A Novel, Comprehensive A129 Mouse Model for Investigating Dengue Vaccines and Evaluating Pathogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus and Cells
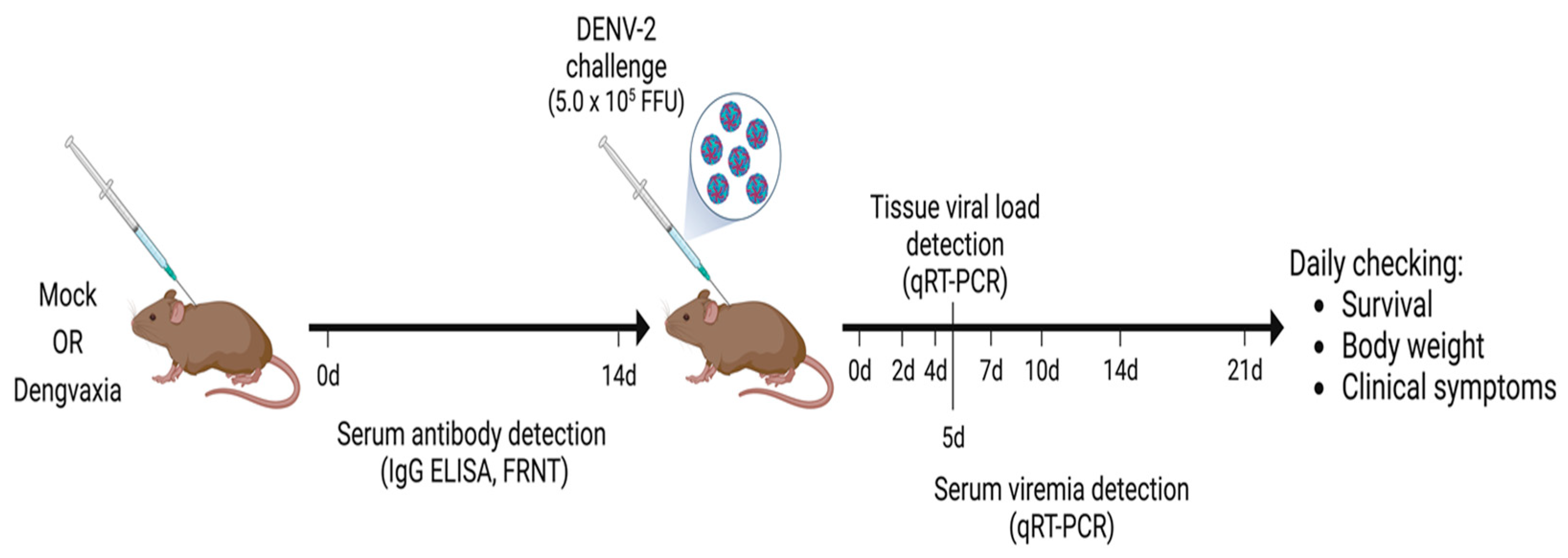
2.2. Immunizations and Virus Challenge of Mice
2.3. Measurement of DENV-Specific Antibodies
2.4. Measurement of DENV Neutralization Antibodies
2.5. Quantification of Viremia Levels via qRT–PCR
2.6. Statistical Analysis
3. Results
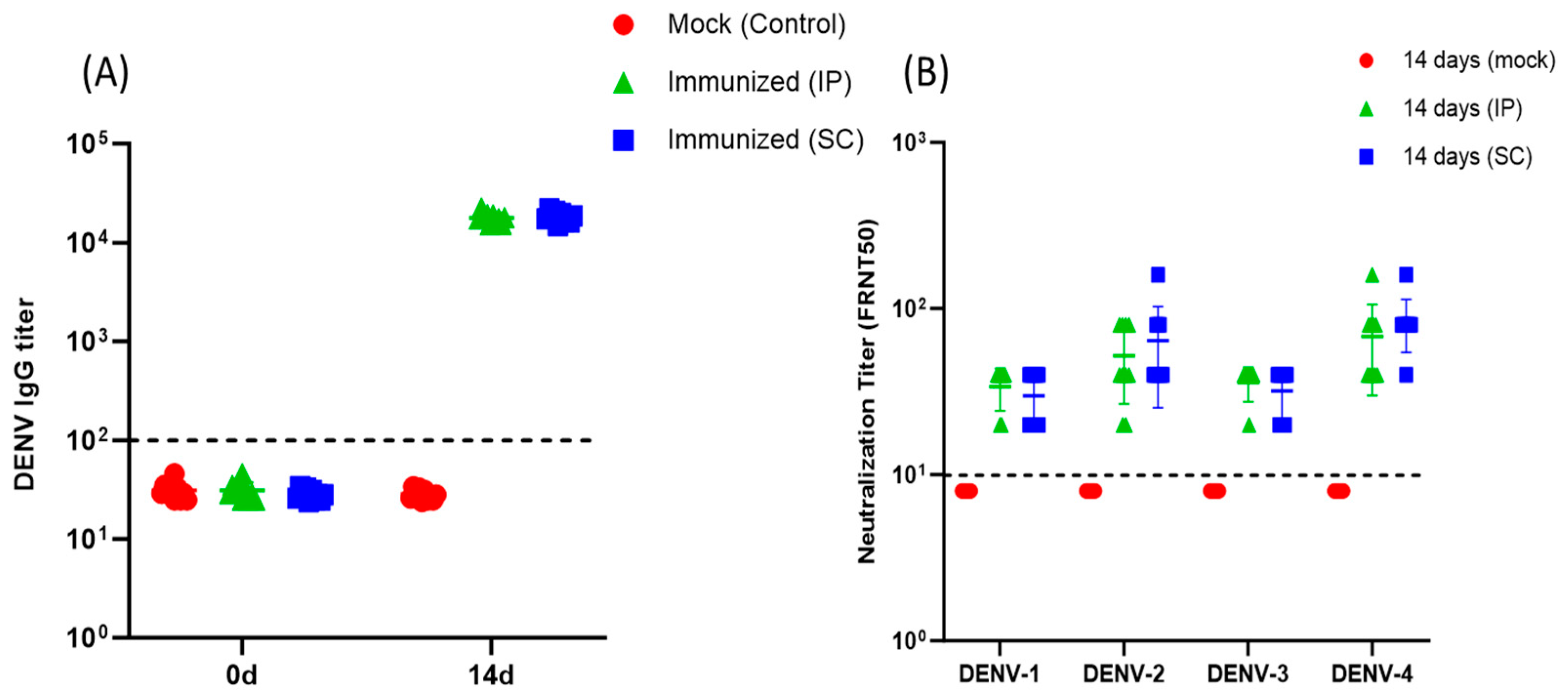
3.1. Humoral Immune Response in A129 Mice after Immunization with Dengvaxia
