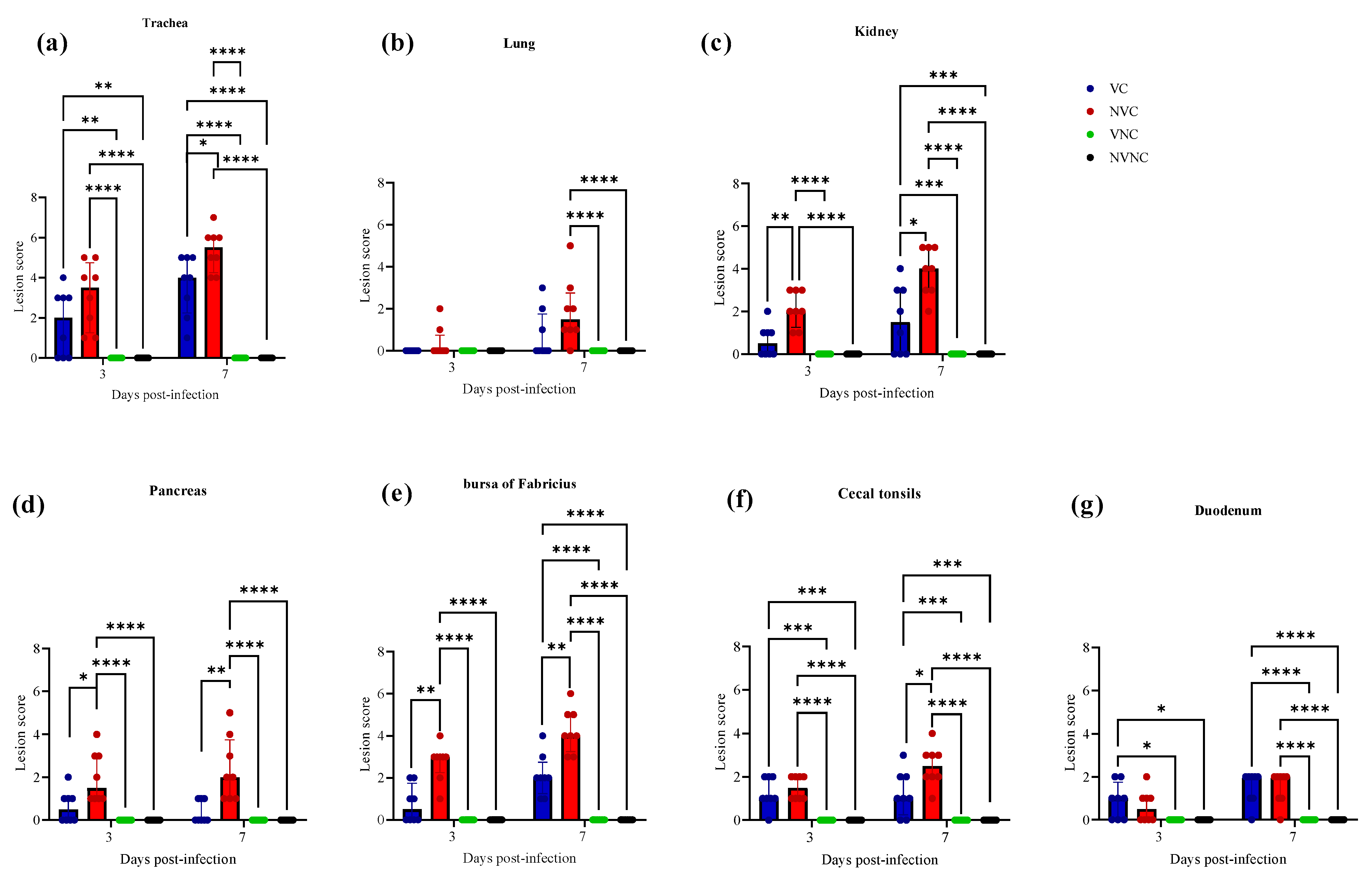
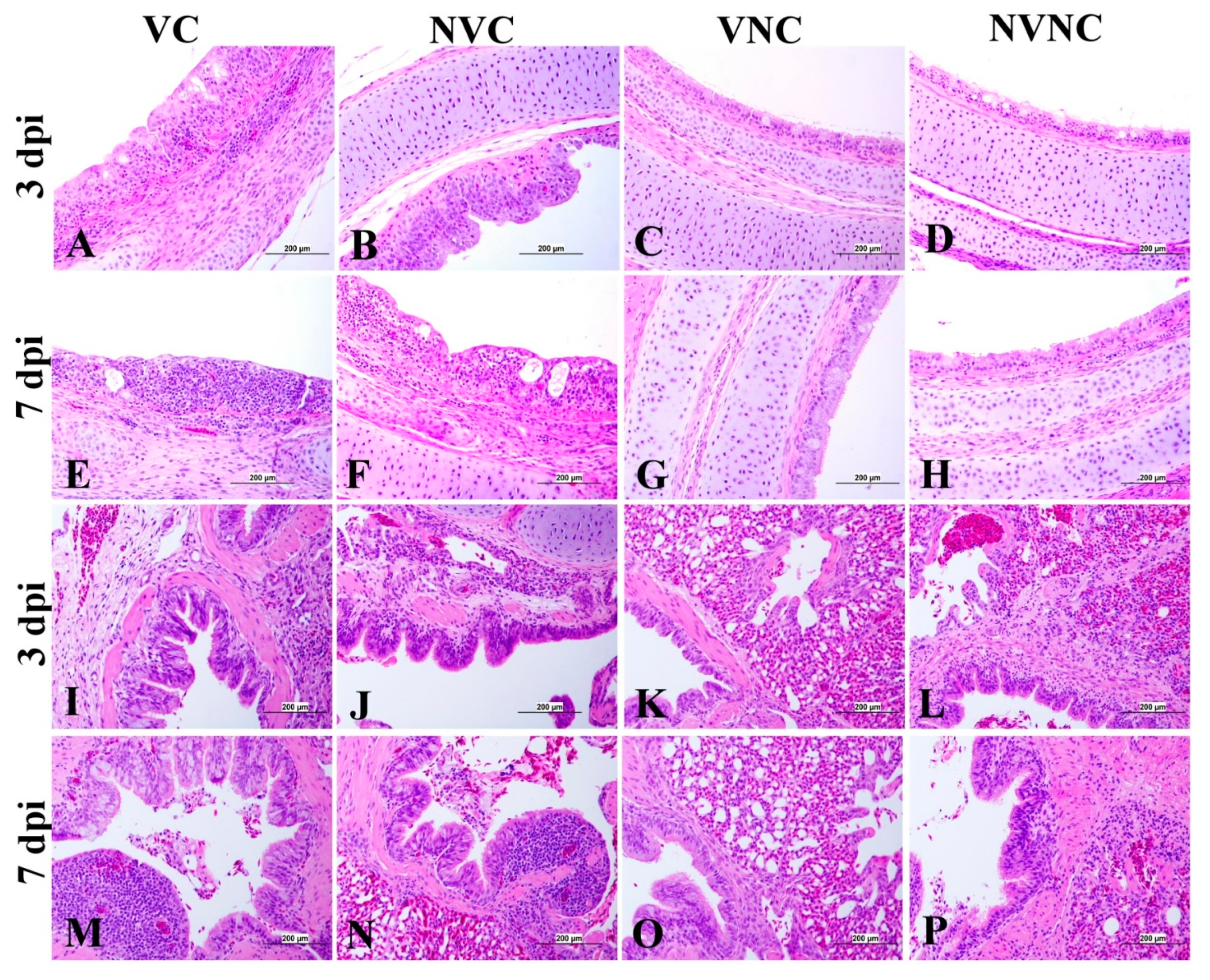
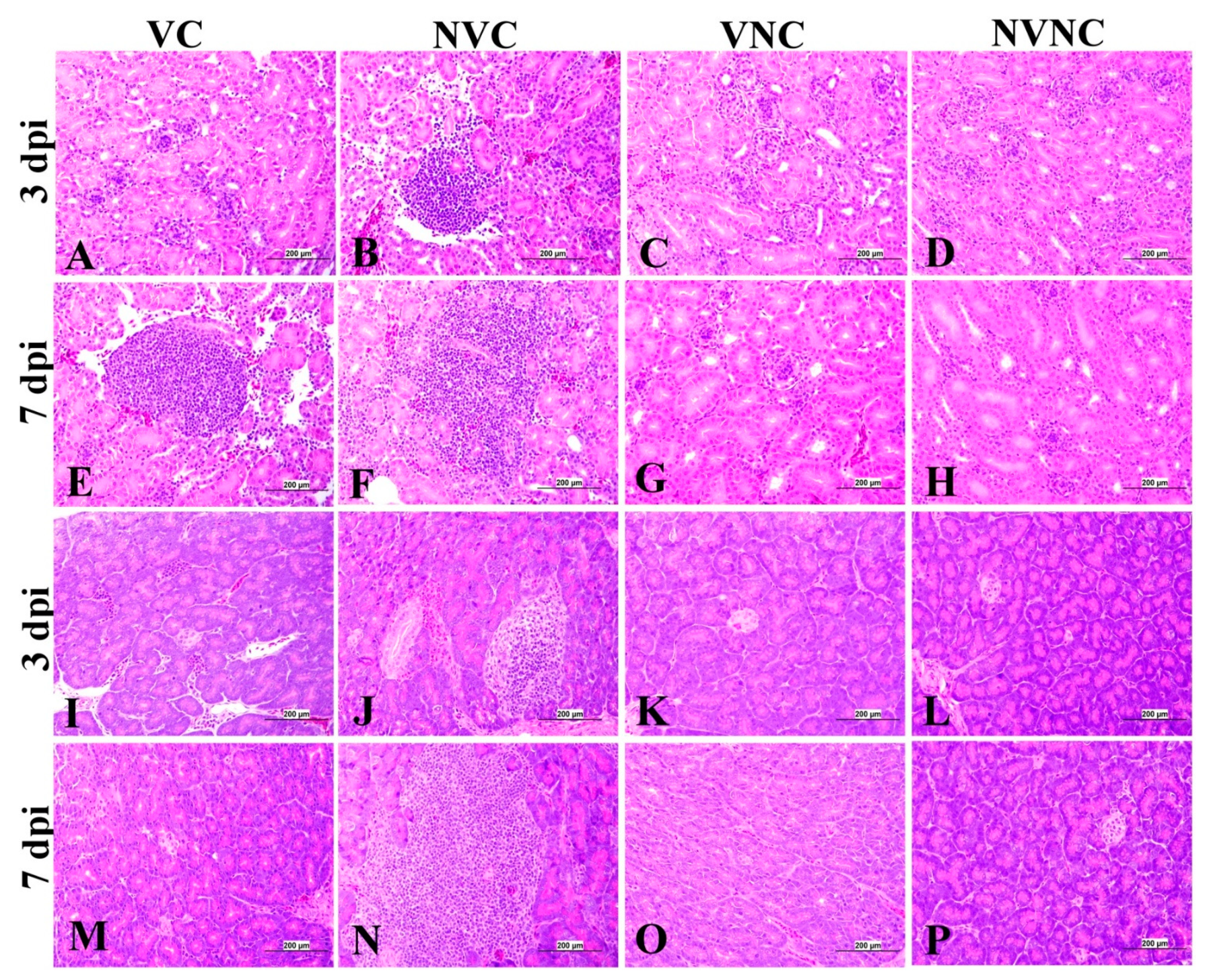
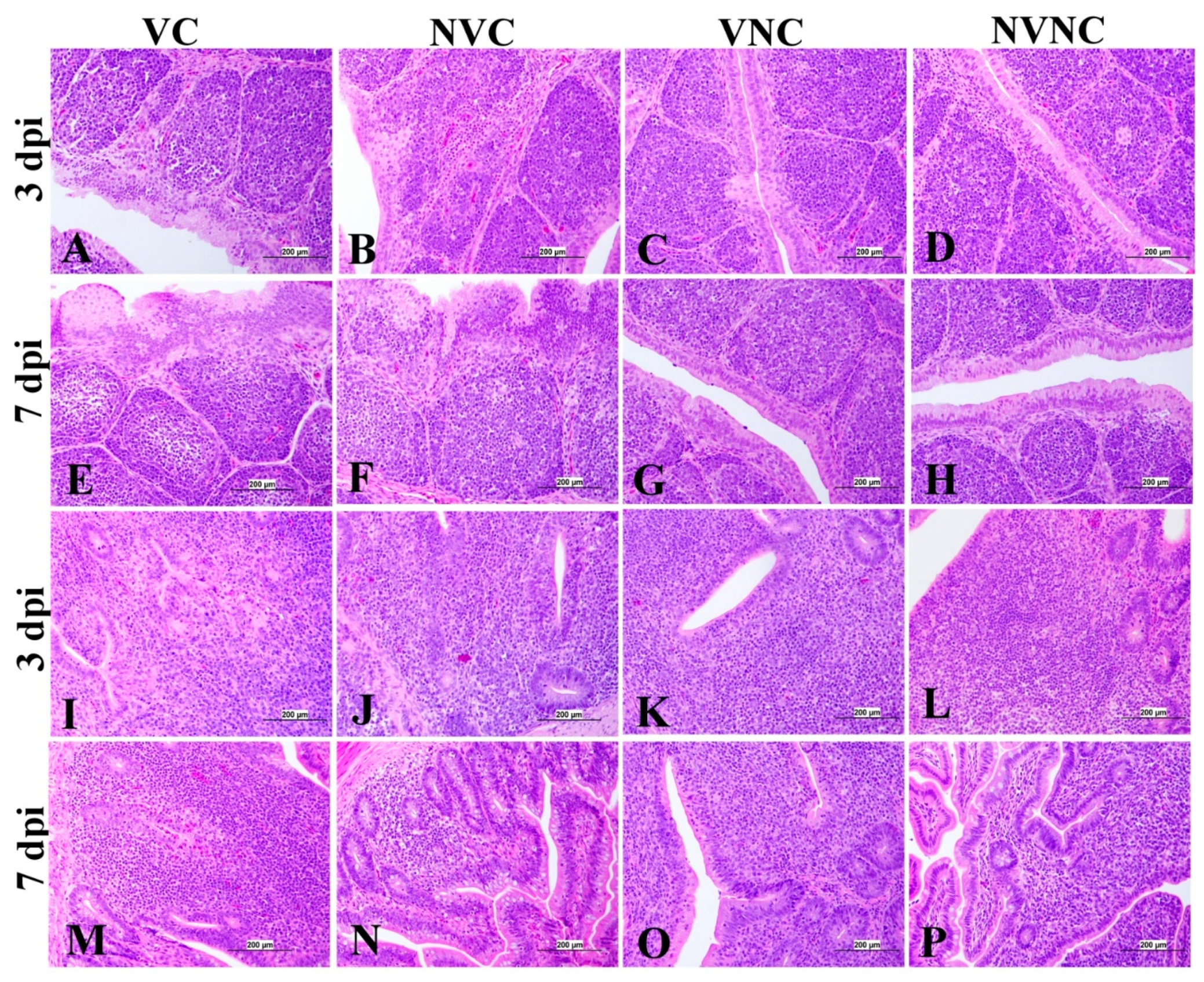
| Organ | Scoring Criteria |
|---|---|
| Trachea | Loss of cilia Epithelial cell degeneration and necrosis Mononuclear cell aggregation in lamina propria Hyperplasia of lining epithelium Loss of mucosa |
| Lung | Bronchitis Focal lymphoplasmocytic infiltration Diffuse lymphoplasmocytic infiltration Parabronchitis |
| Kidney | Tubular necrosis Focal lymphoplasmocytic infiltration Diffuse lymphoplasmocytic infiltration Focal lymphoid follicle aggregation Gout |
| BF | Depletion of lymphoid follicles Epithelial lining hyperplasia with squamous cell metaplasia Epithelial lining degeneration and necrosis |
