Evaluation of Theoretical Frameworks to Detect Correlates of HPV Vaccination in the Midwest, US, Using Structural Equation Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Questionnaire
2.3. Sample Size
2.4. Ethical Consideration
2.5. Data Analysis
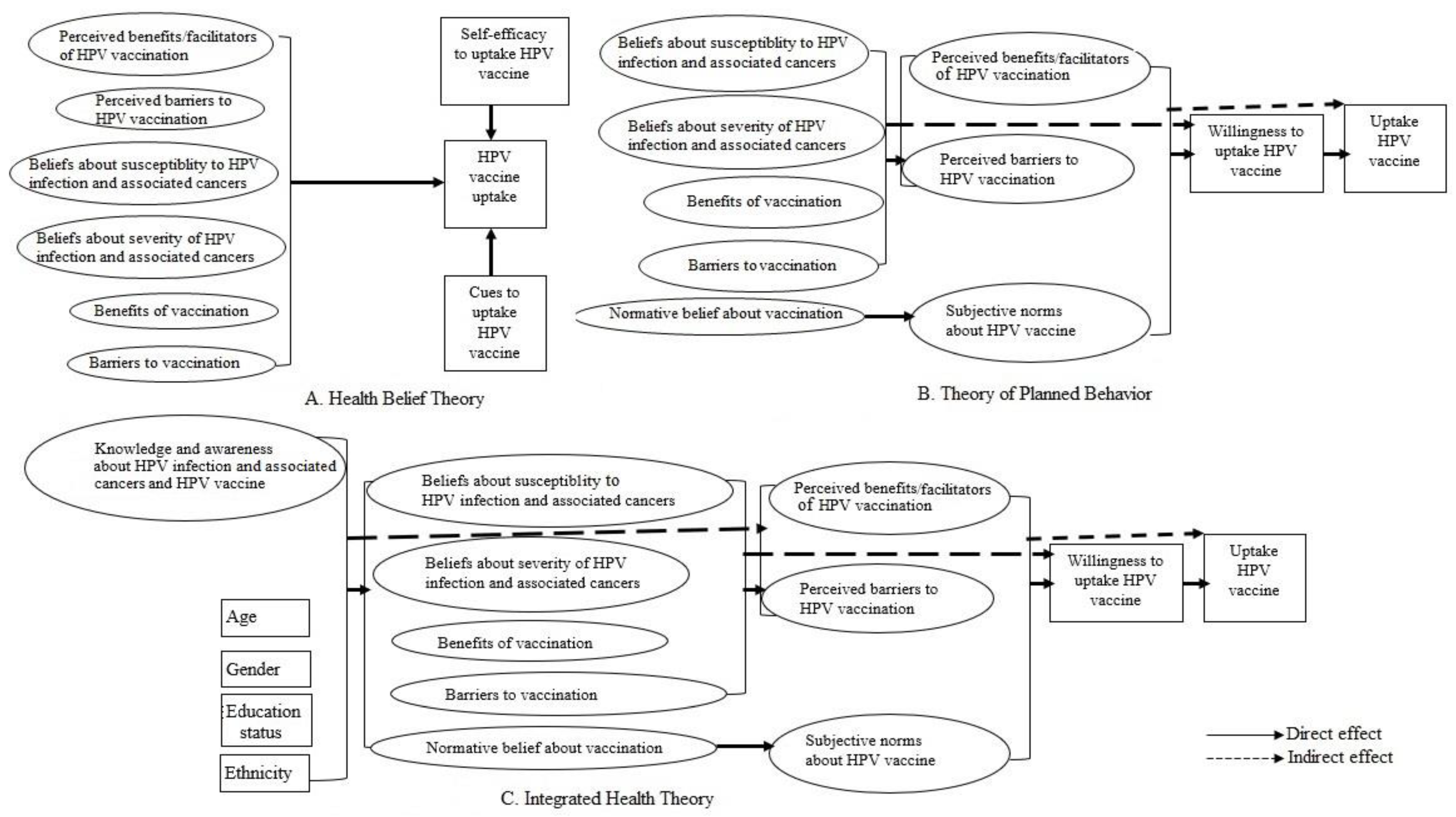
3. Results
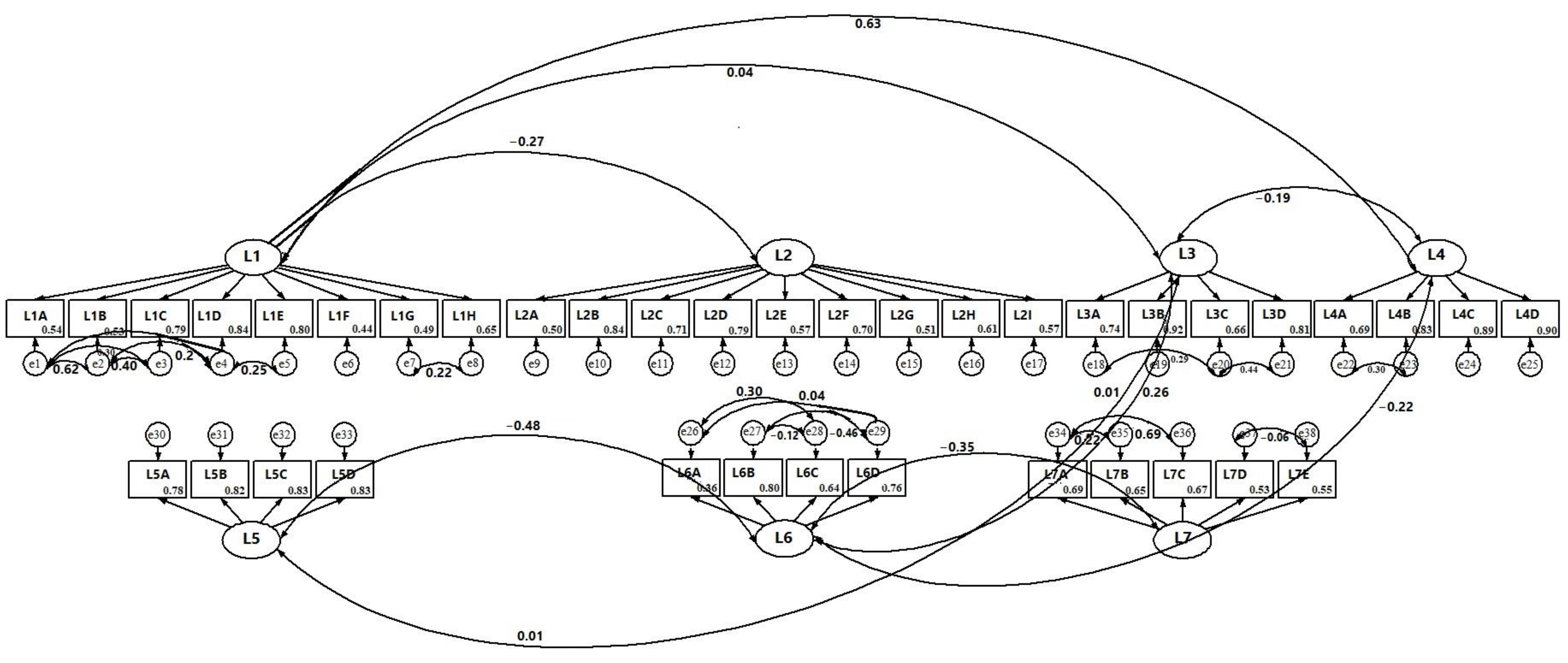
3.1. Measurement Models
3.1.1. Integrated Health Theory
3.1.2. Health Belief Theory and Theory of Planned Behavior
3.2. Structural Model
3.2.1. Integrated Health Theory
3.2.2. Health Belief Theory
3.2.3. Theory of Planned Behavior
3.3. Factors Associated with HPV Vaccination Willingness and Uptake
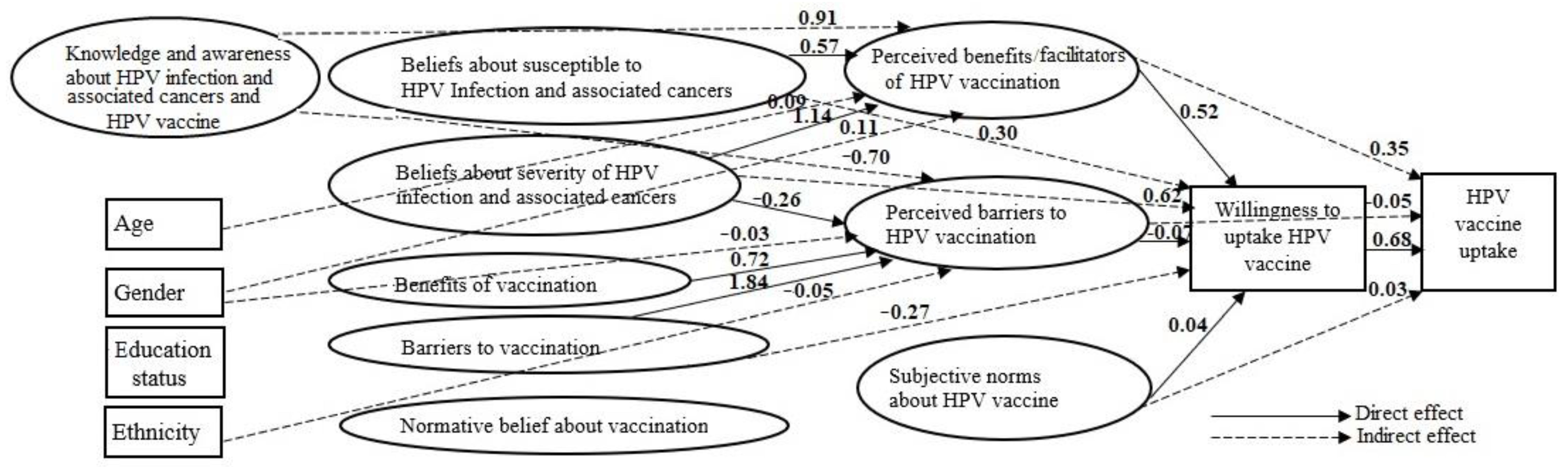
| Exposure | Mediator | Outcome | β (95% CI) |
|---|---|---|---|
| Willingness to uptake HPV vaccine | NA | HPV vaccine uptake | 0.68 (0.63, 0.72) |
| Perceived benefits/facilitators of HPV vaccination | NA | Willingness to uptake HPV vaccine | 0.52 (0.48, 0.57) |
| Perceived barriers to HPV vaccination | NA | Willingness to uptake HPV vaccine | −0.07 (−0.12, −0.02) |
| Subjective norms of HPV vaccination | NA | Willingness to uptake HPV vaccine | 0.04 (0.01, 0.08) |