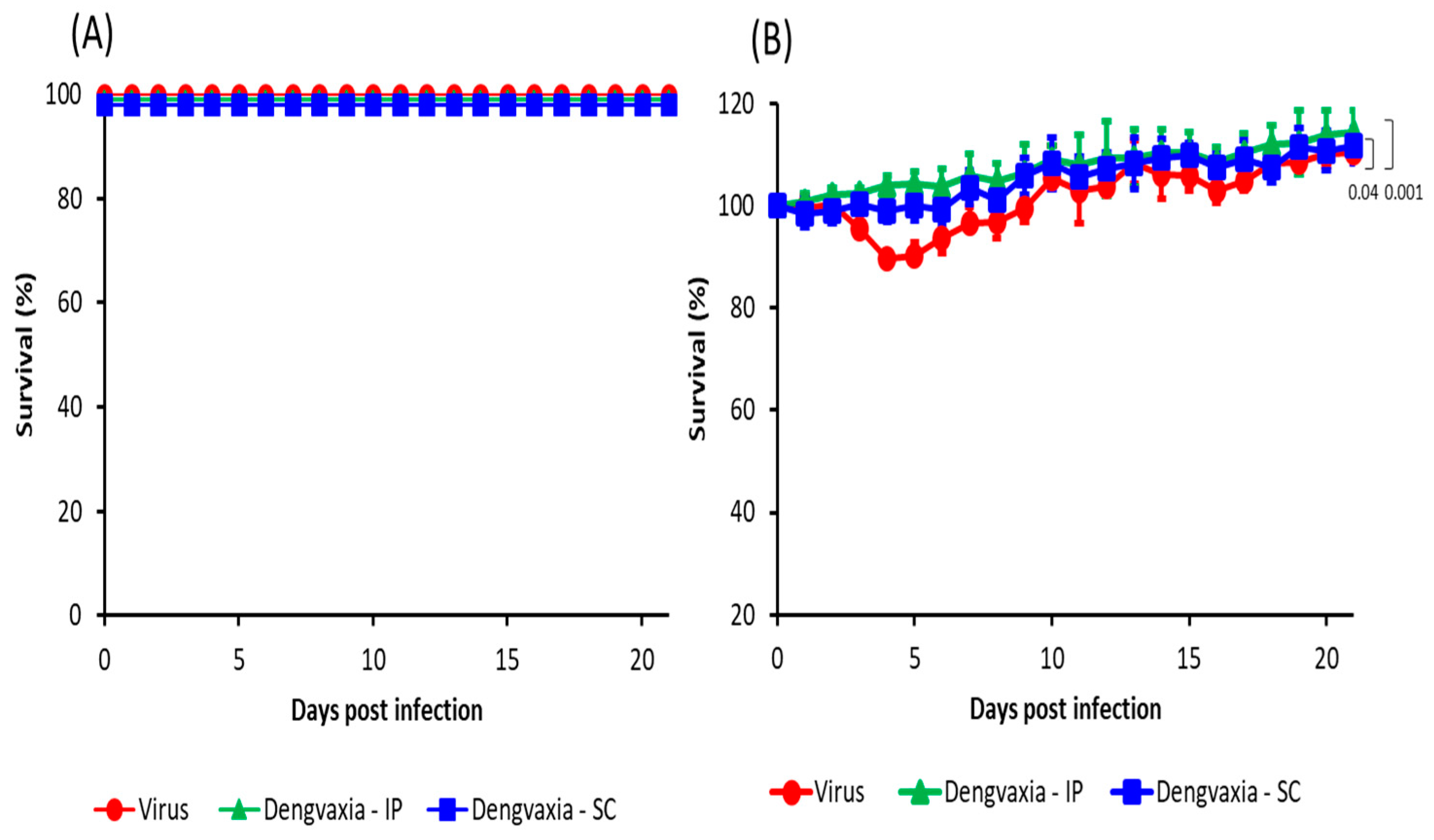
3.2. Morbidity and Mortality in Immunized A129 Mice Following DENV-2 Infection
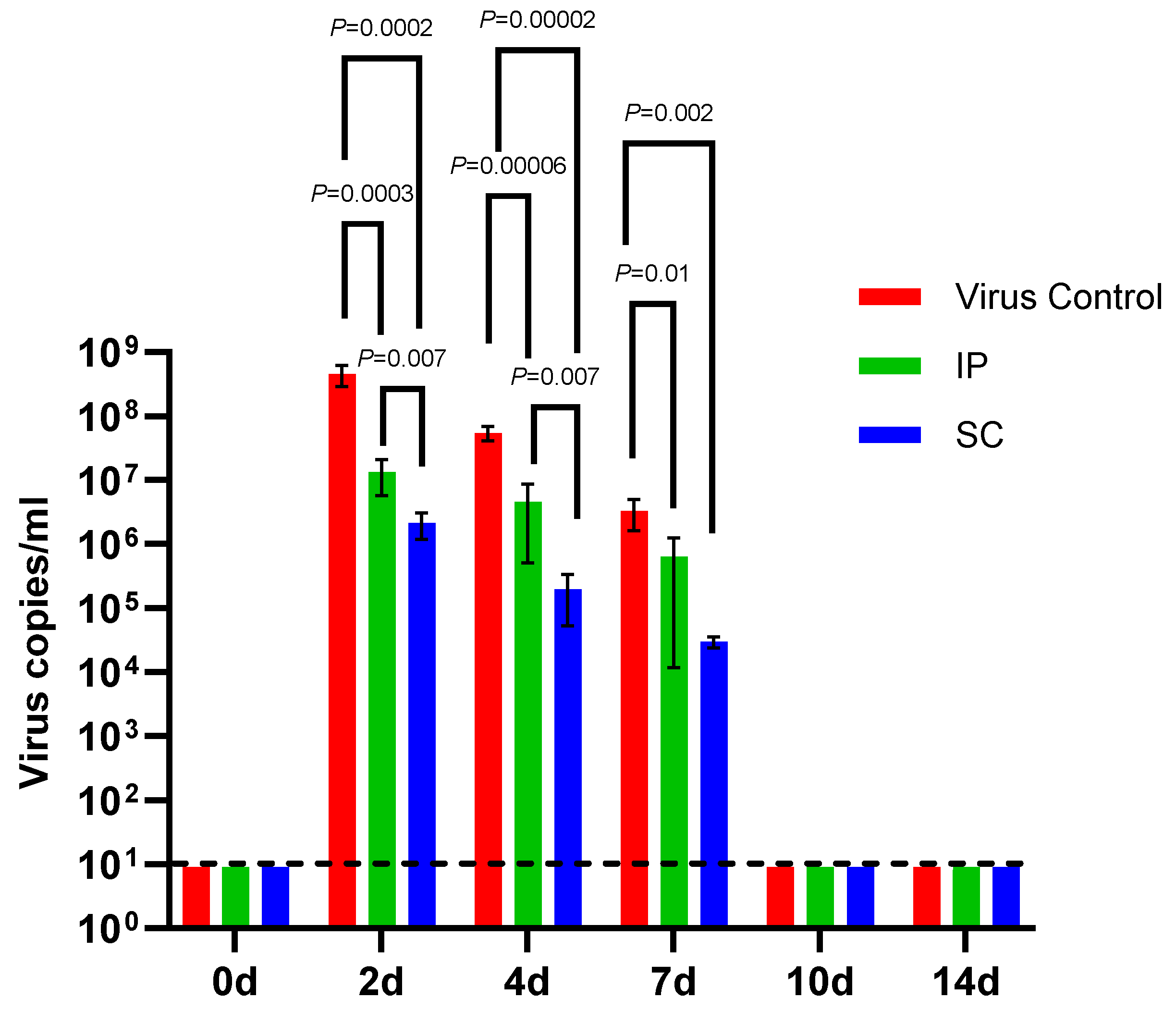
3.3. Viremia in Immunized A129 Mice Following DENV-2 Challenge
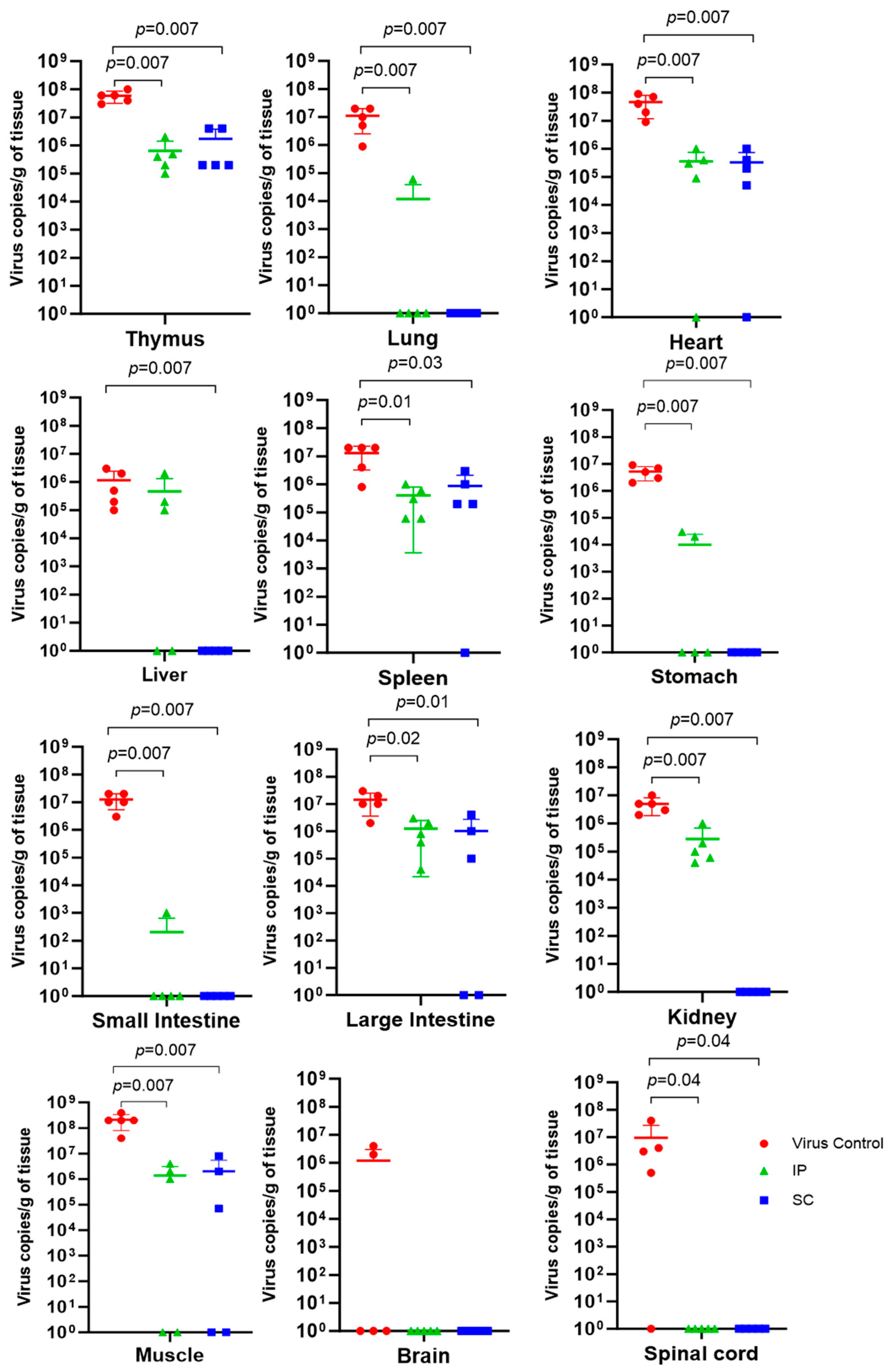
3.4. Viral Load in Organs and Tissues of Immunized A129 Mice Infected with DENV-2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Holmes, E.C. Molecular epidemiology and evolution of emerging infectious diseases. Br. Med. Bull. 1998, 54, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Martina, B.E.; Koraka, P.; Osterhaus, A.D. Dengue virus pathogenesis: An integrated view. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef] [PubMed]
- Hammon, W.M.; Rudnick, A.; Sather, G.E. Viruses associated with epidemic hemorrhagic fevers of the Philippines and Thailand. Science 1960, 131, 1102–1103. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.M.; Anderson, K.B.; Katzelnick, L.C.; Clapham, H.; Johansson, M.A.; Morrison, A.C.; Harris, E.; Paz-Bailey, G.; Waterman, S.H. Knowledge gaps in the epidemiology of severe dengue impede vaccine evaluation. Lancet Infect. Dis. 2022, 22, e42–e51. [Google Scholar] [CrossRef] [PubMed]
- Kliks, S.C.; Nimmanitya, S.; Nisalak, A.; Burke, D.S. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am. J. Trop. Med. Hyg. 1988, 38, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef] [PubMed]
- Rico-Hesse, R. Microevolution and virulence of dengue viruses. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2003; Volume 59, pp. 315–341. [Google Scholar]
- Halstead, S.B. The Alexander D. Langmuir Lecture the pathogenesis of dengue: Molecular epidemiology in infectious disease. Am. J. Epidemiol. 1981, 114, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Noriega, F.; Ochiai, R.L.; L’Azou, M.; Delore, V.; Skipetrova, A.; Verdier, F.; Coudeville, L.; Savarino, S.; Jackson, N. A recombinant live attenuated tetravalent vaccine for the prevention of dengue. Expert. Rev. Vaccines 2017, 16, 671–684. [Google Scholar] [CrossRef]
- Thomas, S.J.; Yoon, I.K. A review of Dengvaxia®: Development to deployment. Hum. Vaccin. Immunother. 2019, 15, 2295–2314. [Google Scholar] [CrossRef]
- Dengue vaccine: WHO position paper—July 2016. Wkly Epidemiol. Rec. 2016, 91, 349–364.
