Impact of Health Workers’ Choice of COVID-19 Vaccine Booster on Immunization Levels in Istanbul, Turkey
Abstract
1. Introduction
2. Materials and Methods
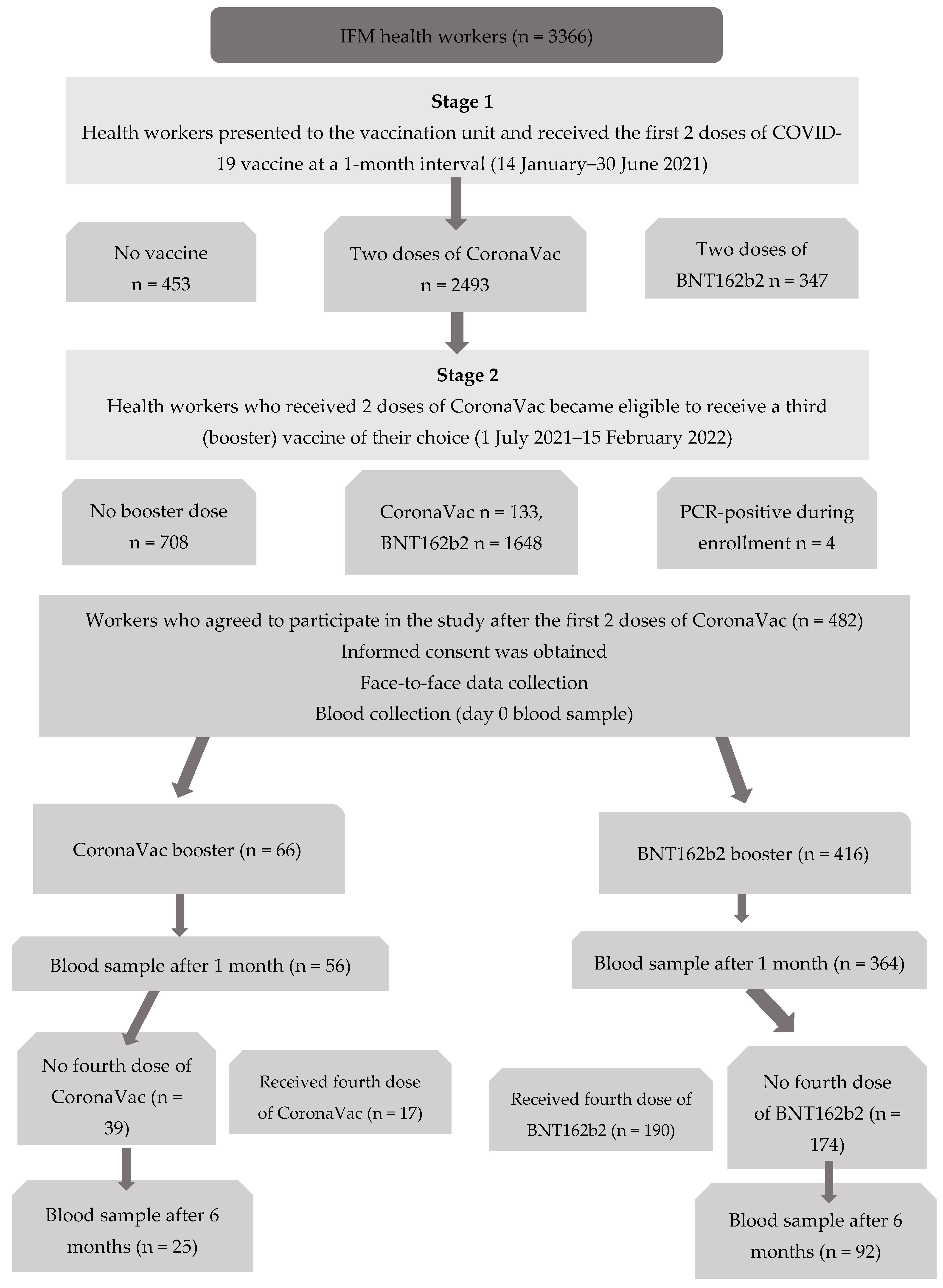
2.1. Study Design and Participants
2.2. Data Collection
2.3. Vaccines
2.3.1. Inactivated SARS-CoV-2 Vaccine (CoronaVac)
2.3.2. BNT162b2 Vaccine
2.4. Evaluation of Immune Response
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- The BNT162b2 vaccine results in higher IgG levels than the CoronaVac vaccine among individuals with a positive history of COVID-19;
- The BNT162b2 vaccine was preferred by health workers with chronic diseases;
- Health workers are less affected by the infodemic because they have easier access to accurate information;
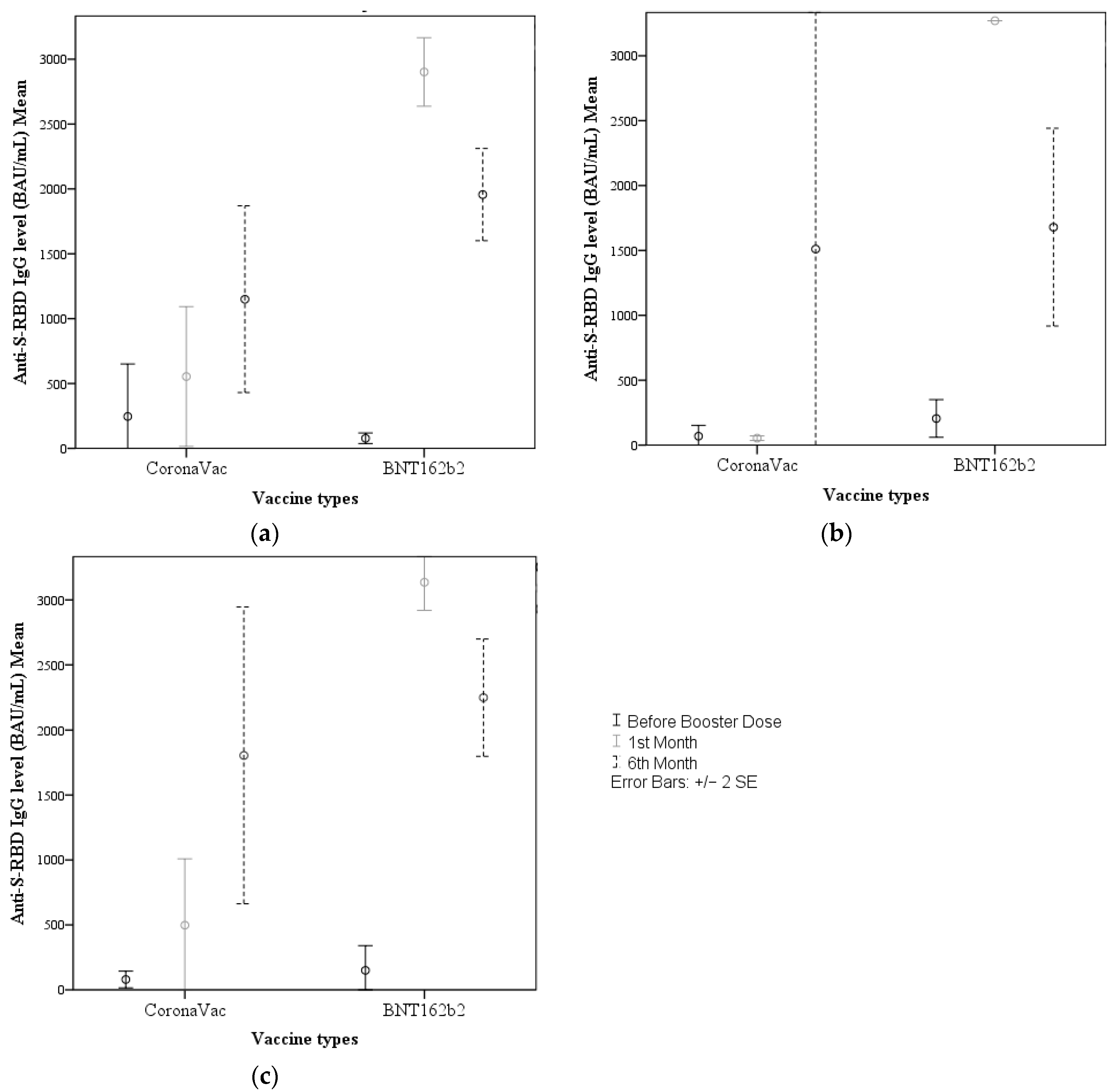
- In participants without chronic diseases, antibody levels increased significantly after both booster vaccines, but among participants with chronic diseases only those boosted with BNT162b2 showed a significant increase in antibody levels;
- Blood samples obtained before and after (1 and 6 months) booster vaccinations demonstrated no difference in the potential to induce an IgG response according to age group or gender;
- In terms of IgG responses to the two booster vaccines in participants without a history of COVID-19 and the entire participant group, no differences were observed within these groups before receiving the booster, whereas all participants who received a BNT162b2 booster