| Thymus | Depletion of the cortical lymphocyte density or relative thickness to the medulla Thymus hemorrhage |
| Spleen | Hyperplasia or hypertrophy in the ellipsoids Lymphoidal apoptosis or necrosis Sinusoidal congestion |
| CT | Lymphoepithelial degeneration and necrosis Subepithelial zone inflammatory cell infiltration Lymphoid apoptosis and necrosis in germinal centers |
| Pancreas | Acinar cell necrosis Lymphoid infiltration Ductal dilation with inflammatory cell infiltration |
| Duodenum | Epithelial degeneration and necrosis Lymphoid infiltration in the lamina propria Submucosal lymphoid cell infiltration |
| Trachea | Lung | Kidney | BF | CT | Spleen | Thymus | Duodenum | Pancreas | |
|---|---|---|---|---|---|---|---|---|---|
| NVNC | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 |
| VC | 5/8 | 2/8 | 8/8 | 7/8 | 7/8 | 0/8 | 0/8 | 1/8 | 0/8 |
| NVC | 6/8 | 2/8 | 8/8 | 8/8 | 8/8 | 3/8 | 0/8 | 3/8 | 1/8 |
| VNC | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 |
| Trachea | Lung | Kidney | BF | CT | Spleen | Thymus | Duodenum | Pancreas | |
|---|---|---|---|---|---|---|---|---|---|
| NVNC | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 |
| VC | 7/8 | 0/8 | 7/8 | 8/8 | 8/8 | 0/8 | 0/8 | 1/8 | 0/8 |
| NVC | 8/8 | 5/8 | 7/8 | 8/8 | 8/8 | 2/8 | 1/8 | 3/8 | 0/8 |