- Hou, J.; Ye, W.; Chen, J. Current Development and Challenges of Tetravalent Live-Attenuated Dengue Vaccines. Front. Immunol. 2022, 13, 840104. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.; Biswal, S.; Sáez-Llorens, X.; Reynales, H.; López-Medina, E.; Borja-Tabora, C.; Bravo, L.; Sirivichayakul, C.; Kosalaraksa, P.; Martinez Vargas, L.; et al. Three-year Efficacy and Safety of Takeda’s Dengue Vaccine Candidate (TAK-003). Clin. Infect. Dis. 2022, 75, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, R.M.; Shresta, S. Mouse models to study dengue virus immunology and pathogenesis. Front. Immunol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Coronel-Ruiz, C.; Gutiérrez-Barbosa, H.; Medina-Moreno, S.; Velandia-Romero, M.L.; Chua, J.V.; Castellanos, J.E.; Zapata, J.C. Humanized Mice in Dengue Research: A Comparison with Other Mouse Models. Vaccines 2020, 8, 39. [Google Scholar] [CrossRef]
- Sánchez, I.J.; Ruiz, B.H. A single nucleotide change in the E protein gene of dengue virus 2 Mexican strain affects neurovirulence in mice. J. Gen. Virol. 1996, 77 Pt. 10, 2541–2545. [Google Scholar] [CrossRef]
- Castillo Ramirez, J.A.; Urcuqui-Inchima, S. Dengue Virus Control of Type I IFN Responses: A History of Manipulation and Control. J. Interferon Cytokine Res. 2015, 35, 421–430. [Google Scholar] [CrossRef]
- Prestwood, T.R.; Morar, M.M.; Zellweger, R.M.; Miller, R.; May, M.M.; Yauch, L.E.; Lada, S.M.; Shresta, S. Gamma interferon (IFN-γ) receptor restricts systemic dengue virus replication and prevents paralysis in IFN-α/β receptor-deficient mice. J. Virol. 2012, 86, 12561–12570. [Google Scholar] [CrossRef]
- Fuchs, J.; Chu, H.; O’Day, P.; Pyles, R.; Bourne, N.; Das, S.C.; Milligan, G.N.; Barrett, A.D.; Partidos, C.D.; Osorio, J.E. Investigating the efficacy of monovalent and tetravalent dengue vaccine formulations against DENV-4 challenge in AG129 mice. Vaccine 2014, 32, 6537–6543. [Google Scholar] [CrossRef]
- Shukla, R.; Beesetti, H.; Brown, J.A.; Ahuja, R.; Ramasamy, V.; Shanmugam, R.K.; Poddar, A.; Batra, G.; Krammer, F.; Lim, J.K.; et al. Dengue and Zika virus infections are enhanced by live attenuated dengue vaccine but not by recombinant DSV4 vaccine candidate in mouse models. EBioMedicine 2020, 60, 102991. [Google Scholar] [CrossRef]
- Ngwe Tun, M.M.; Muthugala, R.; Nabeshima, T.; Rajamanthri, L.; Jayawardana, D.; Attanayake, S.; Soe, A.M.; Dumre, S.P.; Ando, T.; Hayasaka, D.; et al. Unusual, neurological and severe dengue manifestations during the outbreak in Sri Lanka, 2017. J. Clin. Virol. 2020, 125, 104304. [Google Scholar] [CrossRef] [PubMed]
