A Critical Review on Human Malaria and Schistosomiasis Vaccines: Current State, Recent Advancements, and Developments
Abstract
1. Introduction
2. Survey Methodology
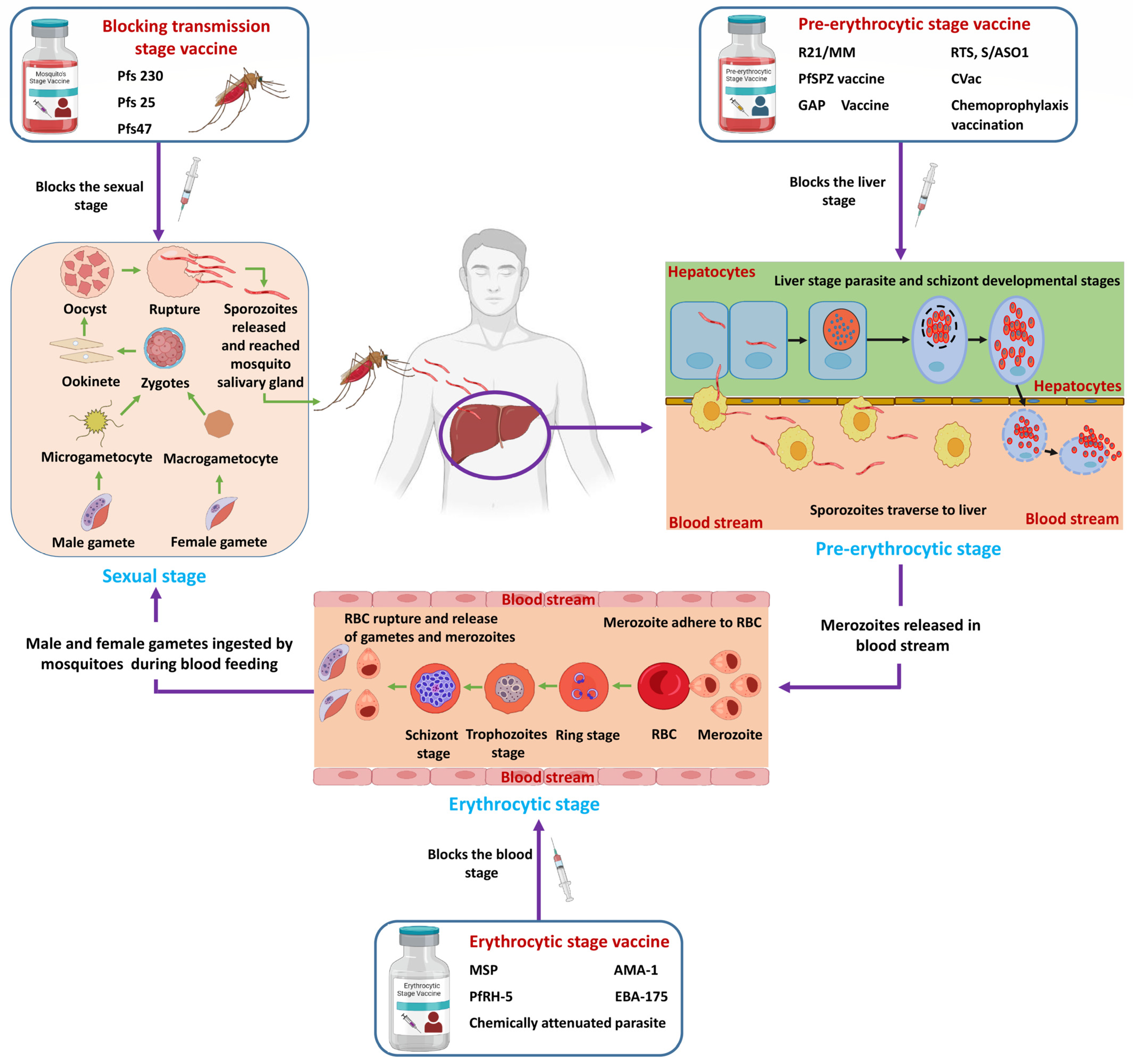
2.1. Malaria Vaccine
2.1.1. Pre-Erythrocytic (Liver) Stage
RTS,S/ASO1 Vaccine
R21/Matrix-M™ Vaccine (R21/MM)
2.1.2. Blood (Erythrocytic) Stage Vaccine
2.1.3. Transmission-Blocking Vaccine
2.2. Schistosoma Vaccine
2.2.1. Current Human Clinical Vaccine
Sh28GST Vaccine
Sm-14
Sm-TSP-2
Sm-p80
3. Challenges for Malaria and Schistosomiasis Vaccines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamed, I.; Kinung’hi, S.; Mwinzi, P.N.; Onkanga, I.O.; Andiego, K.; Muchiri, G.; Odiere, M.R.; Vennervald, B.J.; Olsen, A. Diet and hygiene practices influence morbidity in schoolchildren living in Schistosomiasis endemic areas along Lake Victoria in Kenya and Tanzania—A cross-sectional study. PLoS Negl. Trop. Dis. 2018, 12, e0006373. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.J.; Adnan, M.; Jahan, S.; Redman, W.; Saeed, M.; Patel, M. Neurological disorder and psychosocial aspects of cerebral malaria: What is new on its pathogenesis and complications? A minireview. Folia Parasitol. 2020, 67, 015. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, M.; Watier, L.; Briand, V.; Garcia, A.; Le Hesran, J.Y.; Cot, M. Coinfection with Plasmodium falciparum and Schistosoma haematobium: Additional evidence of the protective effect of Schistosomiasis on malaria in Senegalese children. Am. J. Trop. Med. Hyg. 2014, 90, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Arostegui, M.C.; Wood, C.L.; Jones, I.J.; Chamberlin, A.J.; Jouanard, N.; Faye, D.S.; Kuris, A.M.; Riveau, G.; De Leo, G.A.; Sokolow, S.H. Potential Biological Control of Schistosomiasis by Fishes in the Lower Senegal River Basin. Am. J. Trop. Med. Hyg. 2019, 100, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Okumu, F.; Finda, M. Key Characteristics of Residual Malaria Transmission in Two Districts in South-Eastern Tanzania-Implications for Improved Control. J. Infect. Dis. 2021, 223, S143–S154. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Bhardwaj, J.; Goyal, M.; Prakash, K.; Adnan, M.; Alreshidi, M.M.; Patel, M.; Soni, A.; Redman, W. Immune responses in liver and spleen against Plasmodium yoelii pre-erythrocytic stages in Swiss mice model. J. Adv. Res. 2020, 24, 29–41. [Google Scholar] [CrossRef]
- Sato, S. Plasmodium-a brief introduction to the parasites causing human malaria and their basic biology. J. Physiol. Anthropol. 2021, 40, 1. [Google Scholar] [CrossRef]
- Jagannathan, P.; Kakuru, A. Malaria in 2022: Increasing challenges, cautious optimism. Nat. Commun. 2022, 13, 2678. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2021. 2021. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 6 December 2021).
