Vaccination with Deglycosylated Modified Hemagglutinin Broadly Protects against Influenza Virus Infection in Mice and Ferrets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Virus
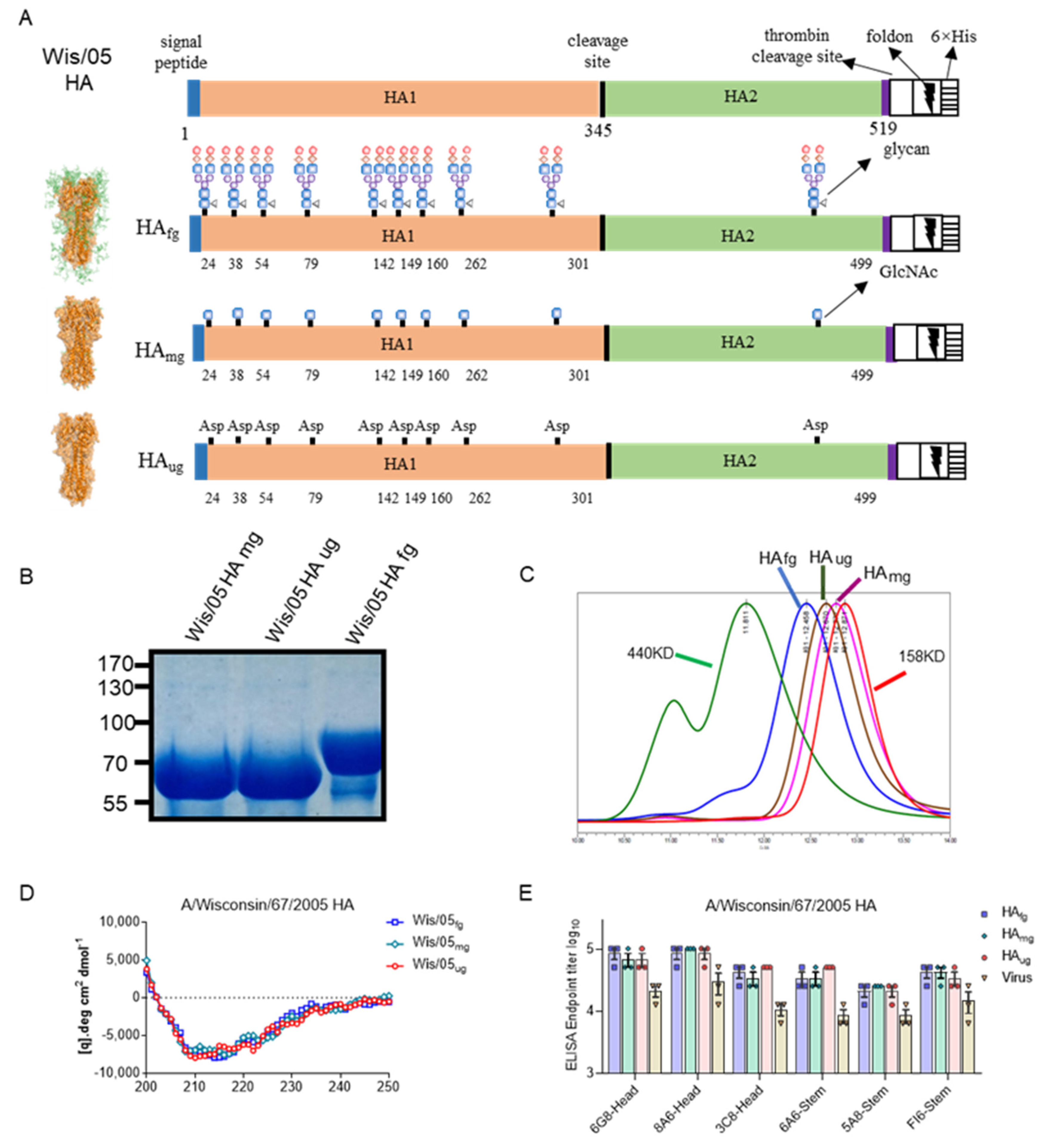
2.2. Construction, Expression and Purification of HA Protein
2.3. Vaccinations and Challenges
2.4. Serological Analysis
2.5. ADCC and Complement Dependent Cytotoxicity (CDC) Assay