had significantly higher IgG levels at 1 and 6 months. Among participants with a history of COVID-19 infection, there was no difference in pre-booster and 6-month IgG levels, while a significant difference was found in favor of BNT162b2 at 1 month.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Information Platform Republic of Turkiye, Ministry of Health. 2022. Available online: https://covid19.saglik.gov.tr/ (accessed on 2 November 2022).
- Sibel Gürbüz, S.A.; Meltem, Ç. COVID-19 Aşı Çalışmaları ve Uygulamaları. In Yeni Koronavirüs Pandemisi Sürecinde Türkiye’de COVID-19 Aşılaması Ve Bağışıklama Hizmetlerinin Durumu; TTB: Ankara, Turkiye, 2021; pp. 45–60. [Google Scholar]
- Republic of Turkiye MoH. COVID-19 Aşısı Ulusal Uygulama Stratejisi. 2022. Available online: https://covid19asi.saglik.gov.tr/TR-77706/covid-19-asisi-ulusal-uygulama-stratejisi.html (accessed on 10 November 2022).
- Şimşek, H.; Kılıç, B. Sağlıkta eşitsizliklerle ilgili temel kavramlar. Türkiye Halk Sağlığı Dergisi. 2012, 10, 116–127. [Google Scholar] [CrossRef]
- Hazard Recognition What Is the Risk to Workers in the United States?: Unıted States Department of Labor Occupational Safety and Health Administration. Available online: https://www.osha.gov/SLTC/covid-19/hazardrecognition.html (accessed on 10 November 2022).
- Integrated Surveillance of COVID-19 in Italy: Istituto Superiore di Sanità EpiCentro—Epidemiology for Public Health. 2020. Available online: https://www.epicentro.iss.it/en/coronavirus/bollettino/Infografica_19giugno%20ENG.pdf (accessed on 1 November 2022).