- Ngwe Tun, M.M.; Thant, K.Z.; Inoue, S.; Kurosawa, Y.; Lwin, Y.Y.; Lin, S.; Aye, K.T.; Thet Khin, P.; Myint, T.; Htwe, K.; et al. Serological characterization of dengue virus infections observed among dengue hemorrhagic fever/dengue shock syndrome cases in upper Myanmar. J. Med. Virol. 2013, 85, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Takasaki, T.; Yamada, K.; Nerome, R.; Tajima, S.; Kurane, I. Development and evaluation of fluorogenic TaqMan reverse transcriptase PCR assays for detection of dengue virus types 1 to 4. J. Clin. Microbiol. 2004, 42, 5935–5937. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.Q.; Yang, X.; Wei, Y.; Chen, J.T.; Wang, X.J.; Peng, H.J. A Review on Dengue Vaccine Development. Vaccines 2020, 8, 63. [Google Scholar] [CrossRef]
- Brewoo, J.N.; Kinney, R.M.; Powell, T.D.; Arguello, J.J.; Silengo, S.J.; Partidos, C.D.; Huang, C.Y.; Stinchcomb, D.T.; Osorio, J.E. Immunogenicity and efficacy of chimeric dengue vaccine (DENVax) formulations in interferon-deficient AG129 mice. Vaccine 2012, 30, 1513–1520. [Google Scholar] [CrossRef]
- Sariol, C.A.; White, L.J. Utility, limitations, and future of non-human primates for dengue research and vaccine development. Front. Immunol. 2014, 5, 452. [Google Scholar] [CrossRef]
- Fumagalli, M.J.; de Souza, W.M.; de Castro-Jorge, L.A.; de Carvalho, R.V.H.; Castro, Í.A.; de Almeida, L.G.N.; Consonni, S.R.; Zamboni, D.S.; Figueiredo, L.T.M. Chikungunya Virus Exposure Partially Cross-Protects against Mayaro Virus Infection in Mice. J. Virol. 2021, 95, e0112221. [Google Scholar] [CrossRef]
- Meier, K.C.; Gardner, C.L.; Khoretonenko, M.V.; Klimstra, W.B.; Ryman, K.D. A mouse model for studying viscerotropic disease caused by yellow fever virus infection. PLoS Pathog. 2009, 5, e1000614. [Google Scholar] [CrossRef]
- Xie, X.; Kum, D.B.; Xia, H.; Luo, H.; Shan, C.; Zou, J.; Muruato, A.E.; Medeiros, D.B.A.; Nunes, B.T.D.; Dallmeier, K.; et al. A Single-Dose Live-Attenuated Zika Virus Vaccine with Controlled Infection Rounds that Protects against Vertical Transmission. Cell Host Microbe 2018, 24, 487–499.e485. [Google Scholar] [CrossRef]
- Samuel, M.A.; Diamond, M.S. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 2005, 79, 13350–13361. [Google Scholar] [CrossRef]
- Davis, E.H.; Beck, A.S.; Li, L.; White, M.M.; Greenberg, M.B.; Thompson, J.K.; Widen, S.G.; Barrett, A.D.T.; Bourne, N. Japanese encephalitis virus live attenuated vaccine strains display altered immunogenicity, virulence and genetic diversity. NPJ Vaccines 2021, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Guirakhoo, F.; Pugachev, K.; Zhang, Z.; Myers, G.; Levenbook, I.; Draper, K.; Lang, J.; Ocran, S.; Mitchell, F.; Parsons, M.; et al. Safety and efficacy of chimeric yellow Fever-dengue virus tetravalent vaccine formulations in nonhuman primates. J. Virol. 2004, 78, 4761–4775. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Briand, O.; Lang, J.; Saville, M.; Jackson, N. Development of the Sanofi Pasteur tetravalent dengue vaccine: One more step forward. Vaccine 2015, 33, 7100–7111. [Google Scholar] [CrossRef] [PubMed]