| Perceived benefits/facilitators of HPV vaccination | Willingness to uptake HPV vaccine | HPV vaccine uptake | 0.35 (0.31, 0.40) |
| Perceived barriers to HPV vaccination | Willingness to uptake HPV vaccine | HPV vaccine uptake | −0.05 (−0.09, −0.01) |
| Subjective norms of HPV vaccination | Willingness to uptake HPV vaccine | HPV vaccine uptake | 0.03 (0.003, 0.06) |
| Beliefs about the benefits of vaccination | Perceived benefits/facilitators of HPV vaccination | Willingness to uptake HPV vaccine | 0.04 (−0.09, 0.16) |
| Beliefs about benefits of vaccination | Perceived barriers to HPV vaccination | Willingness to uptake HPV vaccine | −0.05 (−0.11, 0.005) |
| Total | −0.02 (−0.16, 0.13) | ||
| Beliefs about barriers to vaccination | Perceived benefits/facilitators of HPV vaccination | Willingness to uptake HPV vaccine | −0.14 (−0.23, −0.05) |
| Beliefs about barriers to vaccination | Perceived barriers to HPV vaccination | Willingness to uptake HPV vaccine | −0.13 (−0.26, −0.03) |
| Total | −0.27 (−0.42, −0.12) | ||
| Beliefs about susceptibility to HPV infection and cervical cancer | Perceived benefits/facilitators of HPV vaccination | Willingness to uptake HPV vaccine | 0.30 (0.09, 0.51) |
| Beliefs about susceptibility to HPV infection and cervical cancer | Perceived barriers to HPV vaccination | Willingness to uptake HPV vaccine | 0.002 (−0.02, 0.02) |
| Total | 0.30 (0.09, 0.51) | ||
| Beliefs about severity of HPV infection and cervical cancer | Perceived benefits/facilitators of HPV vaccination | Willingness to uptake HPV vaccine | 0.60 (0.42, 0.78) |
| Beliefs about severity of HPV infection and cervical cancer | Perceived barriers to HPV vaccination | Willingness to uptake HPV vaccine | 0.02 (−0.002, 0.04) |
| Total | 0.62 (0.44, 0.79) | ||
| Normative beliefs about HPV vaccination | Subjective norms of HPV vaccination | Willingness to uptake HPV vaccine | 0.02 (−0.001, 0.03) |
3.4. Factors Associated with the Attitudes about HPV Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancers Associated with Human Papillomavirus, United States—2015–2019. Available online: https://www.cdc.gov/cancer/uscs/about/data-briefs/no31-hpv-assoc-cancers-UnitedStates-2015-2019.htm (accessed on 22 October 2023).