- L’Huillier, A.G.M.B.; Andrey, D.O.; Arm-Vernez, I.; Baggio, S.; Didierlaurent, A.; Eberhardt, C.S.; Eckerle, I.; Grasset-Salomon, C.; Huttner, A.; Posfay-Barbe, K.M.; et al. Geneva Centre for Emerging Viral Diseases. Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: A prospective longitudinal study. Clin. Microbiol. Infect 2021, 27, 1–8. [Google Scholar] [CrossRef]
- Wajnberg, A.A.F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; Stadlbauer, D.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef]
- Galipeau, Y.G.M.; Liu, G.; Driedger, M.; Langlois, M.-A. Humoral Responses and Serological Assays in SARS-CoV-2 Infections. Front. Immunol. 2020, 11, 610688. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.R.-J.S.; Gea-Mallorquí, E. Will SARS-CoV-2 Infection Elicit LongLasting Protective or Sterilising Immunity? Implications for Vaccine Strategies. Front. Immunol. 2020, 11, 571481. [Google Scholar] [CrossRef] [PubMed]
- Lumley, S.F.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Mardsen, B.D.; Cox, S.; James, T.; Warren, F.; et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N. Engl. J. Med. 2021, 384, 533–540. [Google Scholar] [CrossRef] [PubMed]
- CoronaVac COVID-19 Vaccine (Sinovac): Precision Vaccinations. 2022. Available online: https://www.precisionvaccinations.com/vaccines/coronavac-covid-19-vaccine-sinovac (accessed on 1 October 2022).
