Are International Units of Anti-HBs Antibodies Always Indicative of Hepatitis B Virus Neutralizing Activity?
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. Monoclonal Antibodies (mAbs)
2.3. Purification of IgGs from Serum Samples
2.4. Measurement of Anti-HBs, Anti-preS1, and Anti-preS2 Antibodies by ELISA
2.5. Cell Culture and In Vitro HDV Infection Assay
2.6. Detection of Viral Nucleic Acids
2.7. In Vitro Neutralization Assay
2.8. Fluorescence Microscopy
2.9. Statistical Analysis
3. Results
3.1. Neutralization of Virus Infectivity with Monoclonal Anti HBs Antibodies Depends upon HBsAg Subtype
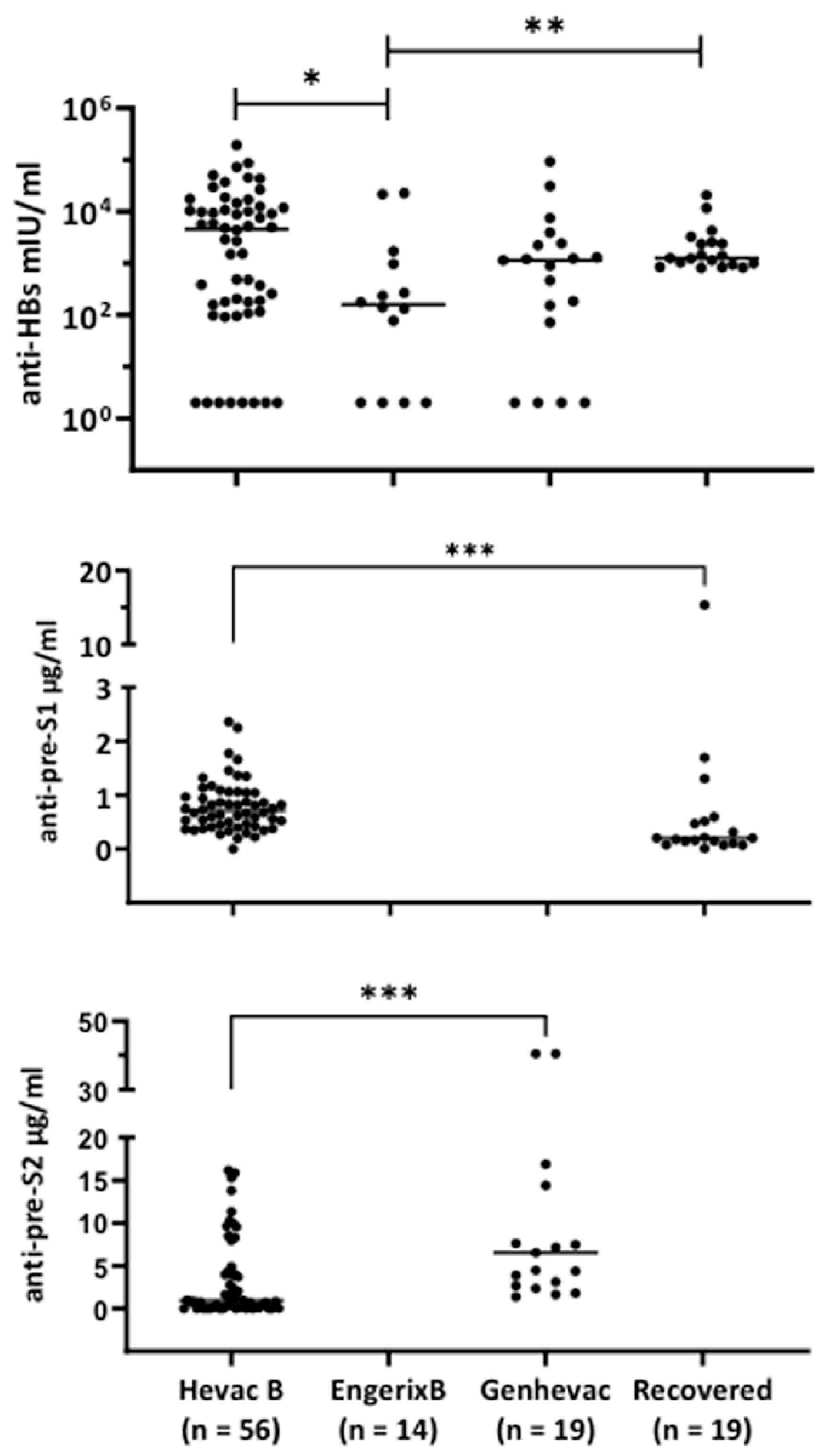
3.2. Characterization of Antibodies Elicited by Hevac-B, Engerix, and Genhevac-B Vaccines or during Acute Infection
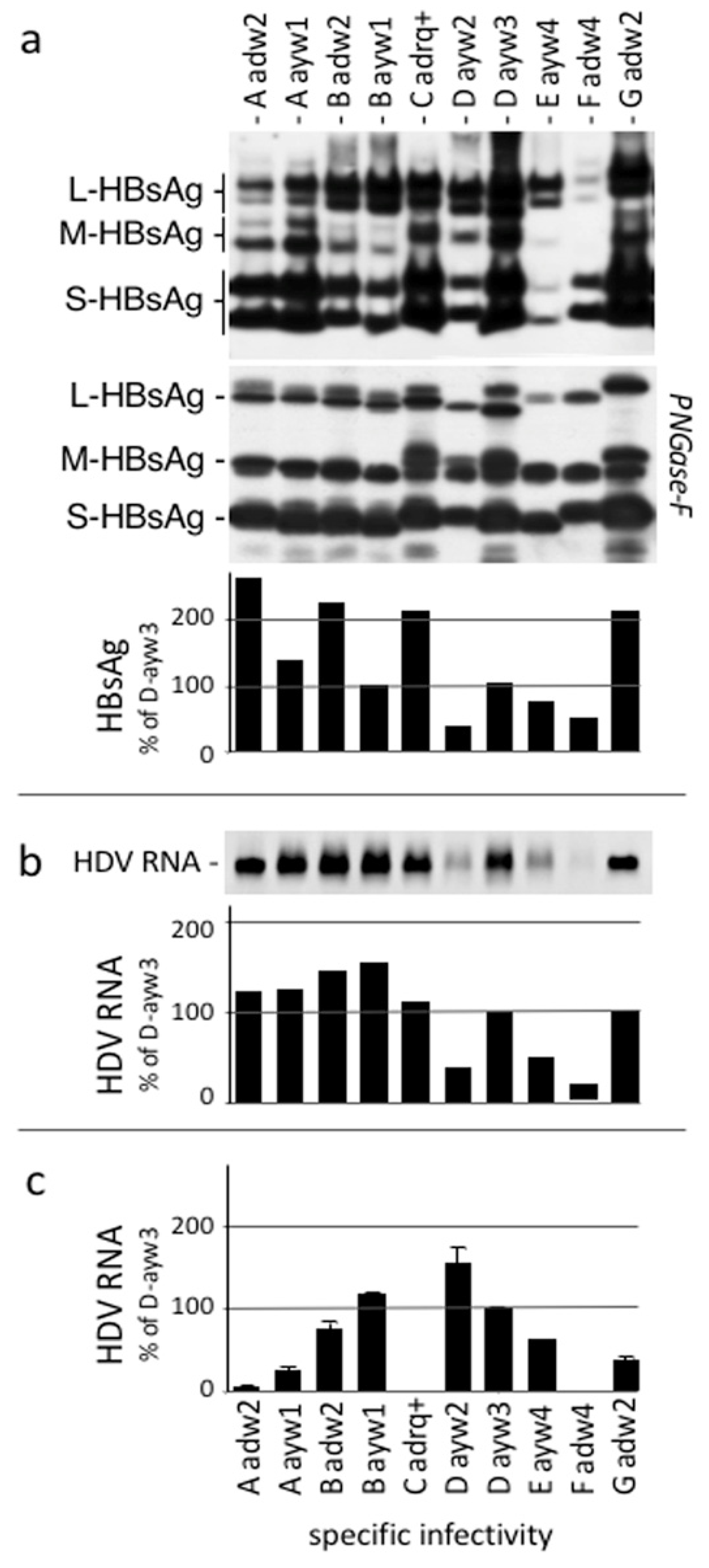
3.3. Establishment of a Quantitative Immunoassay for Measurement In Vitro HDV Infection
3.4. Correlation between Anti-HBs IgG ELISA and Neutralizing Activity
3.5. Correlation between Anti-preS Antibodies Titers and In Vitro Neutralizing Activity
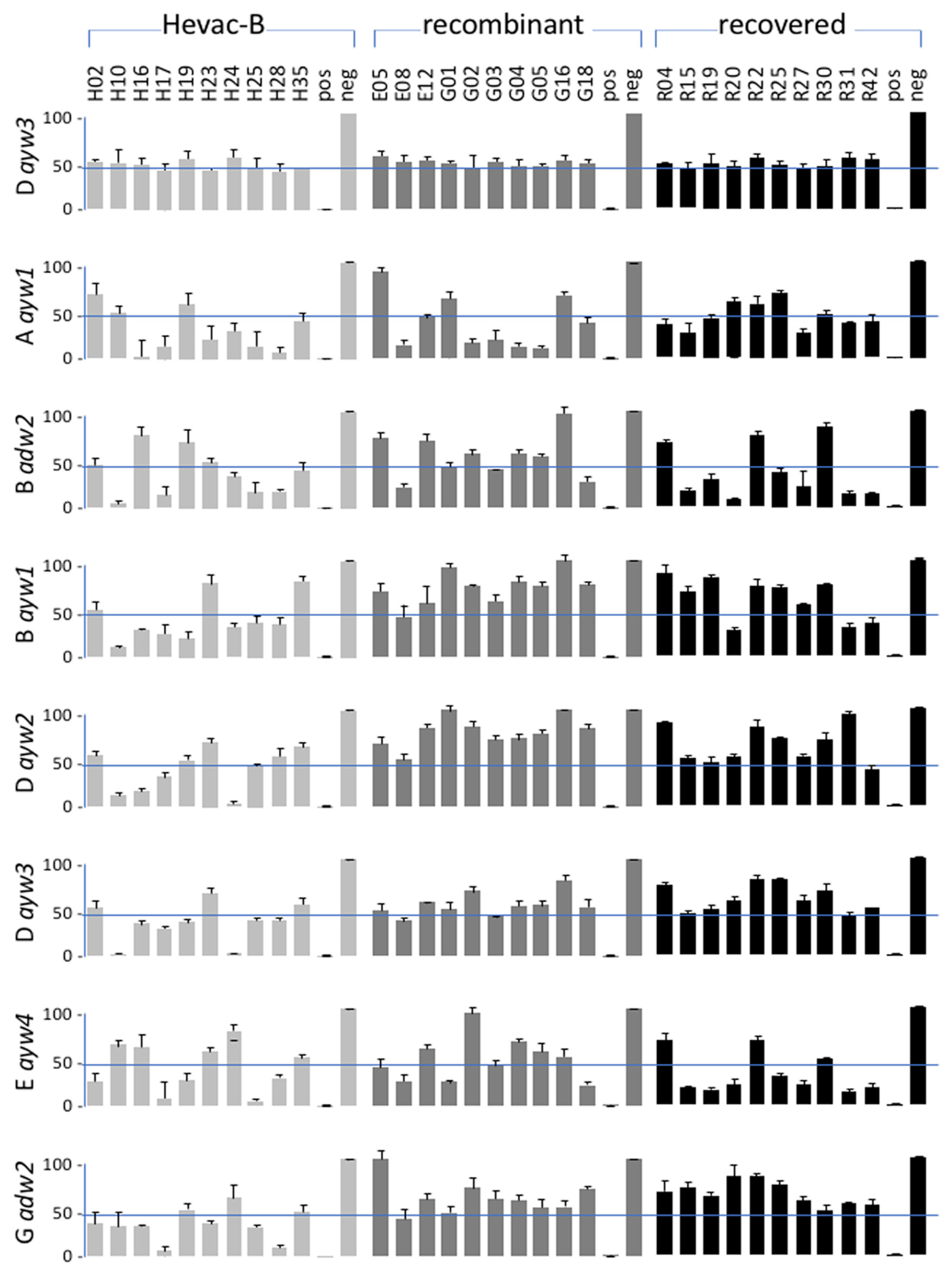
3.6. Neutralizing Activity of Anti-HBV Antibodies according to HBV Genotype of the Inoculum
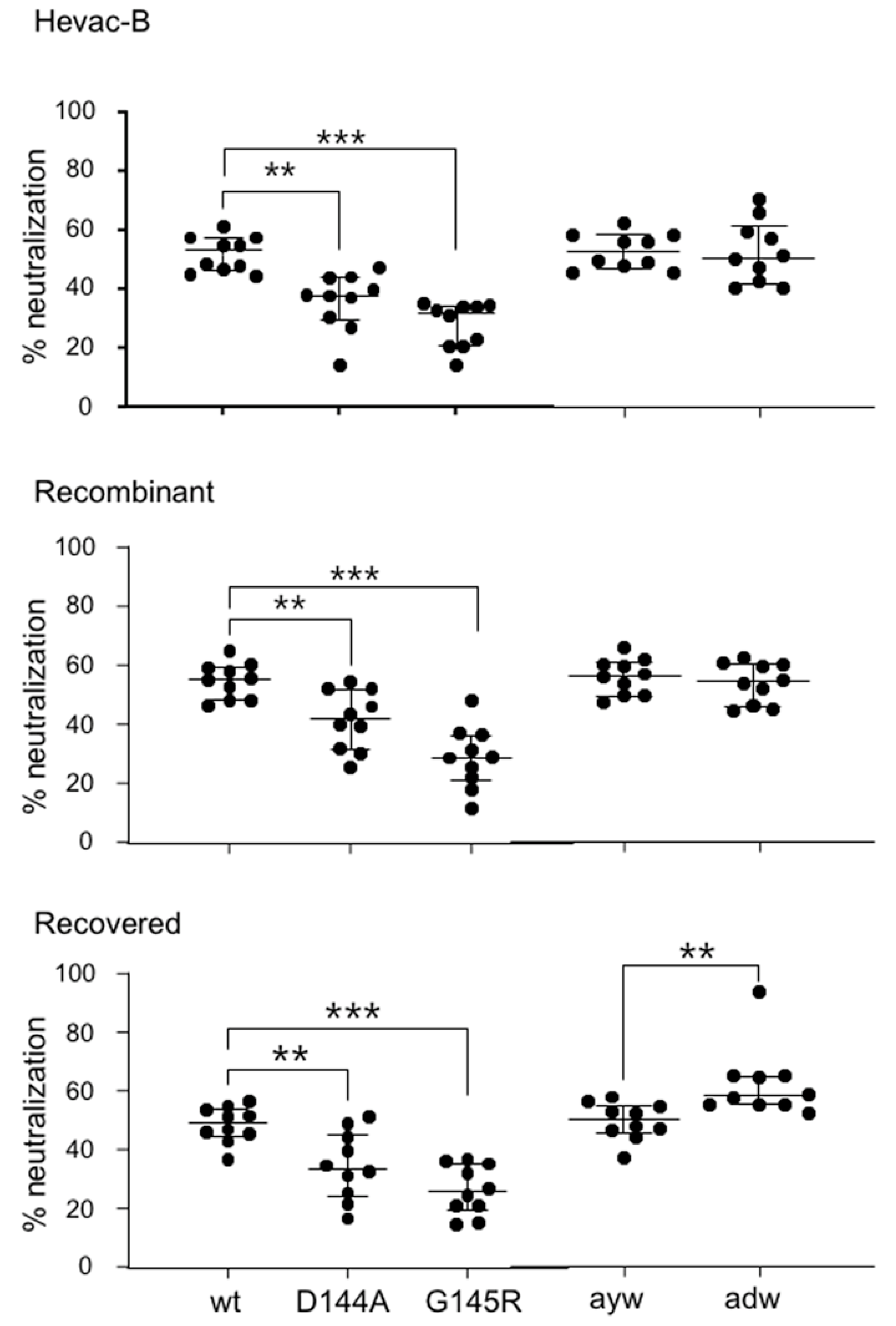
3.7. Neutralizing Activity of Anti-HBs Antibodies against Immune Escape D144A and G145R Variants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerlich, W.H. Medical virology of hepatitis B: How it began and where we are now. Virol. J. 2013, 10, 239. [Google Scholar] [CrossRef]