- Moyer, V.A.; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2012, 156, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.M.; Laprise, J.F.; Gargano, J.W.; Unger, E.R.; Querec, T.D.; Chesson, H.W.; Brisson, M.; Markowitz, L.E. Estimated Prevalence and Incidence of Disease-Associated Human Papillomavirus Types Among 15- to 59-Year-Olds in the United States. Sex. Transm. Dis. 2021, 48, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Senkomago, V.; Henley, S.J.; Thomas, C.C.; Mix, J.M.; Markowitz, L.E.; Saraiya, M. Human Papillomavirus-Attributable Cancers—United States, 2012–2016. MMWR Morb. Mortal Wkly Rep. 2019, 68, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. HPV-Associated Cancer Statistics. Available online: https://www.cdc.gov/cancer/hpv/statistics/index.htm (accessed on 19 January 2021).
- Cancers Associated with Human Papillomavirus by State, 2010–2014. Available online: https://www.cdc.gov/cancer/hpv/pdf/hpv-associated-cancer-incidence-by-state-2010-2014-508.pdf (accessed on 4 November 2023).
- Nebraska Department of Health and Human Services/Cancer Registry Cancer Incidence and Mortality in Nebraska Report 2015. Available online: http://dhhs.ne.gov/Reports/Cancer%20Incidence%20and%20Mortality%20in%20Nebraska%20-%202015.pdf (accessed on 15 January 2022).
- Centers for Disease Control and Prevention. HPV Vaccine. Available online: https://www.cdc.gov/hpv/parents/vaccine-for-hpv.html (accessed on 19 January 2022).
- Gee, J.; Weinbaum, C.; Sukumaran, L.; Markowitz, L.E. Quadrivalent HPV vaccine safety review and safety monitoring plans for nine-valent HPV vaccine in the United States. Hum. Vaccines Immunother. 2016, 12, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. HPV Vaccine Schedule and Dosing. Available online: https://www.cdc.gov/hpv/hcp/schedules-recommendations.html (accessed on 31 October 2023).
- Kash, N.; Lee, M.A.; Kollipara, R.; Downing, C.; Guidry, J.; Tyring, S.K. Safety and Efficacy Data on Vaccines and Immunization to Human Papillomavirus. J. Clin. Med. 2015, 4, 614–633. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, V.S.; Bansal, N.; Liu, Z.; Finalle, R.; Sénécal, M.; Kothari, S.; Trowers, K.; Myers, E. HPV vaccination uptake and administration from 2006 to 2016 in a commercially insured population of the United States. BMC Public Health 2021, 21, 1629. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Mazzucca, S.; Padek, M.; Brownson, R.C. Going beyond the individual: How state-level characteristics relate to HPV vaccine rates in the United States. BMC Public Health 2019, 19, 246. [Google Scholar] [CrossRef]
- Healthy People 2030. Increase the Proportion of Adolescents Who Get Recommended Doses of the HPV Vaccine—IID-08. Available online: https://health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-proportion-adolescents-who-get-recommended-doses-hpv-vaccine-iid-08 (accessed on 30 November 2023).
- NCI. HPV Vaccination. Available online: https://progressreport.cancer.gov/prevention/hpv_immunization (accessed on 2 December 2023).