- Information Sheet Vaccination (Primary Immunization and Booster Vaccinations) against COVID-19: Robert Koch-Institut. 2022. Available online: https://www.rki.de/DE/Content/Infekt/Impfen/Materialien/Downloads-COVID-19/Aufklaerungsbogen-Englisch.pdf?__blob=publicationFile (accessed on 1 April 2023).
- Republic of Turkiye MoH. COVID-19 mRNA Aşısı (BNT162b2) Uygulama Kuralları. 2021. Available online: https://covid19asi.saglik.gov.tr/Eklenti/40481/0/covid-19mrnaasisibnt162b2uygulamakurallarikitapcikpdf.pdf (accessed on 1 October 2022).
- BioNTech. Pfizer Product Monograph Including Patient Medication Information COMIRNATY® COVID-19 Vaccine 2022. Available online: https://covid-vaccine.canada.ca/info/pdf/pfizer-biontech-covid-19-vaccine-pm1-en.pdf (accessed on 29 March 2023).
- COVID-19 Test Directory Updated 29 January 2023. Available online: www.finddx.org/tools-and-resources/dxconnect/test-directories/covid-19-test-directory (accessed on 1 April 2023).
- Del Rio, C.; Malani, P.N.; Omer, S.B. Confronting the delta variant of SARS-CoV-2, summer 2021. JAMA 2021, 326, 1001–1002. [Google Scholar] [CrossRef]
- Çağlayan, D.; Süner, A.F.; Şiyve, N.; Güzel, I.; Irmak, Ç.; Işik, E.; Appak, O.; Celik, M.; Ozturk, G.; Cavus, S.A.; et al. An analysis of antibody response following the second dose of CoronaVac and humoral response after booster dose with BNT162b2 or CoronaVac among healthcare workers in Turkey. J. Med. Virol. 2022, 94, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.-H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccines Immunother. 2022, 18, 2027160. [Google Scholar] [CrossRef]
- Kara, E.; Tanir, F.; Demirhindi, H.; Mete, B.; Kibar, F.; Çetiner, S.; Candevir, A.; Inaltekin, A. Humoral immune response in inactivated SARS-CoV-2 vaccine: When should a booster dose be administered? Mikrobiyoloji Bul. 2022, 56, 2021–2027. [Google Scholar] [CrossRef]
- Hocher, B.; Schönbrunn, A.; Chen, X.; Krämer, B.K.; von Baehr, V. Outliers Matter—Correlation between S1 IgG SARS-CoV-2 Antibodies and Neutralizing SARS-CoV-2 Antibodies. Microorganisms 2022, 10, 2067. [Google Scholar] [CrossRef] [PubMed]
- Kamps, B.S.; Hoffmann, C. Diagnostic Tests and Procedures. In COVID Reference Eng 2020.1; Steinhäuser Verlag: Germany, 2020; pp. 51–62. Available online: https://www.amedeo.com/CovidReference01.pdf (accessed on 1 January 2023).