- Heermann, K.H.; Gerlich, W.H. Surface proteins of hepatitis B viruses. In Molecular Biology of the Hepatitis B Virus; Maclachlan, A., Ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 109–144. [Google Scholar]
- Sureau, C.; Negro, F. The hepatitis delta virus: Replication and pathogenesis. J. Hepatol. 2016, 64, S102–S116. [Google Scholar] [CrossRef]
- Sureau, C.; Salisse, J. A conformational heparan sulfate binding site essential to infectivity overlaps with the conserved hepatitis B virus A-determinant. Hepatology 2013, 57, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H.; et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 2012, 1, e00049. [Google Scholar] [CrossRef]
- Brown, S.E.; Stanley, C.; Howard, C.R.; Zuckerman, A.J.; Steward, M.W. Antibody responses to recombinant and plasma derived hepatitis B vaccines. Br. Med. J. (Clin. Res. Ed.) 1986, 292, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Maupas, P.; Goudeau, A.; Coursaget, P.; Drucker, J.; Bagros, P. Hepatitis B vaccine: Efficacy in high-risk settings, a two-year study. Intervirology 1978, 10, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Szmuness, W.; Stevens, C.E.; Harley, E.J.; Zang, E.A.; Oleszko, W.R.; William, D.C.; Sadovsky, R.; Morrison, J.M.; Kellner, A. Hepatitis B vaccine: Demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N. Engl. J. Med. 1980, 303, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Emini, E.A.; Ellis, R.W.; Miller, W.J.; McAleer, W.J.; Scolnick, E.M.; Gerety, R.J. Production and immunological analysis of recombinant hepatitis B vaccine. J. Infect. 1986, 13 (Suppl. A), 3–9. [Google Scholar] [CrossRef]
- Michel, M.L.; Tiollais, P. Hepatitis B vaccines: Protective efficacy and therapeutic potential. Pathol. Biol. 2010, 58, 288–295. [Google Scholar] [CrossRef]
- Pattyn, J.; Hendrickx, G.; Vorsters, A.; Van Damme, P. Hepatitis B Vaccines. J. Infect. Dis. 2021, 224, S343–S351. [Google Scholar] [CrossRef]
- Cheah, B.C.; Davies, J.; Singh, G.R.; Wood, N.; Jackson, K.; Littlejohn, M.; Davison, B.; McIntyre, P.; Locarnini, S.; Davis, J.S.; et al. Sub-optimal protection against past hepatitis B virus infection where subtype mismatch exists between vaccine and circulating viral genotype in northern Australia. Vaccine 2018, 36, 3533–3540. [Google Scholar] [CrossRef]
- Deepen, R.; Heermann, K.H.; Uy, A.; Thomssen, R.; Gerlich, W.H. Assay of preS epitopes and preS1 antibody in hepatitis B virus carriers and immune persons. Med. Microbiol. Immunol. 1990, 179, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Julithe, R.; Abou-Jaoude, G.; Sureau, C. Modification of the hepatitis B virus envelope protein glycosylation pattern interferes with secretion of viral particles, infectivity, and susceptibility to neutralizing antibodies. J. Virol. 2014, 88, 9049–9059. [Google Scholar] [CrossRef] [PubMed]
