The Association of Bacterin and Recombinant Proteins Induces a Humoral Response in Sheep against Caseous Lymphadenitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Production of Recombinant Proteins
2.3. Experimental Design
2.4. Evaluation of the Humoral Response
2.5. Evaluation of the Cellular Immune Response
2.6. Statistical Analysis
3. Results
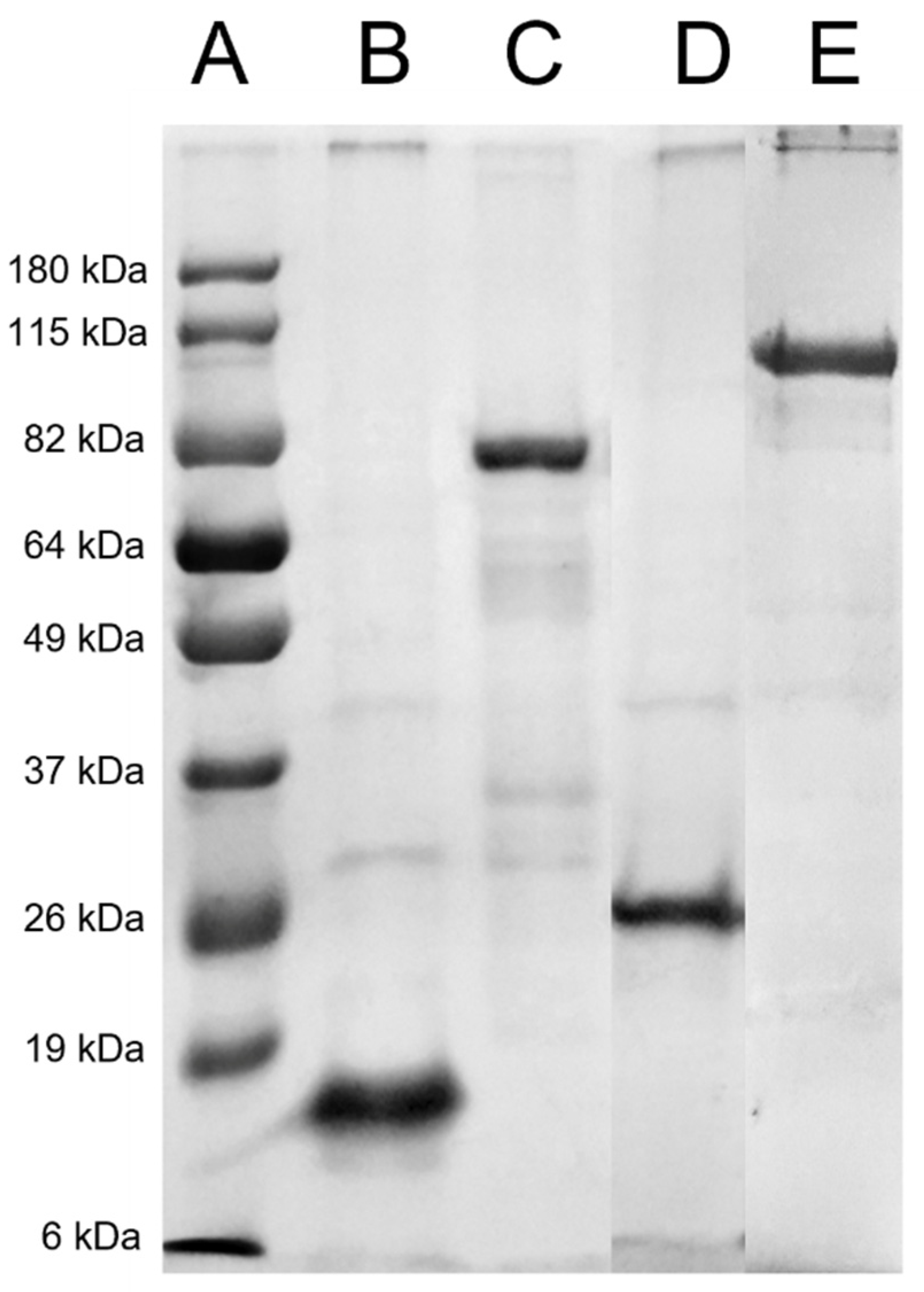
3.1. Recombinant Protein Production
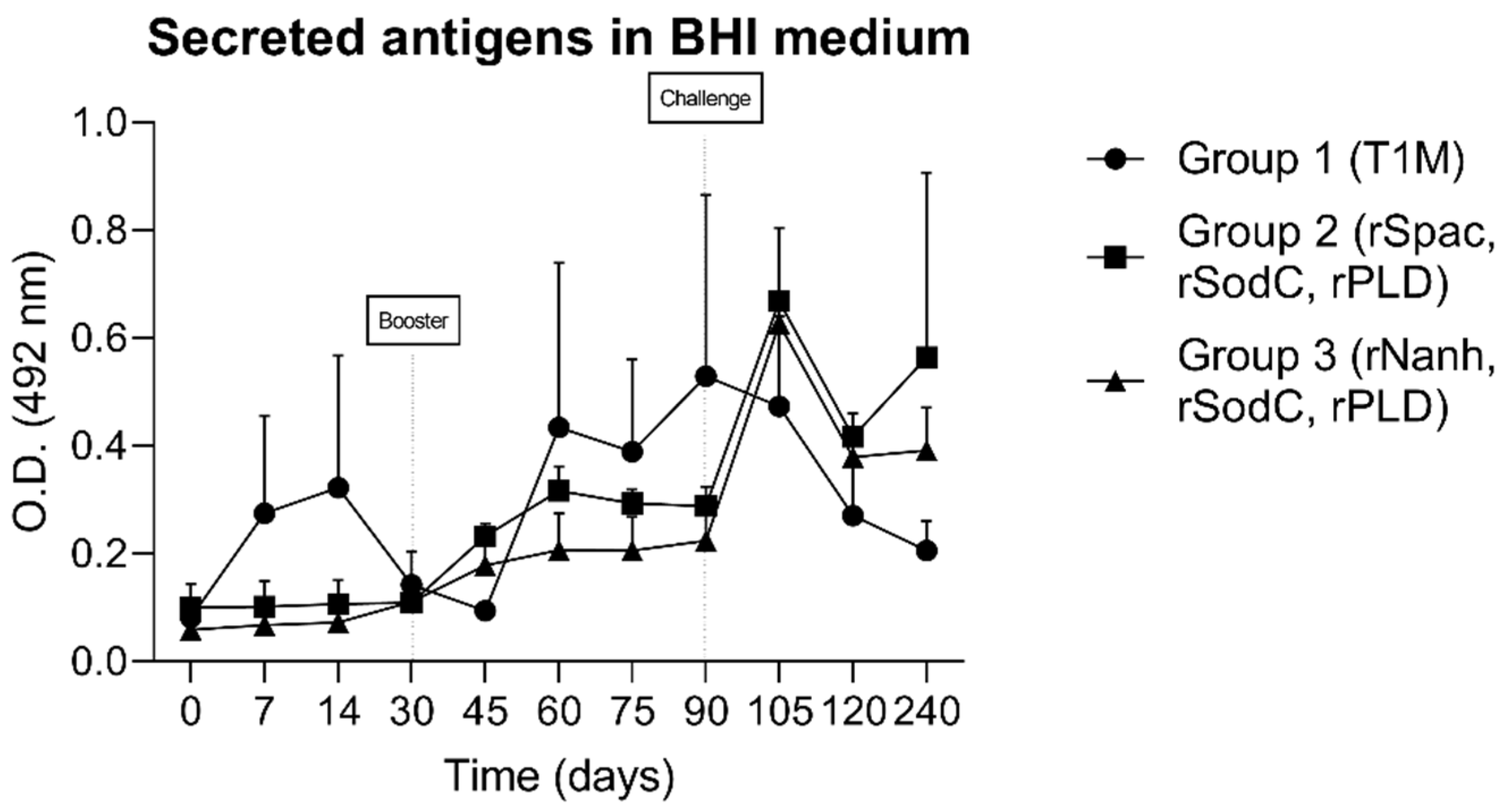
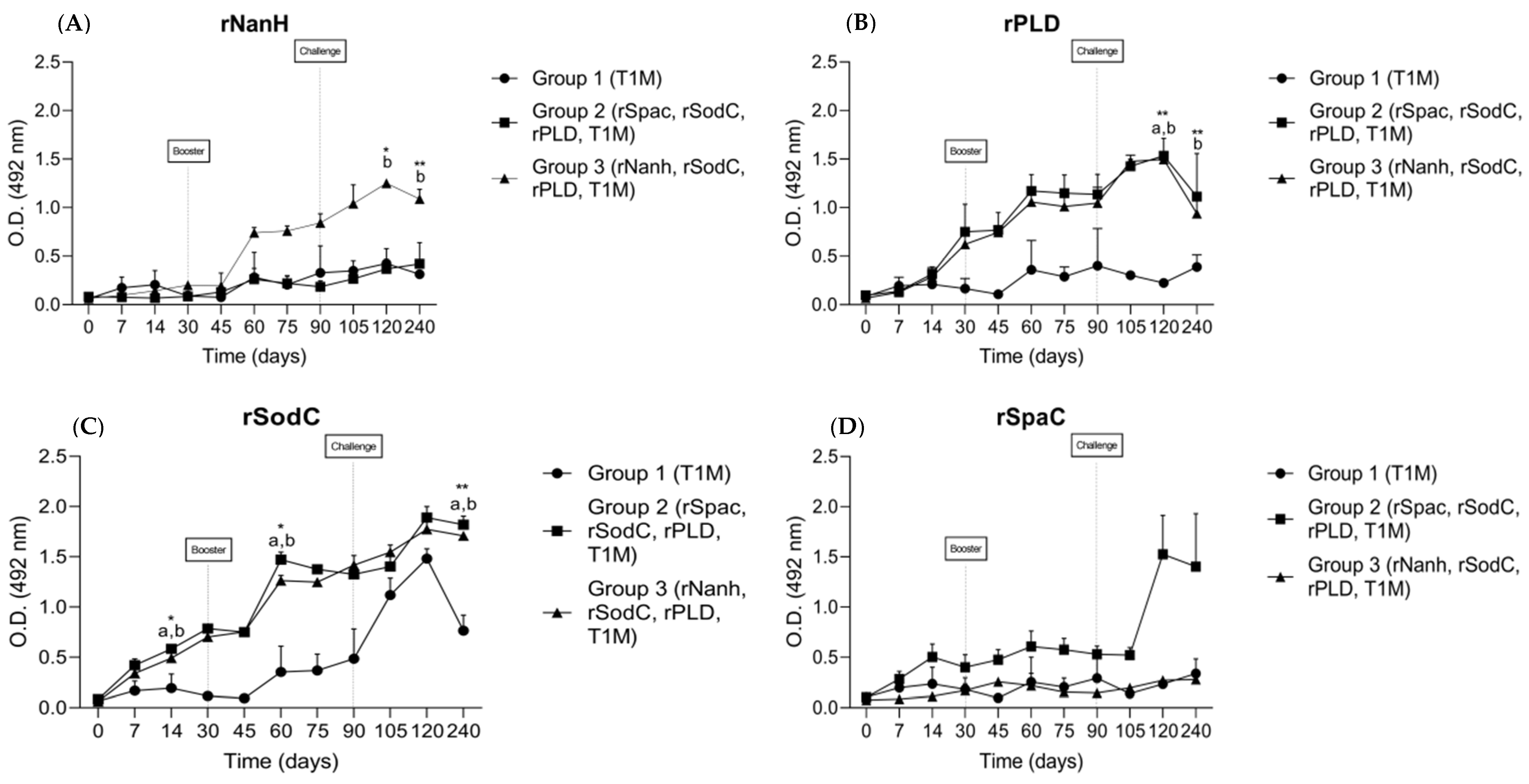
3.2. Humoral Immune Response
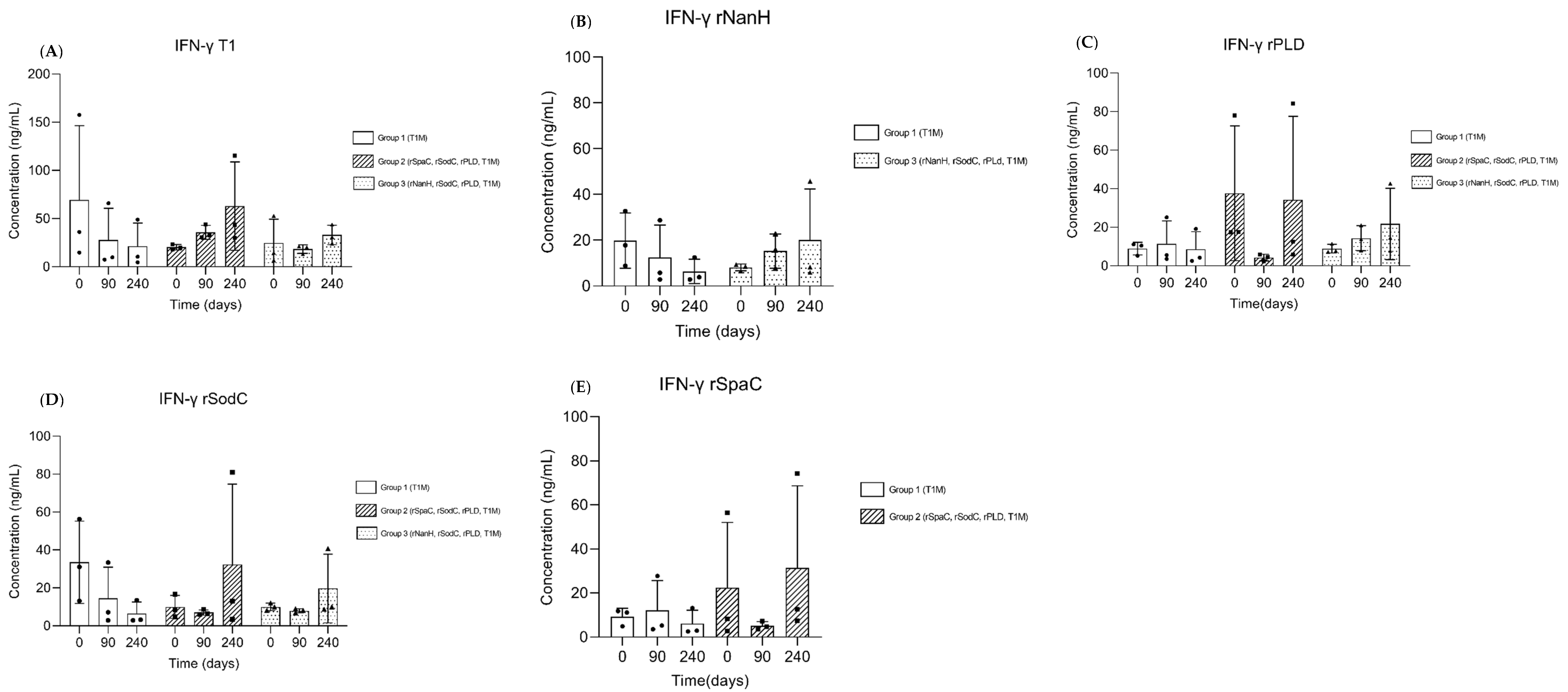
3.3. Cellular Immune Response
3.4. Post-Mortem Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zavoshti, F.R.; Khoojine, A.B.S.; Helan, J.A.; Hassanzadeh, B.; Heydari, A.A. Frequency of caseous lymphadenitis (CLA) in sheep slaughtered in an abattoir in Tabriz: Comparison of bacterial culture and pathological study. Comp. Clin. Pathol. 2012, 21, 667–671. [Google Scholar] [CrossRef]
- Chikhaoui, M.; Khoudja, F.B. Clinico pathological investigation on caseous lymphadenitis in local breed sheep in Algeria. Trop. Anim. Health Prod. 2013, 45, 1641–1643. [Google Scholar] [CrossRef] [PubMed]
- Jesse, F.F.A.; Odhah, M.N.; Abba, Y.; Garba, B.; Mahmood, Z.; Hambali, I.U.; Haron, A.W.; Lila, M.-A.M.; Zamri-Saad, M. Responses of female reproductive hormones and histopathology in the reproductive organs and associated lymph nodes of Boer does challenged with Corynebacterium pseudotuberculosis and its immunogenic corynomycolic acid extract. Microb. Pathog. 2020, 139, 103852. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.R.A.; de Farias, A.E.M.; dos Anjos, D.M.; Lima, A.M.C.; Faccioli-Martins, P.Y.; de Souza, C.J.H.; Pinheiro, R.R.; Alves, F.S.F.; de Azevedo, S.S.; Alves, C.J. Seroepidemiological study of Caseous lymphadenitis in sheep from the Northeast region of Brazil using an indirect ELISA. Trop. Anim. Health Prod. 2020, 52, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Seyffert, N.; Guimarães, A.; Pacheco, L.; Portela, R.; Bastos, B.; Dorella, F.; Heinemann, M.; Lage, A.; Gouveia, A.; Meyer, R.; et al. High seroprevalence of caseous lymphadenitis in Brazilian goat herds revealed by Corynebacterium pseudotuberculosis secreted proteins-based ELISA. Res. Vet. Sci. 2010, 88, 50–55. [Google Scholar] [CrossRef] [PubMed]
- de Farias, A.E.M.; Alves, J.R.A.; Alves, F.S.F.; Pinheiro, R.R.; Faccioli-Martins, P.Y.; Lima, A.M.C.; de Azevedo, S.S.; Alves, C.J. Seroepidemiological characterization and risk factors associated with seroconversion to Corynebacterium pseudotuberculosisin goats from Northeastern Brazil. Trop. Anim. Health Prod. 2019, 51, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.; Sentance, C.; Spooncer, W.; Balan, J.; Morris, S. Inspection of lymph nodes for caseous lymphadenitis and its effect on the density of microbes on sheep carcasses. Meat Sci. 2012, 92, 837–840. [Google Scholar] [CrossRef]
- Oreiby, A.F.; Hegazy, Y.M.; Osman, S.A.; Ghanem, Y.M.; Al-Gaabary, M.H. Caseous lymphadenitis in small ruminants in Egypt: Clinical, epidemiological and prophylactic aspects. Tierärztl. Prax. Ausg. G GroßtiereNutztiere 2014, 42, 271–277. [Google Scholar] [CrossRef]
- Galvão, C.E.; Fragoso, S.P.; de Oliveira, C.E.; Forner, O.; Pereira, R.R.B.; Soares, C.O.; Rosinha, G.M.S. Identification of new Corynebacterium pseudotuberculosis antigens by immunoscreening of gene expression library. BMC Microbiol. 2017, 17, 202. [Google Scholar] [CrossRef]