- Siddiqui, A.J.; Bhardwaj, J.; Hamadou, W.S.; Goyal, M.; Jahan, S.; Ashraf, S.A.; Jamal, A.; Sharma, P.; Sachidanandan, M.; Badraoui, R.; et al. Impact of chemoprophylaxis immunisation under halofantrine (CPS-HF) drug cover in Plasmodium yoelii Swiss mice malaria model. Folia Parasitol. 2022, 69, 003. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Bhardwaj, J.; Hamadou, W.S.; Goyal, M.; Ashraf, S.A.; Jahan, S.; Jamal, A.; Sharma, P.; Sachidanandan, M.; Badraoui, R.; et al. Chemoprophylaxis under sporozoites-lumefantrine (CPS-LMF) immunization induce protective immune responses against Plasmodium yoelii sporozoites infection in mice. 3 Biotech 2021, 11, 465. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Bhardwaj, J.; Puri, S.K. mRNA expression of cytokines and its impact on outcomes after infection with lethal and nonlethal Plasmodium vinckei parasites. Parasitol. Res. 2012, 110, 1517–1524. [Google Scholar] [CrossRef]
- Tizifa, T.A.; Kabaghe, A.N.; McCann, R.S.; van den Berg, H.; Van Vugt, M.; Phiri, K.S. Prevention Efforts for Malaria. Curr. Trop. Med. Rep. 2018, 5, 41–50. [Google Scholar] [CrossRef]
- Dun-Dery, F.; Meissner, P.; Beiersmann, C.; Kuunibe, N.; Winkler, V.; Albrecht, J.; Müller, O. Uptake challenges of intermittent preventive malaria therapy among pregnant women and their health care providers in the Upper West Region of Ghana: A mixed-methods study. Parasite Epidemiol. Control 2021, 15, e00222. [Google Scholar] [CrossRef] [PubMed]
- Nalinya, S.; Musoke, D.; Deane, K. Malaria prevention interventions beyond long-lasting insecticidal nets and indoor residual spraying in low- and middle-income countries: A scoping review. Malar. J. 2022, 21, 31. [Google Scholar] [CrossRef]
- Singh, S.K.; Plieskatt, J.; Chourasia, B.K.; Singh, V.; Bolscher, J.M.; Dechering, K.J.; Adu, B.; López-Méndez, B.; Kaviraj, S.; Locke, E.; et al. The Plasmodium falciparum circumsporozoite protein produced in Lactococcus lactis is pure and stable. J. Biol. Chem. 2020, 295, 403–414. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: Current Status of Two Adjuvanted Malaria Vaccines and the World Health Organization (WHO) Strategy to Eradicate Malaria by 2030. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2023, 29, e939357. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, D.; Kone, A.K.; Traore, K.; Niangaly, A.; Kouriba, B.; Arama, C.; Zeguime, A.; Dolo, A.; Lyke, K.E.; Plowe, C.V.; et al. PfSPZ-CVac malaria vaccine demonstrates safety among malaria-experienced adults: A randomized, controlled phase 1 trial. EClinicalMedicine 2022, 52, 101579. [Google Scholar] [CrossRef] [PubMed]
- Sow, D.; Sylla, K.; Dieng, N.M.; Senghor, B.; Gaye, P.M.; Fall, C.B.; Goumballa, N.; Diallo, A.; Ndiaye, J.L.A.; Parola, P.; et al. Molecular diagnosis of urogenital schistosomiasis in pre-school children, school-aged children and women of reproductive age at community level in central Senegal. Parasites Vectors 2023, 16, 43. [Google Scholar] [CrossRef]
- Lo, N.C.; Bezerra, F.S.M.; Colley, D.G.; Fleming, F.M.; Homeida, M.; Kabatereine, N.; Kabole, F.M.; King, C.H.; Mafe, M.A.; Midzi, N.; et al. Review of 2022 WHO guidelines on the control and elimination of schistosomiasis. Lancet Infect. Dis. 2022, 22, e327–e335. [Google Scholar] [CrossRef]
- Melkus, M.W.; Le, L.; Siddiqui, A.J.; Molehin, A.J.; Zhang, W.; Lazarus, S.; Siddiqui, A.A. Elucidation of Cellular Responses in Non-human Primates With Chronic Schistosomiasis Followed by Praziquantel Treatment. Front. Cell. Infect. Microbiol. 2020, 10, 57. [Google Scholar] [CrossRef]
- Hailegebriel, T.; Nibret, E.; Munshea, A. Prevalence of Schistosoma mansoni and S. haematobium in Snail Intermediate Hosts in Africa: A Systematic Review and Meta-analysis. J. Trop. Med. 2020, 2020, 8850840. [Google Scholar] [CrossRef]
- Chen, C.; Guo, Q.; Fu, Z.; Liu, J.; Lin, J.; Xiao, K.; Sun, P.; Cong, X.; Liu, R.; Hong, Y. Reviews and advances in diagnostic research on Schistosoma japonicum. Acta Trop. 2021, 213, 105743. [Google Scholar] [CrossRef] [PubMed]
- Aula, O.P.; McManus, D.P. Schistosomiasis with a Focus on Africa. Trop. Med. Infect. Dis. 2021, 6, 109. [Google Scholar] [CrossRef]
- Molehin, A.J.; McManus, D.P.; You, H. Vaccines for Human Schistosomiasis: Recent Progress, New Developments and Future Prospects. Int. J. Mol. Sci. 2022, 23, 2255. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.J.; McManus, D.P.; Li, Y.; Williams, G.M.; Bergquist, R.; Ross, A.G. Schistosomiasis elimination: Lessons from the past guide the future. Lancet Infect. Dis. 2010, 10, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef]