- Padoan, A.; Cosma, C.; Bonfante, F.; Della Rocca, F.; Barbaro, F.; Santarossa, C.; Dall’Olmo, L.; Pagliari, M.; Bortolami, A.; Cattelam, A.; et al. SARS-CoV-2 neutralizing antibodies after one or two doses of Comirnaty (BNT162b2, BioNTech/Pfizer): Kinetics and comparison with chemiluminescent assays. Clin. Chim. Acta. 2021, 523, 446–453. [Google Scholar] [CrossRef]
- Nandakumar, V.; Profaizer, T.; Lozier, B.K.; Elgort, M.G.; Larragoite, E.T.; Williams, E.S.; Solis-Leal, A.; Lopez, J.B.; Berges, E.T.; Planelles, V.; et al. Evaluation of a Surrogate Enzyme-Linked Immunosorbent Assay–Based Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) cPass Neutralization Antibody Detection Assay and Correlation with Immunoglobulin G Commercial Serology Assays. Arch. Pathol. Lab. Med. 2021, 145, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Dolscheid-Pommerich, R.; Bartok, E.; Renn, M.; Kümmerer, B.M.; Schulte, B.; Schmithausen, R.M.; Stoffel-Wagner, B.; Streeck, H.; Saschenbrecker, S.; Steinhanger, K.; et al. Correlation between a quantitative anti-SARS-CoV-2 IgG ELISA and neutralization activity. J. Med. Virol. 2022, 94, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, E.; Günal, Ö.; Başbulut, E.; Şen, A. SARS-CoV-2 specific antibody responses in healthcare workers after a third booster dose of CoronaVac or BNT162b2 vaccine. J. Med. Virol. 2022, 94, 3768–3775. [Google Scholar] [CrossRef]
- Keskin, A.U.; Bolukcu, S.; Ciragil, P.; Topkaya, A.E. SARS-CoV-2 specific antibody responses after third CoronaVac or BNT162b2 vaccine following two-dose CoronaVac vaccine regimen. J. Med. Virol. 2022, 94, 39–41. [Google Scholar] [CrossRef]
- Tyagi, K.; Ghosh, A.; Nair, D.; Dutta, K.; Bhandari, P.S.; Ansari, I.A.; Misra, A. Breakthrough COVID19 infections after vaccinations in healthcare and other workers in a chronic care medical facility in New Delhi, India. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 1007–1008. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Alhinai, Z.; Park, S.; Choe, Y.-J.; Michelow, I.C. A global epidemiological analysis of COVID-19 vaccine types and clinical outcomes. Int. J. Infect. Dis. 2022, 124, 206–211. [Google Scholar] [CrossRef]
- Kelkar, A.H.; Blake, J.A.; Cherabuddi, K.; Cornett, H.; McKee, B.L.; Cogle, C.R. Vaccine enthusiasm and hesitancy in cancer patients and the impact of a webinar. Healthcare 2021, 9, 351. [Google Scholar] [CrossRef]
- Ssentongo, P.; Ssentongo, A.E.; Heilbrunn, E.S.; Ba, D.M.; Chinchilli, V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0238215. [Google Scholar] [CrossRef]
- Soares, P.; Rocha, J.V.; Moniz, M.; Gama, A.; Laires, P.A.; Pedro, A.R.; Dias, S.; Leite, A.; Nunes, C. Factors associated with COVID-19 vaccine hesitancy. Vaccines 2021, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Munich Security Conference. 2020. Available online: https://www.who.int/director-general/speeches/detail/munich-security-conference (accessed on 1 October 2022).
- Thompson, M.G. Effectiveness of a Third Dose of mRNA Vaccines Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations among Adults during Periods of Delta and Omicron Variant Predominance—VISION Network, 10 States, August 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 139–145. [Google Scholar]
- Bachmann, M.F.; Dyer, M.R. Therapeutic vaccination for chronic diseases: A new class of drugs in sight. Nat. Rev. Drug Discov. 2004, 3, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Bayram, A.; Demirbakan, H.; Günel Karadeniz, P.; Erdoğan, M.; Koçer, I. Quantitation of antibodies against SARS-CoV-2 spike protein after two doses of CoronaVac in healthcare workers. J. Med. Virol. 2021, 93, 5560–5567. [Google Scholar] [CrossRef] [PubMed]
- Sosyal Güvenlik Kurumu Sağlık Uygulamaları Tebliği. Official Gazette, 24 March 2013; 28597.