- de Pinho, R.B.; Silva, M.T.D.O.; Brenner, G.; Alves, M.S.D.; Azevedo, V.; Portela, R.D.; Borsuk, S. A novel approach for an immunogen against Corynebacterium pseudotuberculosis infection: An Escherichia coli bacterin expressing phospholipase D. Microb. Pathog. 2021, 151, 104746. [Google Scholar] [CrossRef]
- Droppa-Almeida, D.; Vivas, W.L.; Silva, K.K.O.; Rezende, A.F.; Simionatto, S.; Meyer, R.; Lima-Verde, I.B.; Delagostin, O.; Borsuk, S.; Padilha, F.F. Recombinant CP40 from Corynebacterium pseudotuberculosis confers protection in mice after challenge with a virulent strain. Vaccine 2016, 34, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.T.D.O.; de Pinho, R.B.; Fonseca, B.D.R.; Bezerra, F.S.B.; Sousa, F.S.S.; Seixas, F.K.; Collares, T.; Nascimento, R.J.M.; Portela, R.W.; Azevedo, V.A.C.; et al. NanH and PknG putative virulence factors as a recombinant subunit immunogen against Corynebacterium pseudotuberculosis infection in mice. Vaccine 2020, 38, 8099–8106. [Google Scholar] [CrossRef]
- Santana-Jorge, K.T.O.; Santos, T.M.; Tartaglia, N.R.; Aguiar, E.L.; Souza, R.F.S.; Mariutti, R.B.; Eberle, R.J.; Arni, R.K.; Portela, R.W.; Meyer, R.; et al. Putative virulence factors of Corynebacterium pseudotuberculosis FRC41: Vaccine potential and protein expression. Microb. Cell Factories 2016, 15, 83. [Google Scholar] [CrossRef]
- de Pinho, R.B.; Silva, M.T.D.O.; Bezerra, F.S.B.; Borsuk, S. Vaccines for caseous lymphadenitis: Up-to-date and forward-looking strategies. Appl. Microbiol. Biotechnol. 2021, 105, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Vale, V.L.C.; Silva, M.D.C.; De Souza, A.P.; Trindade, S.C.; De Moura-Costa, L.F.; Dos Santos-Lima, E.K.N.; Nascimento, I.L.D.O.; Cardoso, H.S.P.; Marques, E.D.J.; Paule, B.J.A.; et al. Humoral and cellular immune responses in mice against secreted and somatic antigens from a Corynebacterium pseudotuberculosis attenuated strain: Immune response against a C. pseudotuberculosis strain. BMC Vet. Res. 2016, 12, 195. [Google Scholar] [CrossRef]