- Sureau, C.; Moriarty, A.M.; Thornton, G.B.; Lanford, R.E. Production of infectious hepatitis delta virus in vitro and neutralization with antibodies directed against hepatitis B virus pre-S antigens. J. Virol. 1992, 66, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, M.; Sureau, C. Infectivity determinants of the hepatitis B virus pre-S domain are confined to the N-terminal 75 amino acid residues. J. Virol. 2007, 81, 5841–5849. [Google Scholar] [CrossRef]
- Verrier, E.R.; Colpitts, C.C.; Bach, C.; Heydmann, L.; Weiss, A.; Renaud, M.; Durand, S.C.; Habersetzer, F.; Durantel, D.; Abou-Jaoude, G.; et al. A targeted functional RNA interference screen uncovers glypican 5 as an entry factor for hepatitis B and D viruses. Hepatology 2016, 63, 35–48. [Google Scholar] [CrossRef]
- Beilstein, F.; Blanchet, M.; Vaillant, A.; Sureau, C. Nucleic Acid Polymers Are Active against Hepatitis Delta Virus Infection In Vitro. J. Virol. 2018, 92, e01416–e01417. [Google Scholar] [CrossRef]
- Sureau, C. The use of hepatocytes to investigate HDV infection: The HDV/HepaRG model. Methods Mol. Biol. 2010, 640, 463–473. [Google Scholar]
- Chang, M.H.; Chen, C.J.; Lai, M.S.; Hsu, H.M.; Wu, T.C.; Kong, M.S.; Liang, D.C.; Shau, W.Y.; Chen, D.S. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N. Engl. J. Med. 1997, 336, 1855–1859. [Google Scholar] [CrossRef]