- Mavundza, E.J.; Iwu-Jaja, C.J.; Wiyeh, A.B.; Gausi, B.; Abdullahi, L.H.; Halle-Ekane, G.; Wiysonge, C.S. A Systematic Review of Interventions to Improve HPV Vaccination Coverage. Vaccines 2021, 9, 687. [Google Scholar] [CrossRef]
- Loke, A.Y.; Kwan, M.L.; Wong, Y.-T.; Wong, A.K.Y. The Uptake of Human Papillomavirus Vaccination and Its Associated Factors Among Adolescents: A Systematic Review. J. Prim. Care Community Health 2017, 8, 349–362. [Google Scholar] [CrossRef]
- Mansfield, L.N.; Vance, A.; Nikpour, J.A.; Gonzalez-Guarda, R.M. A systematic review of human papillomavirus vaccination among U.S. adolescents. Res. Nurs. Health 2021, 44, 473–489. [Google Scholar] [CrossRef]
- MacDonald, N.E.; SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Montaño, D.E.; Kasprzyk, D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. In Health Behavior and Health Education: Theory, Research, and Practice 2015, 4th ed.; Glanz, K., Rimer, B.K., Viswanath, K., Eds.; Jossey-Bass: San Francisco, CA, USA, 2015; pp. 67–96. [Google Scholar]
- Becker, M.H. The Health Belief Model and personal health behavior. Health Educ. Monogr. 1974, 2, 324–508. [Google Scholar] [CrossRef]
- Bandura, A. Social Foundations of thought and Action: A Social Cognitive Theory; Prentice-Hall: Hoboken, NJ, USA, 1986. [Google Scholar]
- Kilanowski, J.F. Breadth of the Socio-Ecological Model. J. Agromedicine 2017, 22, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wu, J.; Zheng, M. Barriers to and Facilitators of Human Papillomavirus Vaccination Among People Aged 9 to 26 Years: A Systematic Review. Sex. Transm. Dis. 2021, 48, e255–e262. [Google Scholar] [CrossRef]
- Peterson, C.E.; Silva, A.; Holt, H.K.; Balanean, A.; Goben, A.H.; Dykens, J.A. Barriers and facilitators to HPV vaccine uptake among U.S. rural populations: A scoping review. Cancer Causes Control. 2020, 31, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Brewer, N.T.; Fazekas, K.I. Predictors of HPV vaccine acceptability: A theory-informed, systematic review. Prev. Med. 2007, 45, 107–114. [Google Scholar] [CrossRef]
- Li, L.; Wood, C.E.; Kostkova, P. Vaccine hesitancy and behavior change theory-based social media interventions: A systematic review. Transl. Behav. Med. 2022, 12, 243–272. [Google Scholar] [CrossRef]
- Batista Ferrer, H.; Audrey, S.; Trotter, C.; Hickman, M. An appraisal of theoretical approaches to examining behaviours in relation to Human Papillomavirus (HPV) vaccination of young women. Prev. Med. 2015, 81, 122–131. [Google Scholar] [CrossRef]
- Degarege, A.; Krupp, K.; Fennie, K.; Srinivas, V.; Li, T.; Stephens, D.P.; Madhivanan, P. An integrative behavior theory derived model to assess factors affecting HPV vaccine acceptance using structural equation modeling. Vaccine 2019, 37, 945–955. [Google Scholar] [CrossRef]
- Qualtrics, Provo, UT, USA. Available online: https://www.qualtrics.com/ (accessed on 3 December 2023).
- Rodriguez, S.A.; Mullen, P.D.; Lopez, D.M.; Savas, L.S.; Fernández, M.E. Factors associated with adolescent HPV vaccination in the U.S.: A systematic review of reviews and multilevel framework to inform intervention development. Prev. Med. 2020, 131, 105968. [Google Scholar] [CrossRef]
- Holman, D.M.; Benard, V.; Roland, K.B.; Watson, M.; Liddon, N.; Stokley, S. Barriers to human papillomavirus vaccination among U.S. adolescents: A systematic review of the literature. JAMA Pediatr. 2014, 168, 76–82. [Google Scholar] [CrossRef]
- Gopalani, S.V.; Sedani, A.E.; Janitz, A.E.; Clifton, S.C.; Peck, J.D.; Comiford, A.; Campbell, J.E. Barriers and Factors Associated with HPV Vaccination Among American Indians and Alaska Natives: A Systematic Review. J. Community. Health. 2022, 47, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Witte, K. Predicting risk behaviors: Development and validation of a diagnostic scale. J. Health Commun. 1996, 1, 317–342. [Google Scholar] [CrossRef]
- Bentler, P.M.; Chou, C. Practical issues in structural modeling. Sociol. Methods Res. 1987, 16, 78–117. [Google Scholar] [CrossRef]
- Muthén, L.K.; Muthén, B.O. Mplus User’s Guide; Muthén and Muthén: Los Angeles, CA, USA, 1998–2017. [Google Scholar]
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 53–55. [Google Scholar] [CrossRef]
- Bland, J.; Altman, D. Statistics notes: Cronbach’s alpha. BMJ 1997, 314, 275. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F.; Anderson, R.E.; Babin, B.J.; Black, W.C. Multivariate Data Analysis: A Global Perspective, 7th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Boateng, G.O.; Neilands, T.B.; Frongillo, E.A.; Melgar-Quiñonez, H.R.; Young, S.L. Best Practices for Developing and Validating Scales for Health, Social, and Behavioral Research: A Primer. Front. Public. Health 2018, 6, 149. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Muthe’n, B.O.; du Toit, S.H.C.; Spisic, D. Robust Inference Using Weighted Least Squares and Quadratic Estimating Equations in Latent Variable Modeling with Categorical and Continuous Outcomes. Available online: www.statmodel.com/bmuthen/articles/Article_075.pdf (accessed on 15 January 2022).