- de Sá, M.C.A.; da Silva, W.M.; Rodrigues, C.C.S.; Rezende, C.P.; Marchioro, S.B.; Filho, J.T.R.R.; Sousa, T.D.J.; de Oliveira, H.P.; da Costa, M.M.; Figueiredo, H.C.P.; et al. Comparative Proteomic Analyses Between Biofilm-Forming and Non-biofilm-Forming Strains of Corynebacterium pseudotuberculosis Isolated From Goats. Front. Vet. Sci. 2021, 8, 614011. [Google Scholar] [CrossRef]
- Rebouças, M.F.; Loureiro, D.; Bastos, B.; Moura-Costa, L.F.; Hanna, S.A.; Azevedo, V.; Meyer, R.; Portela, R.W. Development of an indirect ELISA to detect Corynebacterium pseudotuberculosis specific antibodies in sheep employing T1 strain culture supernatant as antigen. Pesqui. Vet. Bras. 2013, 33, 1296–1302. [Google Scholar] [CrossRef]
- McKean, S.C.; Davies, J.K.; Moore, R.J. Expression of phospholipase D, the major virulence factor of Corynebacterium pseudotuberculosis, is regulated by multiple environmental factors and plays a role in macrophage death. Microbiology 2007, 153, 2203–2211. [Google Scholar] [CrossRef]
- Nascimento, I.P.; Leite, L.C.C. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 2012, 45, 1102–1111. [Google Scholar] [CrossRef]
- Wu, H.-C.; Yeh, P.-H.; Hsueh, K.-J.; Yang, W.-J.; Chu, C.-Y. Recombinant ApxIV protein enhances protective efficacy against Actinobacilluspleuropneumoniae in mice and pigs. J. Appl. Microbiol. 2018, 124, 1366–1376. [Google Scholar] [CrossRef]
- Rosa, M.C.; Conrad, N.L.; Moraes, C.M.; Leite, F.P.L. Immunogenicity of Streptococcus equi subsp. equi recombinant SeM protein and bacterin in mice. Pesqui. Vet. Bras. 2021, 41, e06910. [Google Scholar] [CrossRef]