- Poovorawan, Y.; Theamboonlers, A.; Vimolket, T.; Sinlaparatsamee, S.; Chaiear, K.; Siraprapasiri, T.; Khwanjaipanich, S.; Owatanapanich, S.; Hirsch, P.; Chunsuttiwat, S. Impact of hepatitis B immunisation as part of the EPI. Vaccine 2000, 19, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Khamduang, W.; Gaudy-Graffin, C.; Ngo-Giang-Huong, N.; Jourdain, G.; Moreau, A.; Borkird, T.; Layangool, P.; Kamonpakorn, N.; Jitphiankha, W.; Kwanchaipanich, R.; et al. Analysis of residual perinatal transmission of hepatitis B virus (HBV) and of genetic variants in human immunodeficiency virus and HBV co-infected women and their offspring. J. Clin. Virol. 2013, 58, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Guo, Y.; Zhou, A.; Zhang, Y.; Cao, J.; Yang, M.; Xiao, F.; Zhang, B.; Du, Y. Immunoprophylaxis failure against vertical transmission of hepatitis B virus in the Chinese population: A hospital-based study and a meta-analysis. Pediatr. Infect. Dis. J. 2014, 33, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gui, X.; Wang, B.; Ji, H.; Yisilafu, R.; Li, F.; Zhou, Y.; Zhang, H.; Liu, X. A study of immunoprophylaxis failure and risk factors of hepatitis B virus mother-to-infant transmission. Eur. J. Pediatr. 