- Byrne, B.M. Structural Equation Modeling with Mplus: Basic Concepts, Applications and Programming; Routledge Academic: New York, NY, USA, 2011. [Google Scholar]
- Carmines, E.G.; McIver, J.P. Analyzing models with unobserved variables. In Social Measurement: Current Issues; Bohrnstedt, G.W., Borgatta, E.F., Eds.; Sage: Beverly Hills, CA, USA, 1981; p. 80. [Google Scholar]
- Marsh, H.W.; Hocevar, D. Application of confirmatory factor analysis to the study of self-concept: First- and higher-order factor models and their invariance across groups. Psychol. Bull. 1985, 97, 562–582. [Google Scholar] [CrossRef]
- Census Reporter. Midwest Region. Available online: https://censusreporter.org/profiles/02000US2-midwest-region/ (accessed on 3 November 2023).
| Constructs | Item Label | Item | Responses/Scores |
|---|---|---|---|
| Benefits/facilitators of HPV vaccination (L1) | L1A | My doctor recommends me to receive HPV vaccine | 0. Strongly disagree, |
| 1. Disagree, | |||
| 2. Neutral, | |||
| 3. Agree, | |||
| 4. Strongly agree | |||
| L1B | My family member recommends/supports me to receive HPV vaccine | ||
| L1C | I believe HPV vaccine is beneficial to my health | ||
| L1D | I believe that HPV vaccine is safe | ||
| L1E | I believe that HPV vaccine is effective | ||
| L1F | I believe that I will become sexually active | ||
| L1G | I believe that HPV infection can cause cervical cancer | ||
| L1H | I believe that HPV vaccine will prevent cervical, oropharyngeal, vaginal, vulvar, penile, anal, and rectal cancers for self and others | ||
| Barriers to HPV vaccination (L2) | L2A | HPV vaccine is too expensive | 0. Strongly disagree, |
| 1. Disagree, | |||
| 2. Neutral, | |||
| 3. Agree, | |||
| 4. Strongly agree | |||
| L2B | My religious belief goes against me getting HPV vaccine | ||
| L2C | Objections in getting HPV vaccine from religious authorities will prevent me from getting the vaccine | ||
| L2D | Friends who disapprove of getting HPV vaccine will prevent me from getting the vaccine | ||
| L2E | I am afraid that HPV vaccine injection may cause pain | ||
| L2F | I will not have time to get HPV vaccine | ||
| L2G | I do not know how to make an appointment to get HPV vaccine | ||
| L2H | My health insurance does not cover the HPV vaccine | ||
| L2I | I am too young for getting vaccination | ||
| Susceptibility to HPV infection and associated cancers (L3) | L3A | I believe I am at risk of getting HPV infection | 0. Strongly disagree, |
| 1. Disagree, | |||
| 2. Neutral, | |||
| 3. Agree, | |||
| 4. Strongly agree | |||
| L3B | I will likely contract HPV infection | ||
| L3C | I am at risk of getting cervical, oropharyngeal, vaginal or penile, anal or rectal cancer | ||
| L3D | I will likely contract cervical, oropharyngeal, vaginal, or penile or rectal cancer | ||
| Severity of HPV infection and associated cancers (L4) | L4A | I believe that HPV infection is severe | 0. Strongly disagree, |
| 1. Disagree, | |||
| 2. Neutral, | |||