| VNC | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isham, I.M.; Hassan, M.S.H.; Abd-Elsalam, R.M.; Ranaweera, H.A.; Mahmoud, M.E.; Najimudeen, S.M.; Ghaffar, A.; Cork, S.C.; Gupta, A.; Abdul-Careem, M.F. Impact of Maternal Antibodies on Infectious Bronchitis Virus (IBV) Infection in Primary and Secondary Lymphoid Organs of Chickens. Vaccines 2023, 11, 1216. https://doi.org/10.3390/vaccines11071216
Isham IM, Hassan MSH, Abd-Elsalam RM, Ranaweera HA, Mahmoud ME, Najimudeen SM, Ghaffar A, Cork SC, Gupta A, Abdul-Careem MF. Impact of Maternal Antibodies on Infectious Bronchitis Virus (IBV) Infection in Primary and Secondary Lymphoid Organs of Chickens. Vaccines. 2023; 11(7):1216. https://doi.org/10.3390/vaccines11071216
Chicago/Turabian StyleIsham, Ishara M., Mohamed S. H. Hassan, Reham M. Abd-Elsalam, Hiruni A. Ranaweera, Motamed E. Mahmoud, Shahnas M. Najimudeen, Awais Ghaffar, Susan C. Cork, Ashish Gupta, and Mohamed Faizal Abdul-Careem. 2023. "Impact of Maternal Antibodies on Infectious Bronchitis Virus (IBV) Infection in Primary and Secondary Lymphoid Organs of Chickens" Vaccines 11, no. 7: 1216. https://doi.org/10.3390/vaccines11071216
APA StyleIsham, I. M., Hassan, M. S. H., Abd-Elsalam, R. M., Ranaweera, H. A., Mahmoud, M. E., Najimudeen, S. M., Ghaffar, A., Cork, S. C., Gupta, A., & Abdul-Careem, M. F. (2023). Impact of Maternal Antibodies on Infectious Bronchitis Virus (IBV) Infection in Primary and Secondary Lymphoid Organs of Chickens. Vaccines, 11(7), 1216. https://doi.org/10.3390/vaccines11071216