- Riveau, G.; Schacht, A.M.; Dompnier, J.P.; Deplanque, D.; Seck, M.; Waucquier, N.; Senghor, S.; Delcroix-Genete, D.; Hermann, E.; Idris-Khodja, N.; et al. Safety and efficacy of the rSh28GST urinary schistosomiasis vaccine: A phase 3 randomized, controlled trial in Senegalese children. PLoS Negl. Trop. Dis. 2018, 12, e0006968. [Google Scholar] [CrossRef]
- Langenberg, M.C.C.; Hoogerwerf, M.A.; Koopman, J.P.R.; Janse, J.J.; Kos-van Oosterhoud, J.; Feijt, C.; Jochems, S.P.; de Dood, C.J.; van Schuijlenburg, R.; Ozir-Fazalalikhan, A.; et al. A controlled human Schistosoma mansoni infection model to advance novel drugs, vaccines and diagnostics. Nat. Med. 2020, 26, 326–332. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.F.; Li, W.; Yang, K. Development of New Technologies for Risk Identification of Schistosomiasis Transmission in China. Pathogens 2022, 11, 224. [Google Scholar] [CrossRef]
- Laurens, M.B. RTS,S/AS01 vaccine (Mosquirix™): An overview. Hum. Vaccines Immunother. 2020, 16, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Marques-da-Silva, C.; Peissig, K.; Kurup, S.P. Pre-Erythrocytic Vaccines against Malaria. Vaccines 2020, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef] [PubMed]
- Chulanetra, M.; Chaicumpa, W. Revisiting the Mechanisms of Immune Evasion Employed by Human Parasites. Front. Cell. Infect. Microbiol. 2021, 11, 702125. [Google Scholar] [CrossRef]
- Long, C.A.; Zavala, F. Immune Responses in Malaria. Cold Spring Harb. Perspect. Med. 2017, 7, a025577. [Google Scholar] [CrossRef]
- Molina-Franky, J.; Cuy-Chaparro, L.; Camargo, A.; Reyes, C.; Gómez, M.; Salamanca, D.R.; Patarroyo, M.A.; Patarroyo, M.E. Plasmodium falciparum pre-erythrocytic stage vaccine development. Malar. J. 2020, 19, 56. [Google Scholar] [CrossRef]
- Roman, F.P.; Coccia, M.; Schuerman, L. Commentary in reply to a publication on Plasmodium falciparum pre-erythrocytic stage vaccine development. Malar. J. 2020, 19, 261. [Google Scholar] [CrossRef]
- Nunes-Cabaço, H.; Moita, D.; Prudêncio, M. Five decades of clinical assessment of whole-sporozoite malaria vaccines. Front. Immunol. 2022, 13, 977472. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.M.; Sack, B.K.; Dankwa, D.; Minkah, N.; Nguyen, T.; Cardamone, H.; Kappe, S.H.I. A Plasmodium Parasite with Complete Late Liver Stage Arrest Protects against Preerythrocytic and Erythrocytic Stage Infection in Mice. Infect. Immun. 2018, 86, 1–18. [Google Scholar] [CrossRef]
- Nadeem, A.Y.; Shehzad, A.; Islam, S.U.; Al-Suhaimi, E.A. Mosquirix™ RTS, S/AS01 Vaccine Development, Immunogenicity, and Efficacy. Vaccines 2022, 10, 713. [Google Scholar] [CrossRef]
- Vaughan, A.M.; Kappe, S.H.I. Genetically attenuated malaria parasites as vaccines. Expert Rev. Vaccines 2017, 16, 765–767. [Google Scholar] [CrossRef]
- Mikolajczak, S.A.; Lakshmanan, V.; Fishbaugher, M.; Camargo, N.; Harupa, A.; Kaushansky, A.; Douglass, A.N.; Baldwin, M.; Healer, J.; O’Neill, M.; et al. A next-generation genetically attenuated Plasmodium falciparum parasite created by triple gene deletion. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Zanghì, G.; Vembar, S.S.; Baumgarten, S.; Ding, S.; Guizetti, J.; Bryant, J.M.; Mattei, D.; Jensen, A.T.R.; Rénia, L.; Goh, Y.S.; et al. A Specific PfEMP1 Is Expressed in P. falciparum Sporozoites and Plays a Role in Hepatocyte Infection. Cell Rep. 2018, 22, 2951–2963. [Google Scholar] [CrossRef] [PubMed]
- Draper, S.J.; Sack, B.K.; King, C.R.; Nielsen, C.M.; Rayner, J.C.; Higgins, M.K.; Long, C.A.; Seder, R.A. Malaria vaccines: Recent advances and new horizons. Cell Host Microbe 2018, 24, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Kazmin, D.; Nakaya, H.I.; Lee, E.K.; Johnson, M.J.; Van Der Most, R.; Van Den Berg, R.A.; Ballou, W.R.; Jongert, E.; Wille-Reece, U.; Ockenhouse, C. Systems analysis of protective immune responses to RTS, S malaria vaccination in humans. Proc. Natl. Acad. Sci. USA 2017, 114, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Song, G.; Beale, K.; Yan, J.; Garst, E.; Feng, J.; Lund, E.; Catteruccia, F.; Springer, T.A. Design and assessment of TRAP-CSP fusion antigens as effective malaria vaccines. PLoS ONE 2020, 15, e0216260. [Google Scholar] [CrossRef]
- Zavala, F. RTS,S: The first malaria vaccine. J. Clin. Investig. 2022, 132, e156588. [Google Scholar] [CrossRef]
- Beeson, J.G.; Kurtovic, L. The RTS,S malaria vaccine: Current impact and foundation for the future. Sci. Transl. Med. 2022, 14, eabo6646. [Google Scholar] [CrossRef]
- Cotton, M. The Mosquirix (RTS.S) malaria vaccine. Trop. Dr. 2020, 50, 107. [Google Scholar] [CrossRef]
- Agnandji, S.T.; Lell, B.; Fernandes, J.F.; Abossolo, B.P.; Methogo, B.G.; Kabwende, A.L.; Adegnika, A.A.; Mordmüller, B.; Issifou, S.; Kremsner, P.G.; et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 2012, 367, 2284–2295. [Google Scholar] [CrossRef]