| 3. Agree, | |||
| 4. Strongly agree | |||
| L4B | I believe that HPV infection is serious. | ||
| L4C | I believe that cervical, oropharyngeal, vaginal, vulvar, penile, anal, and rectal cancers are severe | ||
| L4D | I believe that cervical, oropharyngeal, vaginal, vulvar, penile, anal, and rectal cancers are serious | ||
| Benefits of vaccination (L5) | L5A | Vaccines are effective in preventing disease | 0. Strongly disagree, |
| 1. Disagree, | |||
| 2. Neutral, | |||
| 3. Agree, | |||
| 4. Strongly agree | |||
| L5B | It is very important that I receive all the necessary routine vaccines | ||
| L5C | Vaccine is one way that I can ensure good health | ||
| L5D | I have a responsibility to get vaccinated for the protection of others | ||
| Barriers to vaccination (L6) | L6A | I am concerned about vaccine side effects | 0. Strongly disagree, |
| 1. Disagree, | |||
| 2. Neutral, | |||
| 3. Agree, | |||
| 4. Strongly agree | |||
| L6B | It is better to get the disease and get protected naturally | ||
| L6C | There are too many vaccines available to take | ||
| L6D | I have a negative experience with vaccination | ||
| Awareness and knowledge about HPV infection and associated cancers and HPV vaccine (L7) | L7A | Have you ever heard about the HPV infection? | 1. Yes, 2 = No |
| L7B | Have you ever heard about cervical cancer, anal, penial, vaginal, vulva, oropharynx? | 1. Yes, 0 = No | |
| L7C | Have you ever heard about the HPV vaccine? | 1. Yes, 0 = No | |
| L7D | HPV infection can cause cervical, oropharyngeal, vaginal, vulvar, penile, anal, and rectal cancers | 1. True, 0 = False | |
| L7E | HPV vaccine can prevent cervical, oropharyngeal, vaginal, vulvar, penile, anal, and rectal cancers | 1. True, 0 = False |
| Variables | Categories | Illinois (n = 259) | Ohio (n = 243) | Michigan (n = 187) | Indiana (n = 126) | Missouri (n= 117) | Wisconsin (n = 97) | Minnesota (n = 91) | Kansas (n = 58) | Iowa (n = 53) | Nebraska (n= 42) | South Dakota (n = 17) | North Dakota (n = 16) | Total (n = 1306) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age in years | 13–17 | 42.9 | 40.3 | 41.2 | 38.1 | 47.9 | 44.3 | 52.75 | 53.5 | 49.1 | 59.5 | 41.18 | 56.3 | 44.3 |
| 18–22 | 40.9 | 43.2 | 42.8 | 48.4 | 38.5 | 41.2 | 28.6 | 36.2 | 43.4 | 23.8 | 35.3 | 37.5 | 40.5 | |
| 23–26 | 16.2 | 16.5 | 16.0 | 13.5 | 13.7 | 14.4 | 18.7 | 10.3 | 7.6 | 16.7 | 23.5 | 6.3 | 14.4 | |
| Gender | Male | 45.2 | 55.1 | 51.9 | 50.8 | 53.0 | 55.67 | 47.3 | 43.1 | 45.3 | 54.8 | 47.1 | 68.8 | 50.7 |
| Female | 51.7 | 42.4 | 40.1 | 43.7 | 42.7 | 43.3 | 48.4 | 53.4 | 45.3 | 40.4 | 41.2 | 31.3 | 45.0 | |
| Other | 3.1 | 2.5 | 8.0 | 5.6 | 4.3 | 1.0 | 4.4 | 3.5 | 9.4 | 4.8 | 11.8 | 0.0 | 4.3 | |
| Educational attainment | Less than high school | 34.4 | 37.4 | 36.9 | 35.7 | 42.7 | 46.4 | 41.8 | 46.6 | 50.9 | 47.6 | 64.7 | 43.8 | 39.7 |
| High school graduate or GED | 35.5 | 34.1 | 33.2 | 38.1 | 35.0 | 29.9 | 37.4 | 25.9 | 30.2 | 28.6 | 11.8 | 31.3 | 33.6 | |