- Fink, A.L.; Klein, S.L. Sex and gender impact immune responses to vaccines among the elderly. Physiology 2015, 30, 408–416. [Google Scholar] [CrossRef]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef]
- Chen, L.-K. COVID-19 vaccination and frailty in older adults. Arch. Gerontol. Geriatr. 2021, 96, 104487. [Google Scholar] [CrossRef]
- Uwamino, Y.; Kurafuji, T.; Sato, Y.; Tomita, Y.; Shibata, A.; Tanabe, A.; Yatabe, K.; Noguchi, M.; Arai, T.; Ohno, A.; et al. Young age, female sex, and presence of systemic adverse reactions are associated with high post-vaccination antibody titer after two doses of BNT162b2 mRNA SARS-CoV-2 vaccination: An observational study of 646 Japanese healthcare workers and university staff. Vaccine 2022, 40, 1019–1025. [Google Scholar]
- Anichini, G.; Terrosi, C.; Gandolfo, C.; Gori Savellini, G.; Fabrizi, S.; Miceli, G.B.; Cusi, M.G. SARS-CoV-2 antibody response in persons with past natural infection. N. Engl. J. Med. 2021, 385, 90–92. [Google Scholar] [CrossRef]
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaun, V.; Mandelboim, M.; Doolam, R.; Amit, S.; et al. BNT162b2 vaccine-induced immune responses and dynamics vary among age groups, sex and co-morbidities: A longitudinal prospective cohort study. Lancet Respir. Med. 2021, 9, 999–1009. [Google Scholar] [CrossRef]
- Sterlin, D.; Malaussena, A.; Gorochov, G. IgA dominates the early neutralizing antibody response to SARS-CoV-2 virus. Med. Sci. 2021, 37, 968–970. [Google Scholar]
- Wisnewski, A.V.; Luna, J.C.; Redlich, C.A. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS ONE 2021, 16, e0249499. [Google Scholar] [CrossRef]
- Nickel, O.; Rockstroh, A.; Wolf, J.; Landgraf, S.; Kalbitz, S.; Kellner, N.; Borte, A.; Pietschm, C.; Fertney, J.; Lubbert, C.; et al. Evaluation of the systemic and mucosal immune response induced by COVID-19 and the BNT162b2 mRNA vaccine for SARS-CoV-2. PLoS ONE 2022, 17, e0263861. [Google Scholar] [CrossRef] [PubMed]
- Buonfrate, D.; Piubelli, C.; Gobbi, F.; Martini, D.; Bertoli, G.; Ursini, T.; Moro, L.; Ronzoni, N.; Angheben, A.; Rodari, P.; et al. Antibody response induced by the BNT162b2 mRNA COVID-19 vaccine in a cohort of health-care workers, with or without prior SARS-CoV-2 infection: A prospective study. Clin. Microbiol. Infect. 2021, 27, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Pawłowska, M.; Rogalska-Płońska, M.; Bociąga-Jasik, M.; Kłos, K.; Piekarska, A.; Zarebska-Michaluk, D. Effect of COVID-19 on anti-S antibody response in healthcare workers six months post-vaccination. Vaccines 2021, 9, 1325. [Google Scholar] [CrossRef]
| Sociodemographic Characteristics | CoronaVac (n = 25) | BNT162b2 (n = 92) | p | ||