- Hsueh, K.-J.; Cheng, L.-T.; Lee, J.-W.; Chung, Y.-C.; Chung, W.-B.; Chu, C.-Y. Immunization with Streptococcus suis bacterin plus recombinant Sao protein in sows conveys passive immunity to their piglets. BMC Vet. Res. 2017, 13, 15. [Google Scholar] [CrossRef][Green Version]
- Khorasani, A.; Madadgar, O.; Soleimanjahi, H.; Keyvanfar, H.; Mahravani, H. Evaluation of the efficacy of a new oil-based adjuvant ISA 61 VG FMD vaccine as a potential vaccine for cattle. Iran. J. Vet. Res. 2016, 17, 8–12. [Google Scholar] [CrossRef]
- Zafra, R.; Buffoni, L.; Pérez-Caballero, R.; Molina-Hernández, V.; Ruiz-Campillo, M.T.; Pérez, J.; Martínez-Moreno, Á.; Moreno, F.J.M. Efficacy of a multivalent vaccine against Fasciola hepatica infection in sheep. Vet. Res. 2021, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dorella, F.A.; Pacheco, L.G.; Seyffert, N.; Portela, R.W.; Meyer, R.; Miyoshi, A.; Azevedo, V. Antigens of Corynebacterium pseudotuberculosis and prospects for vaccine development. Expert Rev. Vaccines 2009, 8, 205–213. [Google Scholar] [CrossRef]
- Corrêa, J.I.; Stocker, A.; Trindade, S.C.; Vale, V.; Brito, T.; Bastos, B.; Raynal, J.T.; de Miranda, P.M.; de Alcantara, A.C.; Freire, S.M.; et al. In vivo and in vitro expression of five genes involved in Corynebacterium pseudotuberculosis virulence. AMB Express 2018, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Lopes Bastos, B.; Dias Portela, R.W.; Alves Dorella, F.; Ribeiro, D.; Seyffert, N.; de Paula Castro, T.L.; Miyoshi, A.; Costa Oliveira, S.; Meyer, S.; Azevedo, V. Corynebacterium pseudotuberculosis: Immunological Responses in Animal Models and Zoonotic Potential. J. Clin. Cell. Immunol. 2012, S4, 5. [Google Scholar] [CrossRef]