| Some college | 20.5 | 22.63 | 20.3 | 18.25 | 17.1 | 19.6 | 13.2 | 22.4 | 17.0 | 14.3 | 23.5 | 18.8 | 19.5 | |
| Graduate school | 9.7 | 5.8 | 9.6 | 7.9 | 5.1 | 4.1 | 7.7 | 5.2 | 1.9 | 9.5 | 0.0 | 6.3 | 7.1 | |
| Race | White | 48.3 | 60.9 | 53.5 | 54.8 | 62.6 | 68.0 | 62.6 | 65.5 | 71.7 | 69.1 | 64.7 | 62.5 | 58.1 |
| Black or African American | 31.3 | 26.3 | 29.4 | 28.6 | 16.5 | 9.3 | 16.5 | 12.1 | 5.7 | 9.5 | 11.8 | 18.8 | 24.0 | |
| American Indian or Alaska natives | 1.9 | 1.2 | 2.1 | 2.4 | 3.5 | 5.15 | 3.3 | 5.2 | 1.9 | 4.76 | 23.5 | 12.5 | 5.3 | |
| Asian | 5.41 | 4.9 | 5.4 | 5.6 | 8.8 | 7.2 | 8.8 | 3.4 | 7.5 | 2.4 | 0.0 | 6.3 | 3.1 | |
| Native Hawaiian or Pacific Islander | 0.0 | 1.23 | 1.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.9 | 0.0 | 0.0 | 0.0 | 0.5 | |
| Other | 13.1 | 5.4 | 8.0 | 8.7 | 8.8 | 10.3 | 8.79 | 13.79 | 11.32 | 14.29 | 0.0 | 0.0 | 8.9 | |
| Ethnicity | Hispanic or Latino | 23.17 | 14.0 | 10.2 | 16.7 | 11.97 | 21.7 | 19.8 | 31.0 | 9.4 | 21.4 | 5.9 | 18.8 | 17.1 |
| Non-Hispanic or non-Latino | 76.8 | 86.0 | 89.8 | 83.3 | 88.0 | 78.4 | 80.2 | 69.0 | 90.6 | 78.6 | 94.1 | 81.3 | 82.9 | |
| HPV vaccination status | Fully vaccinated (3 doses) | 15.1 | 6.3 | 13.4 | 15.1 | 10.3 | 14.4 | 15.4 | 15.5 | 11.3 | 9.52 | 0.0 | 6.3 | 13.6 |
| Fully vaccinated (2 doses) | 16.2 | 18.8 | 16.1 | 9.5 | 16.2 | 15.5 | 18.7 | 13.8 | 7.6 | 23.8 | 29.4 | 18.8 | 16.8 | |
| Partially vaccinated (one does) | 10.0 | 12.5 | 13.4 | 8.7 | 5.98 | 7.2 | 6.6 | 6.9 | 9.4 | 9.52 | 5.9 | 12.50 | 9.5 | |
| Unvaccinated | 22.4 | 25.0 | 21.9 | 26.2 | 29.9 | 25.8 | 27.5 | 20.7 | 24.5 | 23.8 | 23.5 | 25.0 | 24.8 | |
| Do not know | 36.3 | 37.5 | 35.3 | 40.5 | 37.6 | 37.1 | 31.9 | 43.1 | 47.2 | 33.3 | 41.2 | 37.5 | 37.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degarege, A.; Watanabe-Galloway, S.; Mansilla, K.; Sileshi, R.M.; Peters, E.S. Evaluation of Theoretical Frameworks to Detect Correlates of HPV Vaccination in the Midwest, US, Using Structural Equation Modeling. Vaccines 2023, 11, 1856. https://doi.org/10.3390/vaccines11121856
Degarege A, Watanabe-Galloway S, Mansilla K, Sileshi RM, Peters ES. Evaluation of Theoretical Frameworks to Detect Correlates of HPV Vaccination in the Midwest, US, Using Structural Equation Modeling. Vaccines. 2023; 11(12):1856. https://doi.org/10.3390/vaccines11121856
Chicago/Turabian StyleDegarege, Abraham, Shinobu Watanabe-Galloway, Kristyne Mansilla, Rahel M. Sileshi, and Edward S. Peters. 2023. "Evaluation of Theoretical Frameworks to Detect Correlates of HPV Vaccination in the Midwest, US, Using Structural Equation Modeling" Vaccines 11, no. 12: 1856. https://doi.org/10.3390/vaccines11121856
APA StyleDegarege, A., Watanabe-Galloway, S., Mansilla, K., Sileshi, R. M., & Peters, E. S. (2023). Evaluation of Theoretical Frameworks to Detect Correlates of HPV Vaccination in the Midwest, US, Using Structural Equation Modeling. Vaccines, 11(12), 1856. https://doi.org/10.3390/vaccines11121856