|---|---|---|---|---|---|
| Number | % * | Number | % * | ||
| Gender | 0.035 a | ||||
| Male | 12 | 48.0% | 24 | 26.1% | |
| Female | 13 | 52.0% | 68 | 73.9% | |
| Age Group | 0.007 a | ||||
| 20–39 | 6 | 24.0% | 50 | 54.3% | |
| ≥40 | 19 | 76.0% | 42 | 45.7% | |
| Department | - | ||||
| Basic medical sciences | 7 | 28.0% | 9 | 9.8% | |
| Internal medicine divisions | 7 | 28.0% | 47 | 51.1% | |
| Surgical divisions | 6 | 24.0% | 27 | 29.3% | |
| Other | 5 | 20.0% | 9 | 9.8% | |
| Occupation | - | ||||
| Physician | 2 | 8.0% | 14 | 15.2% | |
| Nurse | 4 | 16.0% | 22 | 23.9% | |
| Other health worker | 17 | 68.0% | 52 | 56.5% | |
| Office worker | 2 | 8.0% | 4 | 4.4% | |
| Chronic Disease | 0.138 a | ||||
| No | 22 | 88.0% | 68 | 73.9% | |
| Yes | 3 | 12.0% | 24 | 26.1% | |
| Vaccination Status | |||||
| Influenza/Pneumococcal Vaccine | 0.433 a | ||||
| No | 18 | 72.0% | 73 | 79.3% | |
| Yes | 7 | 28.0% | 19 | 20.7% | |
| History of COVID-19 Infection | |||||
| None | 16 | 64.0% | 54 | 58.7% | 0.631 a |
| Before booster | 3 | 12.0% | 13 | 14.1% | 0.783 a |
| After booster | 8 | 32.0% | 31 | 33.7% | 0.873 a |
| IgG titer before booster (BAU/mL), median (min–max) | 31.2 (10.9–3270.0) | 28.8 (10.9–2979.8) | 0.88 b | ||
| Sociodemographic Characteristics | CoronaVac (n = 25) | BNT162b2 (n = 92) | ||||||
|---|---|---|---|---|---|---|---|---|
| Before Booster (BAU/mL) | One Month after Booster (BAU/mL) | Six Months after Booster (BAU/mL) | p a | Before Booster (BAU/mL) | One Month after Booster (BAU/mL) | Six Months after Booster (BAU/mL) | p a | |
| Gender | ||||||||
| Male | 34.4 (10.9–3270.1) | 88.5 (0–3270.0) | 438.2 (11.6–3270.0) | 0.121 | 29.9 (10.9–758.4) | 3270.0 (0.0–3270.0) | 2769.1 (44.9–3270.0) | <0.001 |
| Female | 17.7 (10.9–150.0) | 115.3 (36.6–1223.4) | 1231.5 (15.0–3270.0) | 0.009 | 28.8 (10.9–32979.8) | 3270.0 (0.0–3270.0) | 2392.0 (81.3–3270.0) | <0.001 |
| p b | 0.722 | 0.828 | 0.911 | 0.538 | 0.693 | 0.768 | ||
| Age Groups | ||||||||
| 20–39 years | 43.4 (10.9–3270.0) | 124.7 (0.0–3270.0) | 83.7 (28.1–3270.0) | 0.738 | 27.4 (10.9–758.4) | 3270.0 (0.0–3270.0) | 2159.0 (44.9–3270.0) | <0.001 |
| ≥40 | 17.7 (10.9–272.3) | 104.0 (36.6– 3270.0) | 1231.5 (11.6–3270.0) | <0.001 | 31.5 (10.9–2979.8) | 3270.0 (0.0–3270.0) | 3270 (116.6–3270.0) | <0.001 |
| p b | 0.262 | 0.874 | 0.648 | 0.55 | 0.346 | 0.207 | ||
| Chronic Disease | ||||||||
| No | 24.4 (10.9–3270.0) | 93.3 (0.0–3270.0) | 518.2 (11.6–3270.0) | 0.001 | 27.9 (10.9–3270.0) | 3270.0 (0.0–3270.0) | 2246.7 (44.9–3270.0) | <0.001 |
| Yes | 42.7 (10.9–278.6) | 104.0 (73.0–191.2) | 3270.0 (80.0–3270.0) | 0.717 | 36.8 (10.9–612.6) | 3270.0 (0.0–3270.0) | 3270.0 (140.6–3270.0) | <0.001 |