- Agnandji, S.T.; Asante, K.P.; Lyimo, J.; Vekemans, J.; Soulanoudjingar, S.S.; Owusu, R.; Shomari, M.; Leach, A.; Fernandes, J.; Dosoo, D.; et al. Evaluation of the safety and immunogenicity of the RTS,S/AS01E malaria candidate vaccine when integrated in the expanded program of immunization. J. Infect. Dis. 2010, 202, 1076–1087. [Google Scholar] [CrossRef]
- Olotu, A.; Fegan, G.; Wambua, J.; Nyangweso, G.; Awuondo, K.O.; Leach, A.; Lievens, M.; Leboulleux, D.; Njuguna, P.; Peshu, N.; et al. Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure. N. Engl. J. Med. 2013, 368, 1111–1120. [Google Scholar] [CrossRef]
- Vandoolaeghe, P.; Schuerman, L. The RTS,S/AS01 malaria vaccine in children 5 to 17 months of age at first vaccination. Expert Rev. Vaccines 2016, 15, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO) and the Malaria Vaccine Funders Group. The Malaria Vaccine Technology Roadmap. Nov, A.a. 2013. Available online: https://www.malariavaccine.org/sites/mvi/files/content/page/files/TRM_update_nov13.pdf (accessed on 23 February 2022).
- Samuels, A.M.; Ansong, D.; Kariuki, S.K.; Adjei, S.; Bollaerts, A.; Ockenhouse, C.; Westercamp, N.; Lee, C.K.; Schuerman, L.; Bii, D.K.; et al. Efficacy of RTS,S/AS01(E) malaria vaccine administered according to different full, fractional, and delayed third or early fourth dose regimens in children aged 5-17 months in Ghana and Kenya: An open-label, phase 2b, randomised controlled trial. Lancet Infect. Dis. 2022, 22, 1329–1342. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Anbalagan, L.C.; Pannu, A.K. Towards Eradication of Malaria: Is the WHO’s RTS,S/AS01 Vaccination Effective Enough? Risk Manag. Healthc. Policy 2021, 14, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Datoo, M.S.; Natama, H.M.; Somé, A.; Bellamy, D.; Traoré, O.; Rouamba, T.; Tahita, M.C.; Ido, N.F.A.; Yameogo, P.; Valia, D.; et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: A phase 1/2b randomised controlled trial. Lancet Infect. Dis. 2022, 22, 1728–1736. [Google Scholar] [CrossRef]
- Moorthy, V.; Binka, F. R21/Matrix-M: A second malaria vaccine? Lancet 2021, 397, 1782–1783. [Google Scholar] [CrossRef]
- NCT05252845; Safety and Immunogenicity of the Malaria Vaccine, R21/MatrixM in Healthy Thai Adults (R21/Matrix-M). 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05252845 (accessed on 23 February 2022).
- NCT04704830; R21/Matrix-M in African Children against Clinical Malaria. Available online: https://clinicaltrials.gov/ct2/show/NCT04704830 (accessed on 12 January 2021).
- Salinas, N.D.; Tang, W.K.; Tolia, N.H. Blood-Stage Malaria Parasite Antigens: Structure, Function, and Vaccine Potential. J. Mol. Biol. 2019, 431, 4259–4280. [Google Scholar] [CrossRef]
- Campo, J.J.; Aponte, J.J.; Skinner, J.; Nakajima, R.; Molina, D.M.; Liang, L.; Sacarlal, J.; Alonso, P.L.; Crompton, P.D.; Felgner, P.L.; et al. RTS,S vaccination is associated with serologic evidence of decreased exposure to Plasmodium falciparum liver- and blood-stage parasites. Mol. Cell. Proteom. MCP 2015, 14, 519–531. [Google Scholar] [CrossRef]
- Sack, B.K.; Keitany, G.J.; Vaughan, A.M.; Miller, J.L.; Wang, R.; Kappe, S.H.I. Mechanisms of stage-transcending protection following immunization of mice with late liver stage-arresting genetically attenuated malaria parasites. PLoS Pathog. 2015, 11, e1004855. [Google Scholar] [CrossRef]
- Good, M.F.; Miller, L.H. Interpreting challenge data from early phase malaria blood stage vaccine trials. Expert Rev. Vaccines 2018, 17, 189–196. [Google Scholar] [CrossRef]
- Ntege, E.H.; Takashima, E.; Morita, M.; Nagaoka, H.; Ishino, T.; Tsuboi, T. Blood-stage malaria vaccines: Post-genome strategies for the identification of novel vaccine candidates. Expert Rev. Vaccines 2017, 16, 769–779. [Google Scholar] [CrossRef]
- Graves, P.; Gelband, H. Vaccines for preventing malaria (SPf66). Cochrane Database Syst. Rev. 2006, 2006, Cd005966. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, D.R.; Gómez, M.; Camargo, A.; Cuy-Chaparro, L.; Molina-Franky, J.; Reyes, C.; Patarroyo, M.A.; Patarroyo, M.E. Plasmodium falciparum Blood Stage Antimalarial Vaccines: An Analysis of Ongoing Clinical Trials and New Perspectives Related to Synthetic Vaccines. Front. Microbiol. 2019, 10, 2712. [Google Scholar] [CrossRef] [PubMed]
- Blank, A.; Fürle, K.; Jäschke, A.; Mikus, G.; Lehmann, M.; Hüsing, J.; Heiss, K.; Giese, T.; Carter, D.; Böhnlein, E. Immunization with full-length Plasmodium falciparum merozoite surface protein 1 is safe and elicits functional cytophilic antibodies in a randomized first-in-human trial. NPJ Vaccines 2020, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Alves, K.C.S.; Guimarães, J.M.; Almeida, M.E.M.d.; Mariúba, L.A.M. Plasmodium falciparum merozoite surface protein 3 as a vaccine candidate: A brief review. Rev. Inst. Med. Trop. Sao Paulo 2022, 64. [Google Scholar] [CrossRef] [PubMed]