- Paule, B.J.A.; Azevedo, V.; Regis, L.F.; Carminati, R.; Bahia, C.R.; Vale, V.L.C.; Moura-Costa, L.F.; Freire, S.M.; Nascimento, I.; Schaer, R.; et al. Experimental Corynebacterium pseudotuberculosis primary infection in goats: Kinetics of IgG and interferon-γ production, IgG avidity and antigen recognition by Western blotting. Vet. Immunol. Immunopathol. 2003, 96, 129–139. [Google Scholar] [CrossRef]
- Bezerra, F.S.B.; Silva, M.T.D.O.; Rezende, A.D.F.S.; Lopes, A.S.; de Pinho, R.B.; Seixas, F.K.; Collares, T.V.; Portela, R.W.D.; Azevedo, V.A.D.C.; Borsuk, S. Saponin-adjuvanted recombinant vaccines containing rCP00660, rCP09720 or rCP01850 proteins against Corynebacterium pseudotuberculosis infection in mice. Vaccine 2021, 39, 2568–2574. [Google Scholar] [CrossRef]
- Bezerra, F.S.B.; Rezende, A.D.F.S.; Silva, M.T.D.O.; Sena-Lopes, Â.; Roesch-Ely, M.; Henriques, J.A.P.; Padilha, F.F.; Azevedo, V.A.C.; Portela, R.W.D.; Seixas, F.K.; et al. The combination of Brazilian red propolis and recombinant protein rCP01850 in the immunoprophylaxis of Corynebacterium pseudotuberculosis infection in mice. Microb. Pathog. 2020, 149, 104354. [Google Scholar] [CrossRef]
- Silva, M.T.D.O.; Bezerra, F.; de Pinho, R.B.; Begnini, K.R.; Seixas, F.K.; Collares, T.; Portela, R.; Azevedo, V.; Dellagostin, O.; Borsuk, S. Association of Corynebacterium pseudotuberculosis recombinant proteins rCP09720 or rCP01850 with rPLD as immunogens in caseous lymphadenitis immunoprophylaxis. Vaccine 2018, 36, 74–83. [Google Scholar] [CrossRef] [PubMed]
| Group | N | D 0 b | D 30 | D 90 | D 240 |
|---|---|---|---|---|---|
| Group 1 (T1M a) | 3 | First dose c | Booster c | Challenge infection | Euthanasia |