2014, 173, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Golsaz-Shirazi, F.; Mohammadi, H.; Amiri, M.M.; Khoshnoodi, J.; Kardar, G.A.; Jeddi-Tehrani, M.; Shokri, F. Localization of immunodominant epitopes within the “a” determinant of hepatitis B surface antigen using monoclonal antibodies. Arch. Virol. 2016, 161, 2765–2772. [Google Scholar] [CrossRef] [PubMed]
- Hehle, V.; Beretta, M.; Bourgine, M.; Ait-Goughoulte, M.; Planchais, C.; Morisse, S.; Vesin, B.; Lorin, V.; Hieu, T.; Stauffer, A.; et al. Potent human broadly neutralizing antibodies to hepatitis B virus from natural controllers. J. Exp. Med. 2020, 217, e20200840. [Google Scholar] [CrossRef] [PubMed]
- Salisse, J.; Sureau, C. A function essential to 584 viral entry underlies the hepatitis B virus “a” determinant. J. Virol. 2009, 83, 9321–9328. [Google Scholar] [CrossRef]
- Glebe, D.; Aliakbari, M.; Krass, P.; Knoop, E.V.; Valerius, K.P.; Gerlich, W.H. Pre-s1 antigen-dependent infection of Tupaia hepatocyte cultures with human hepatitis B virus. J. Virol. 2003, 77, 9511–9521. [Google Scholar] [CrossRef] [PubMed]
- Shouval, D.; Roggendorf, H.; Roggendorf, M. Enhanced immune response to hepatitis B vaccination through immunization with a Pre-S1/Pre-S2/S Vaccine. Med. Microbiol. Immunol. 2015, 204, 57–68. [Google Scholar] [CrossRef]
- Safadi, R.; Khoury, T.; Saed, N.; Hakim, M.; Jamalia, J.; Nijim, Y.; Farah, N.; Nuser, T.; Natur, N.; Mahamid, M.; et al. Efficacy of Birth Dose Vaccination in Preventing Mother-to-Child Transmission of Hepatitis B: A Randomized Controlled Trial Comparing Engerix-B and Sci-B-Vac. Vaccines 2021, 9, 331. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aronthippaitoon, Y.; Szerman, N.; Ngo-Giang-Huong, N.; Laperche, S.; Ungeheuer, M.-N.; Sureau, C.; Khamduang, W.; Gaudy-Graffin, C. Are International Units of Anti-HBs Antibodies Always Indicative of Hepatitis B Virus Neutralizing Activity? Vaccines 2023, 11, 791. https://doi.org/10.3390/vaccines11040791
Aronthippaitoon Y, Szerman N, Ngo-Giang-Huong N, Laperche S, Ungeheuer M-N, Sureau C, Khamduang W, Gaudy-Graffin C. Are International Units of Anti-HBs Antibodies Always Indicative of Hepatitis B Virus Neutralizing Activity? Vaccines. 2023; 11(4):791. https://doi.org/10.3390/vaccines11040791
Chicago/Turabian StyleAronthippaitoon, Yada, Nathan Szerman, Nicole Ngo-Giang-Huong, Syria Laperche, Marie-Noelle Ungeheuer, Camille Sureau, Woottichai Khamduang, and Catherine Gaudy-Graffin. 2023. "Are International Units of Anti-HBs Antibodies Always Indicative of Hepatitis B Virus Neutralizing Activity?" Vaccines 11, no. 4: 791. https://doi.org/10.3390/vaccines11040791
APA StyleAronthippaitoon, Y., Szerman, N., Ngo-Giang-Huong, N., Laperche, S., Ungeheuer, M.-N., Sureau, C., Khamduang, W., & Gaudy-Graffin, C. (2023). Are International Units of Anti-HBs Antibodies Always Indicative of Hepatitis B Virus Neutralizing Activity? Vaccines, 11(4), 791. https://doi.org/10.3390/vaccines11040791