- Obaldia III, N.; Stockelman, M.G.; Otero, W.; Cockrill, J.A.; Ganeshan, H.; Abot, E.N.; Zhang, J.; Limbach, K.; Charoenvit, Y.; Doolan, D.L. A Plasmodium vivax plasmid DNA-and adenovirus-vectored malaria vaccine encoding blood-stage antigens AMA1 and MSP142 in a prime/boost heterologous immunization regimen partially protects Aotus monkeys against blood-stage challenge. Clin. Vaccine Immunol. 2017, 24, e00539-16. [Google Scholar]
- Ellis, R.D.; Wu, Y.; Martin, L.B.; Shaffer, D.; Miura, K.; Aebig, J.; Orcutt, A.; Rausch, K.; Zhu, D.; Mogensen, A. Phase 1 study in malaria naïve adults of BSAM2/Alhydrogel®+ CPG 7909, a blood stage vaccine against P. falciparum malaria. PLoS ONE 2012, 7, e46094. [Google Scholar] [CrossRef]
- Ragotte, R.J.; Higgins, M.K.; Draper, S.J. The RH5-CyRPA-Ripr complex as a malaria vaccine target. Trends Parasitol. 2020, 36, 545–559. [Google Scholar] [CrossRef]
- Wright, K.E.; Hjerrild, K.A.; Bartlett, J.; Douglas, A.D.; Jin, J.; Brown, R.E.; Illingworth, J.J.; Ashfield, R.; Clemmensen, S.B.; de Jongh, W.A. Structure of malaria invasion protein RH5 with erythrocyte basigin and blocking antibodies. Nature 2014, 515, 427–430. [Google Scholar] [CrossRef]
- Ndwiga, L.; Osoti, V.; Ochwedo, K.O.; Wamae, K.; Bejon, P.; Rayner, J.C.; Githinji, G.; Ochola-Oyier, L.I. The Plasmodium falciparum Rh5 invasion protein complex reveals an excess of rare variant mutations. Malar. J. 2021, 20, 278. [Google Scholar] [CrossRef]
- Minassian, A.M.; Silk, S.E.; Barrett, J.R.; Nielsen, C.M.; Miura, K.; Diouf, A.; Loos, C.; Fallon, J.K.; Michell, A.R.; White, M.T. Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination. Med 2021, 2, 701–719.e719. [Google Scholar] [CrossRef]
- Ragotte, R.J.; Pulido, D.; Lias, A.M.; Quinkert, D.; Alanine, D.G.W.; Jamwal, A.; Davies, H.; Nacer, A.; Lowe, E.D.; Grime, G.W.; et al. Heterotypic interactions drive antibody synergy against a malaria vaccine candidate. Nat. Commun. 2022, 13, 933. [Google Scholar] [CrossRef] [PubMed]
- Healer, J.; Thompson, J.K.; Mackwell, K.L.; Browne, C.D.; Seager, B.A.; Ngo, A.; Lowes, K.N.; Silk, S.E.; Pulido, D.; King, L.D.W.; et al. RH5.1-CyRPA-Ripr antigen combination vaccine shows little improvement over RH5.1 in a preclinical setting. Front. Cell. Infect. Microbiol. 2022, 12, 1049065. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.O.; Silk, S.E.; Elias, S.C.; Miura, K.; Diouf, A.; Galaway, F.; de Graaf, H.; Brendish, N.J.; Poulton, I.D.; Griffiths, O.J.; et al. Human vaccination against RH5 induces neutralizing antimalarial antibodies that inhibit RH5 invasion complex interactions. JCI Insight 2017, 2, e96381. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.M.; Barrett, J.R.; Davis, C.; Fallon, J.K.; Goh, C.; Michell, A.R.; Griffin, C.; Kwok, A.; Loos, C.; Darko, S.; et al. Delayed boosting improves human antigen-specific Ig and B cell responses to the RH5.1/AS01B malaria vaccine. JCI Insight 2023, 8, e163859. [Google Scholar] [CrossRef]
- Zheng, J.; Pan, H.; Gu, Y.; Zuo, X.; Ran, N.; Yuan, Y.; Zhang, C.; Wang, F. Prospects for malaria vaccines: Pre-erythrocytic stages, blood stages, and transmission-blocking stages. BioMed Res. Int. 2019, 2019, 9751471. [Google Scholar] [CrossRef] [PubMed]
- Acquah, F.K.; Obboh, E.K.; Asare, K.; Boampong, J.N.; Nuvor, S.V.; Singh, S.K.; Theisen, M.; Williamson, K.C.; Amoah, L.E. Antibody responses to two new Lactococcus lactis-produced recombinant Pfs48/45 and Pfs230 proteins increase with age in malaria patients living in the Central Region of Ghana. Malar. J. 2017, 16, 306. [Google Scholar] [CrossRef]
- Unyene, M.S.A.; Agatha, A. Malaria vaccine development: A challenge for Africa. J. Dent. Med. Sci. 2013, 8, 40–50. [Google Scholar] [CrossRef]
- Singh, K.; Burkhardt, M.; Nakuchima, S.; Herrera, R.; Muratova, O.; Gittis, A.G.; Kelnhofer, E.; Reiter, K.; Smelkinson, M.; Veltri, D.; et al. Structure and function of a malaria transmission blocking vaccine targeting Pfs230 and Pfs230-Pfs48/45 proteins. Commun. Biol. 2020, 3, 395. [Google Scholar] [CrossRef]
- Scaria, P.V.; Chen, B.B.; Rowe, C.G.; Alani, N.; Muratova, O.V.; Barnafo, E.K.; Lambert, L.E.; Zaidi, I.U.; Lees, A.; Rausch, K.M. Comparison of carrier proteins to conjugate malaria transmission blocking vaccine antigens, Pfs25 and Pfs230. Vaccine 2020, 38, 5480–5489. [Google Scholar] [CrossRef]
- Healy, S.A.; Anderson, C.; Swihart, B.J.; Mwakingwe, A.; Gabriel, E.E.; Decederfelt, H.; Hobbs, C.V.; Rausch, K.M.; Zhu, D.; Muratova, O. Pfs230 yields higher malaria transmission–blocking vaccine activity than Pfs25 in humans but not mice. J. Clin. Investig. 2021, 131, e146221. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Leneghan, D.B.; Miura, K.; Nikolaeva, D.; Brian, I.J.; Dicks, M.D.; Fyfe, A.J.; Zakutansky, S.E.; de Cassan, S.; Long, C.A. Enhancing immunogenicity and transmission-blocking activity of malaria vaccines by fusing Pfs25 to IMX313 multimerization technology. Sci. Rep. 2016, 6, 18848. [Google Scholar] [CrossRef]