| p b | 0.643 | 0.738 | 0.266 | 0.179 | 0.645 | 0.262 | ||
| Influenza/PneumococcalVaccination Status | ||||||||
| No | 17.5 (10.9–272.3) | 87.6 (0.0–3270.0) | 167.4 (11.6–3270.0) | 0.001 | 29.9 (10.9–2979.8) | 3270.0 (0.0–3270.0) | 3067.0 (44.9–3270.0) | <0.001 |
| Yes | 53.2 (10.9–3270.0) | 115.3 (36.6–3270.0) | 2669.4 (22.7–3270.0) | 0.772 | 23.1 (10.9–466.5) | 3270.0 (0.0–3270.0) | 1427.2 (140.6–3270.0) | <0.001 |
| p b | 0.12 | 0.785 | 0.321 | 0.35 | 0.437 | 0.166 | ||
| History of COVID-19 Infection | Booster Vaccine | Before Booster Dose (BAU/mL) | One Month after Booster (BAU/mL) | Six Months after Booster (BAU/mL) | p a |
|---|---|---|---|---|---|
| Negative history (n = 70) | CoronaVac (n = 16) | 24.4 (10.9–3270.0) | 88.5 (0.0–3270.0) | 167.4 (11.6–3270.0) | 0.019 |
| BNT162b2 (n = 54) | 24.7 (10.9–707.6) | 3270.0 (0.0–3270.0) | 2255.2 (44.9–3270.0) | <0.001 | |
| p b | 0.729 | <0.001 | 0.007 | ||
| Positive history (n = 47) | CoronaVac (n = 9) | 45.1 (10.9–272.3) | 115.3 (0.0–1995.8) | 3270 (11.6–3270.0) | 0.045 |
| BNT162b2 (n = 38) | 41.4 (10.9–2979.8) | 3270.0 (0.0–3270.0) | 2874.8 (81.3–3270.0) | <0.001 | |
| p b | 0.683 | <0.001 | 0.708 | ||
| All participants (n = 117) | CoronaVac (n = 25) | 31.2 (10.9–3270.0) | 104.0 (0.0–3270.0) | 788.9 (11.6–3270) | 0.001 |
| BNT162b2 (n = 92) | 28.8 (10.9–2979.8) | 3270.0 (0.0–3270.0) | 2469.4 (44.9–3270.0) | <0.001 | |
| p b | 0.88 | <0.001 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ören, M.M.; Canbaz, S.; Meşe, S.; Ağaçfidan, A.; Demir, Ö.S.; Karaca, E.; Doğruyol, A.R.; Otçu, G.H.; Tükek, T.; Özgülnar, N. Impact of Health Workers’ Choice of COVID-19 Vaccine Booster on Immunization Levels in Istanbul, Turkey. Vaccines 2023, 11, 935. https://doi.org/10.3390/vaccines11050935
Ören MM, Canbaz S, Meşe S, Ağaçfidan A, Demir ÖS, Karaca E, Doğruyol AR, Otçu GH, Tükek T, Özgülnar N. Impact of Health Workers’ Choice of COVID-19 Vaccine Booster on Immunization Levels in Istanbul, Turkey. Vaccines. 2023; 11(5):935. https://doi.org/10.3390/vaccines11050935
Chicago/Turabian StyleÖren, Meryem Merve, Sevgi Canbaz, Sevim Meşe, Ali Ağaçfidan, Ömer Serdil Demir, Esra Karaca, Ayşe Rumeysa Doğruyol, Gökçe Hazar Otçu, Tufan Tükek, and Nuray Özgülnar. 2023. "Impact of Health Workers’ Choice of COVID-19 Vaccine Booster on Immunization Levels in Istanbul, Turkey" Vaccines 11, no. 5: 935. https://doi.org/10.3390/vaccines11050935
APA StyleÖren, M. M., Canbaz, S., Meşe, S., Ağaçfidan, A., Demir, Ö. S., Karaca, E., Doğruyol, A. R., Otçu, G. H., Tükek, T., & Özgülnar, N. (2023). Impact of Health Workers’ Choice of COVID-19 Vaccine Booster on Immunization Levels in Istanbul, Turkey. Vaccines, 11(5), 935. https://doi.org/10.3390/vaccines11050935