- Guirakhoo, F.; Arroyo, J.; Pugachev, K.V.; Miller, C.; Zhang, Z.X.; Weltzin, R.; Georgakopoulos, K.; Catalan, J.; Ocran, S.; Soike, K.; et al. Construction, safety, and immunogenicity in nonhuman primates of a chimeric yellow fever-dengue virus tetravalent vaccine. J. Virol. 2001, 75, 7290–7304. [Google Scholar] [CrossRef] [PubMed]
- Gallichotte, E.N.; Henein, S.; Nivarthi, U.; Delacruz, M.; Scobey, T.; Bonaparte, M.; Moser, J.; Munteanu, A.; Baric, R.; de Silva, A.M. Vaccine-induced antibodies to contemporary strains of dengue virus type 4 show a mechanistic correlate of protective immunity. Cell Rep. 2022, 39, 110930. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Grifoni, A.; Sette, A.; Weiskopf, D. Human T Cell Response to Dengue Virus Infection. Front. Immunol. 2019, 10, 2125. [Google Scholar] [CrossRef]
- Friberg, H.; Gargulak, M.; Kong, A.; Lin, L.; Martinez, L.J.; Schmidt, A.C.; Paris, R.M.; Jarman, R.G.; Diaz, C.; Thomas, S.J.; et al. Characterization of B-cell and T-cell responses to a tetravalent dengue purified inactivated vaccine in healthy adults. NPJ Vaccines 2022, 7, 132. [Google Scholar] [CrossRef]
- Shresta, S.; Sharar, K.L.; Prigozhin, D.M.; Beatty, P.R.; Harris, E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J. Virol. 2006, 80, 10208–10217. [Google Scholar] [CrossRef]
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Endy, T.P.; Raengsakulrach, B.; Rothman, A.L.; Ennis, F.A.; et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000, 181, 2–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngwe Tun, M.M.; Nwe, K.M.; Balingit, J.C.; Takamatsu, Y.; Inoue, S.; Pandey, B.D.; Urano, T.; Kohara, M.; Tsukiyama-Kohara, K.; Morita, K. A Novel, Comprehensive A129 Mouse Model for Investigating Dengue Vaccines and Evaluating Pathogenesis. Vaccines 2023, 11, 1857. https://doi.org/10.3390/vaccines11121857
Ngwe Tun MM, Nwe KM, Balingit JC, Takamatsu Y, Inoue S, Pandey BD, Urano T, Kohara M, Tsukiyama-Kohara K, Morita K. A Novel, Comprehensive A129 Mouse Model for Investigating Dengue Vaccines and Evaluating Pathogenesis. Vaccines. 2023; 11(12):1857. https://doi.org/10.3390/vaccines11121857
Chicago/Turabian StyleNgwe Tun, Mya Myat, Khine Mya Nwe, Jean Claude Balingit, Yuki Takamatsu, Shingo Inoue, Basu Dev Pandey, Takeshi Urano, Michinori Kohara, Kyoko Tsukiyama-Kohara, and Kouichi Morita. 2023. "A Novel, Comprehensive A129 Mouse Model for Investigating Dengue Vaccines and Evaluating Pathogenesis" Vaccines 11, no. 12: 1857. https://doi.org/10.3390/vaccines11121857
APA StyleNgwe Tun, M. M., Nwe, K. M., Balingit, J. C., Takamatsu, Y., Inoue, S., Pandey, B. D., Urano, T., Kohara, M., Tsukiyama-Kohara, K., & Morita, K. (2023). A Novel, Comprehensive A129 Mouse Model for Investigating Dengue Vaccines and Evaluating Pathogenesis. Vaccines, 11(12), 1857. https://doi.org/10.3390/vaccines11121857