- Chichester, J.A.; Green, B.J.; Jones, R.M.; Shoji, Y.; Miura, K.; Long, C.A.; Lee, C.K.; Ockenhouse, C.F.; Morin, M.J.; Streatfield, S.J. Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: A Phase 1 dose-escalation study in healthy adults. Vaccine 2018, 36, 5865–5871. [Google Scholar] [CrossRef] [PubMed]
- Brod, F.; Miura, K.; Taylor, I.; Li, Y.; Marini, A.; Salman, A.M.; Spencer, A.J.; Long, C.A.; Biswas, S. Combination of RTS,S and Pfs25-IMX313 Induces a Functional Antibody Response Against Malaria Infection and Transmission in Mice. Front. Immunol. 2018, 9, 2780. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.H.; Tang, W.K.; Burkhardt, M.; Galson, J.D.; Muratova, O.; Salinas, N.D.; Alves e Silva, T.L.; Reiter, K.; MacDonald, N.J.; Nguyen, V. A human monoclonal antibody blocks malaria transmission and defines a highly conserved neutralizing epitope on gametes. Nat. Commun. 2021, 12, 1750. [Google Scholar] [CrossRef]
- Alkema, M.; Reuling, I.J.; de Jong, G.M.; Lanke, K.; Coffeng, L.E.; van Gemert, G.J.; van de Vegte-Bolmer, M.; de Mast, Q.; van Crevel, R.; Ivinson, K.; et al. A Randomized Clinical Trial to Compare Plasmodium falciparum Gametocytemia and Infectivity After Blood-Stage or Mosquito Bite-Induced Controlled Malaria Infection. J. Infect. Dis. 2021, 224, 1257–1265. [Google Scholar] [CrossRef]
- Molehin, A.J. Schistosomiasis vaccine development: Update on human clinical trials. J. Biomed. Sci. 2020, 27, 28. [Google Scholar] [CrossRef]
- Cioli, D.; Pica-Mattoccia, L.; Basso, A.; Guidi, A. Schistosomiasis control: Praziquantel forever? Mol. Biochem. Parasitol. 2014, 195, 23–29. [Google Scholar] [CrossRef]
- Vale, N.; Gouveia, M.J.; Rinaldi, G.; Brindley, P.J.; Gärtner, F.; Correia da Costa, J.M. Praziquantel for Schistosomiasis: Single-Drug Metabolism Revisited, Mode of Action, and Resistance. Antimicrob. Agents Chemother. 2017, 61, 1–16. [Google Scholar] [CrossRef]
- Aruleba, R.T.; Adekiya, T.A.; Oyinloye, B.E.; Masamba, P.; Mbatha, L.S.; Pretorius, A.; Kappo, A.P. PZQ Therapy: How Close are we in the Development of Effective Alternative Anti-schistosomal Drugs? Infect. Disord. Drug Targets 2019, 19, 337–349. [Google Scholar] [CrossRef]
- Pinto-Almeida, A.; Mendes, T.M.; Ferreira, P.; Abecasis, A.B.; Belo, S.; Anibal, F.F.; Allegretti, S.M.; Galinaro, C.A.; Carrilho, E.; Afonso, A. A Comparative Proteomic Analysis of Praziquantel-Susceptible and Praziquantel-Resistant Schistosoma mansoni Reveals Distinct Response Between Male and Female Animals. Front. Trop. Dis. 2021, 2, 664642. [Google Scholar]
- Graham, M.; Ayabina, D.; Lucas, T.C.; Collyer, B.S.; Medley, G.F.; Hollingsworth, T.D.; Toor, J. SCHISTOX: An individual based model for the epidemiology and control of schistosomiasis. Infect. Dis. Model. 2021, 6, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Tedla, B.A.; Pickering, D.; Becker, L.; Loukas, A.; Pearson, M.S. Vaccination with Schistosoma mansoni cholinesterases reduces the parasite burden and egg viability in a mouse model of schistosomiasis. Vaccines 2020, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Ogongo, P.; Nyakundi, R.K.; Chege, G.K.; Ochola, L. The road to elimination: Current state of schistosomiasis research and progress towards the end game. Front. Immunol. 2022, 13, 846108. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A.; Hadjichrysanthou, C.; Truscott, J.E.; Anderson, R.M. Developing a mathematical model for the evaluation of the potential impact of a partially efficacious vaccine on the transmission dynamics of Schistosoma mansoni in human communities. Parasites Vectors 2017, 10, 294. [Google Scholar] [CrossRef]
- Hambrook, J.R.; Hanington, P.C. Immune Evasion Strategies of Schistosomes. Front. Immunol. 2020, 11, 624178. [Google Scholar] [CrossRef]
- McManus, D.P.; Bergquist, R.; Cai, P.; Ranasinghe, S.; Tebeje, B.M.; You, H. Schistosomiasis-from immunopathology to vaccines. Semin. Immunopathol. 2020, 42, 355–371. [Google Scholar] [CrossRef]
- Pooe, K.; Thulo, M.; Makumbe, H.; Akumadu, B.; Otun, O.; Aloke, C.; Achilonu, I. Biophysical description of Bromosulfophthalein interaction with the 28-kDa glutathione transferase from Schistosoma japonicum. Mol. Biochem. Parasitol. 2022, 252, 111524. [Google Scholar] [CrossRef]
- Al-Naseri, A.; Al-Absi, S.; El Ridi, R.; Mahana, N. A comprehensive and critical overview of schistosomiasis vaccine candidates. J. Parasit. Dis. 2021, 45, 557–580. [Google Scholar] [CrossRef]
- Nelwan, M. Vaccine Development for Schistosomiasis. 2021. Available online: https://ssrn.com/abstract=3808477 (accessed on 20 March 2021).