| Group 2 (rSpaC + rSodC + rPLD + T1M) | 3 | First dose | Booster | Challenge infection | Euthanasia |
| Group 3 (rNanH + rSodC + rPLD + T1M) | 3 | First dose | Booster | Challenge infection | Euthanasia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, L.S.; Lopes, N.d.R.; Pereira, V.C.; Andrade, C.L.B.; Torres, A.J.L.; Ribeiro, M.B.; Freire, S.M.; Santos, R.M.d.; D’ávila, M.; Nascimento, R.M.; et al. The Association of Bacterin and Recombinant Proteins Induces a Humoral Response in Sheep against Caseous Lymphadenitis. Vaccines 2022, 10, 1406. https://doi.org/10.3390/vaccines10091406
Moreira LS, Lopes NdR, Pereira VC, Andrade CLB, Torres AJL, Ribeiro MB, Freire SM, Santos RMd, D’ávila M, Nascimento RM, et al. The Association of Bacterin and Recombinant Proteins Induces a Humoral Response in Sheep against Caseous Lymphadenitis. Vaccines. 2022; 10(9):1406. https://doi.org/10.3390/vaccines10091406
Chicago/Turabian StyleMoreira, Luan Santana, Natália da Rocha Lopes, Vitor Cordeiro Pereira, Caio Lopes Borges Andrade, Alex José Leite Torres, Marcos Borges Ribeiro, Songeli Menezes Freire, Ramon Mendes dos Santos, Milena D’ávila, Roberto Meyer Nascimento, and et al. 2022. "The Association of Bacterin and Recombinant Proteins Induces a Humoral Response in Sheep against Caseous Lymphadenitis" Vaccines 10, no. 9: 1406. https://doi.org/10.3390/vaccines10091406
APA StyleMoreira, L. S., Lopes, N. d. R., Pereira, V. C., Andrade, C. L. B., Torres, A. J. L., Ribeiro, M. B., Freire, S. M., Santos, R. M. d., D’ávila, M., Nascimento, R. M., & Marchioro, S. B. (2022). The Association of Bacterin and Recombinant Proteins Induces a Humoral Response in Sheep against Caseous Lymphadenitis. Vaccines, 10(9), 1406. https://doi.org/10.3390/vaccines10091406