- Maizels, R.M. Identifying novel candidates and configurations for human helminth vaccines. Expert Rev. Vaccines 2021, 20, 1389–1393. [Google Scholar] [CrossRef]
- Driciru, E.; Koopman, J.P.R.; Cose, S.; Siddiqui, A.A.; Yazdanbakhsh, M.; Elliott, A.M.; Roestenberg, M. Immunological considerations for Schistosoma vaccine development: Transitioning to endemic settings. Front. Immunol. 2021, 12, 635985. [Google Scholar] [CrossRef]
- Eyayu, T.; Zeleke, A.J.; Worku, L. Current status and future prospects of protein vaccine candidates against Schistosoma mansoni infection. Parasite Epidemiol. Control 2020, 11, e00176. [Google Scholar] [CrossRef]
- Merrifield, M.; Hotez, P.J.; Beaumier, C.M.; Gillespie, P.; Strych, U.; Hayward, T.; Bottazzi, M.E. Advancing a vaccine to prevent human schistosomiasis. Vaccine 2016, 34, 2988–2991. [Google Scholar] [CrossRef] [PubMed]
- Santini-Oliveira, M.; Machado Pinto, P.; Santos, T.D.; Vilar, M.M.; Grinsztejn, B.; Veloso, V.; Paes-de-Almeida, E.C.; Amaral, M.A.Z.; Ramos, C.R.; Marroquin-Quelopana, M.; et al. Development of the Sm14/GLA-SE Schistosomiasis Vaccine Candidate: An Open, Non-Placebo-Controlled, Standardized-Dose Immunization Phase Ib Clinical Trial Targeting Healthy Young Women. Vaccines 2022, 10, 1724. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Selgrad, M.; Wex, T.; Romi, B.; Borgogni, E.; Spensieri, F.; Zedda, L.; Ruggiero, P.; Pancotto, L.; Censini, S.; et al. Efficacy, immunogenicity, and safety of a parenteral vaccine against Helicobacter pylori in healthy volunteers challenged with a Cag-positive strain: A randomised, placebo-controlled phase 1/2 study. Lancet. Gastroenterol. Hepatol. 2018, 3, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Tendler, M.; Almeida, M.S.; Vilar, M.M.; Pinto, P.M.; Limaverde-Sousa, G. Current status of the Sm14/GLA-SE schistosomiasis vaccine: Overcoming barriers and paradigms towards the first anti-parasitic human (itarian) vaccine. Trop. Med. Infect. Dis. 2018, 3, 121. [Google Scholar] [CrossRef] [PubMed]
- Tendler, M.; Almeida, M.; Simpson, A. Development of the Brazilian anti schistosomiasis vaccine based on the recombinant fatty acid binding protein Sm14 plus GLA-SE adjuvant. Front. Immunol. 2015, 6, 218. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.S.; Ribeiro, A.P.D.; Cardoso, F.C.; Martins, V.P.; Figueiredo, B.C.; Assis, N.R.; Morais, S.B.; Caliari, M.V.; Loukas, A.; Oliveira, S.C. A multivalent chimeric vaccine composed of Schistosoma mansoni Sm TSP-2 and Sm29 was able to induce protection against infection in mice. Parasite Immunol. 2014, 36, 303–312. [Google Scholar] [CrossRef]
- Jia, X.; Schulte, L.; Loukas, A.; Pickering, D.; Pearson, M.; Mobli, M.; Jones, A.; Rosengren, K.J.; Daly, N.L.; Gobert, G.N.; et al. Solution structure, membrane interactions, and protein binding partners of the tetraspanin Sm-TSP-2, a vaccine antigen from the human blood fluke Schistosoma mansoni. J. Biol. Chem. 2014, 289, 7151–7163. [Google Scholar] [CrossRef]
- Panzner, U.; Boissier, J. Natural Intra- and Interclade Human Hybrid Schistosomes in Africa with Considerations on Prevention through Vaccination. Microorganisms 2021, 9, 1465. [Google Scholar] [CrossRef]
- Li, G.; Hoeweler, L.; Keegan, B.; Peng, J.; Scholte, L.; Hotez, P.; Bottazzi, M.E.; Diemert, D.; Bethony, J. Potency testing for a recombinant protein vaccine early in clinical development: Lessons from the Schistosoma mansoni Tetraspanin 2 vaccine. Vaccine X 2021, 8, 100100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Molehin, A.J.; Rojo, J.U.; Sudduth, J.; Ganapathy, P.K.; Kim, E.; Siddiqui, A.J.; Freeborn, J.; Sennoune, S.R.; May, J.; et al. Sm-p80-based schistosomiasis vaccine: Double-blind preclinical trial in baboons demonstrates comprehensive prophylactic and parasite transmission-blocking efficacy. Ann. N. Y. Acad. Sci. 2018, 1425, 38–51. [Google Scholar] [CrossRef]
- Zhang, W.; Ahmad, G.; Molehin, A.J.; Torben, W.; Le, L.; Kim, E.; Lazarus, S.; Siddiqui, A.J.; Carter, D.; Siddiqui, A.A. Schistosoma mansoni antigen Sm-p80: Prophylactic efficacy using TLR4 agonist vaccine adjuvant glucopyranosyl lipid A-Alum in murine and non-human primate models. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2018, 66, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Siddiqui, S.Z. Sm-p80-based schistosomiasis vaccine: Preparation for human clinical trials. Trends Parasitol. 2017, 33, 194–201. [Google Scholar] [CrossRef]
- Zhang, W.; Le, L.; Ahmad, G.; Molehin, A.J.; Siddiqui, A.J.; Torben, W.; Karmakar, S.; Rojo, J.U.; Sennoune, S.; Lazarus, S.; et al. Fifteen Years of Sm-p80-Based Vaccine Trials in Nonhuman Primates: Antibodies From Vaccinated Baboons Confer Protection in vivo and in vitro From Schistosoma mansoni and Identification of Putative Correlative Markers of Protection. Front. Immunol. 2020, 11, 1246. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.J.; Molehin, A.J.; Zhang, W.; Ganapathy, P.K.; Kim, E.; Rojo, J.U.; Redman, W.K.; Sennoune, S.R.; Sudduth, J.; Freeborn, J.; et al. Sm-p80-based vaccine trial in baboons: Efficacy when mimicking natural conditions of chronic disease, praziquantel therapy, immunization, and Schistosoma mansoni re-encounter. Ann. N. Y. Acad. Sci. 2018, 1425, 19–37. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Siddiqui, A.J.; Goyal, M.; Prakash, K.; Soni, A.; Puri, S.K. Repetitive live sporozoites inoculation under arteether chemoprophylaxis confers protection against subsequent sporozoite challenge in rodent malaria model. Acta Trop. 2016, 158, 130–138. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Bhardwaj, J.; Goyal, M.; Prakash, K.; Soni, A.; Tiwari, V.; Puri, S.K. Assessment of real-time method to detect liver parasite burden under different experimental conditions in mice infected with Plasmodium yoelii sporozoites. Microb Pathog. 2015, 89, 35–42. [Google Scholar] [CrossRef]
- Hassan, A.O.; Oso, O.V.; Obeagu, E.I.; Adeyemo, A.T. Malaria Vaccine: Prospects and Challenges. Madonna Univ. J. Med. Health Sci. 2022, 2, 22–40. [Google Scholar]
- Mariano, R.M.d.S.; Gonçalves, A.A.M.; Oliveira, D.S.d.; Ribeiro, H.S.; Pereira, D.F.S.; Santos, I.S.; Lair, D.F.; Silva, A.V.d.; Galdino, A.S.; Chávez-Fumagalli, M.A. A Review of Major Patents on Potential Malaria Vaccine Targets. Pathogens 2023, 12, 247. [Google Scholar] [CrossRef]
- You, H.; Jones, M.K.; Gordon, C.A.; Arganda, A.E.; Cai, P.; Al-Wassiti, H.; Pouton, C.W.; McManus, D.P. The mRNA Vaccine Technology Era and the Future Control of Parasitic Infections. Clin. Microbiol. Rev. 2023, 36, e0024121. [Google Scholar] [CrossRef] [PubMed]
| Malaria Parasite Vaccine | Vaccine Categorization | Current Vaccine Status |
|---|---|---|
| Liver stage (pre-erythrocytic) | ||
| R21/MM | Adjuvanted protein vaccine (virus-like particles based on the PfCSP strain NF54 fused to the N-terminus of HBsAg) | Phase III |
| RTS, S/ASO1 | Subunit | Licensed |
| PfSPZ vaccine | Whole organism (radiation attenuated) | Phase II |
| CVac | Whole organism (chemically attenuated) | Phase I |
| GAP vaccine | Whole organism (genetically attenuated) | Phase I |
| Blood stage (erythrocytic) | ||
| MSP | Subunit | Preclinical |
| AMA-1 | Subunit | Preclinical |
| PfRH-5 | Subunit | Phase Ib |
| Chemically attenuated parasite | Whole organism | Preclinical |
| Transmission blocking antigen (mosquito stage) | ||
| Pfs230 | Subunit | Phase I |
| Pfs25 | Subunit | Phase I |
| Pfs47 | Subunit | Preclinical |
| Schistosoma Parasite Vaccine | Vaccine Categorization | Current Vaccine Status |
|---|---|---|
| Sh28GST | Recombinant 28-kDa glutathione S-transferase from S. haematobium | Phase III |
| Sm-14 | Recombinant 14-kDa (rSm-14) S. mansoni fatty-acid-binding protein | Phase III |
| Sm-TSP-2 | Recombinant Sm-TSP-2/Alhydrogel vaccine | Phase Ib |
| Sm-p80 | Recombinant | Phase I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, A.J.; Bhardwaj, J.; Saxena, J.; Jahan, S.; Snoussi, M.; Bardakci, F.; Badraoui, R.; Adnan, M. A Critical Review on Human Malaria and Schistosomiasis Vaccines: Current State, Recent Advancements, and Developments. Vaccines 2023, 11, 792. https://doi.org/10.3390/vaccines11040792
Siddiqui AJ, Bhardwaj J, Saxena J, Jahan S, Snoussi M, Bardakci F, Badraoui R, Adnan M. A Critical Review on Human Malaria and Schistosomiasis Vaccines: Current State, Recent Advancements, and Developments. Vaccines. 2023; 11(4):792. https://doi.org/10.3390/vaccines11040792
Chicago/Turabian StyleSiddiqui, Arif Jamal, Jyoti Bhardwaj, Juhi Saxena, Sadaf Jahan, Mejdi Snoussi, Fevzi Bardakci, Riadh Badraoui, and Mohd Adnan. 2023. "A Critical Review on Human Malaria and Schistosomiasis Vaccines: Current State, Recent Advancements, and Developments" Vaccines 11, no. 4: 792. https://doi.org/10.3390/vaccines11040792
APA StyleSiddiqui, A. J., Bhardwaj, J., Saxena, J., Jahan, S., Snoussi, M., Bardakci, F., Badraoui, R., & Adnan, M. (2023). A Critical Review on Human Malaria and Schistosomiasis Vaccines: Current State, Recent Advancements, and Developments. Vaccines, 11(4), 792. https://doi.org/10.3390/vaccines11040792