2.6. Statistical Analyses
3. Results
3.1. Deglycosylated Modified HA Proteins Treated with Different Glycosidases Could Retain Structural Integrity and Primary Epitope Conformations
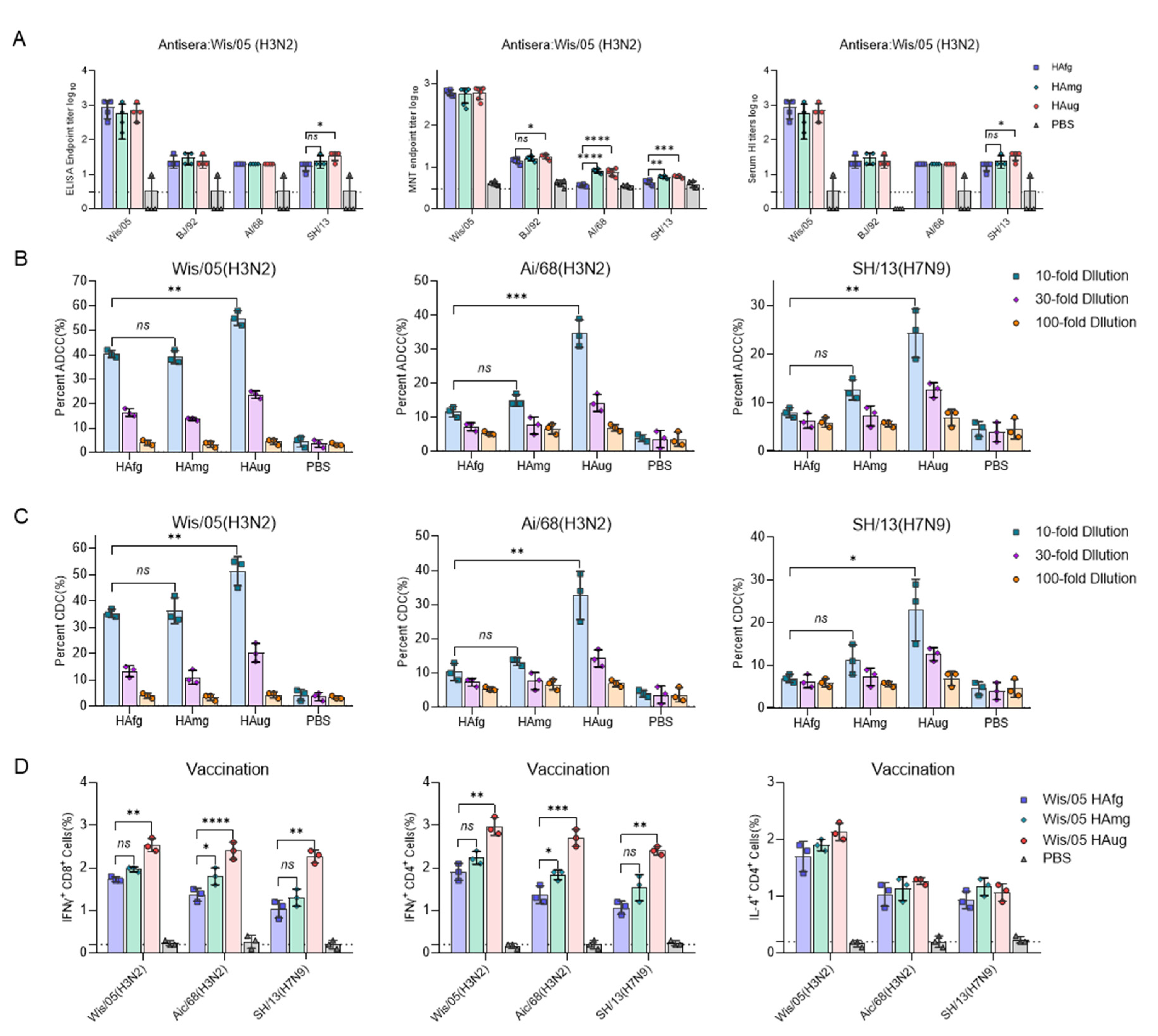
3.2. Vaccination of Mice with Deglycosylated Modified HA Elicits a Strong T-Cell Response and Antibody-Dependent Effector Functions against H3 and H7 Viruses
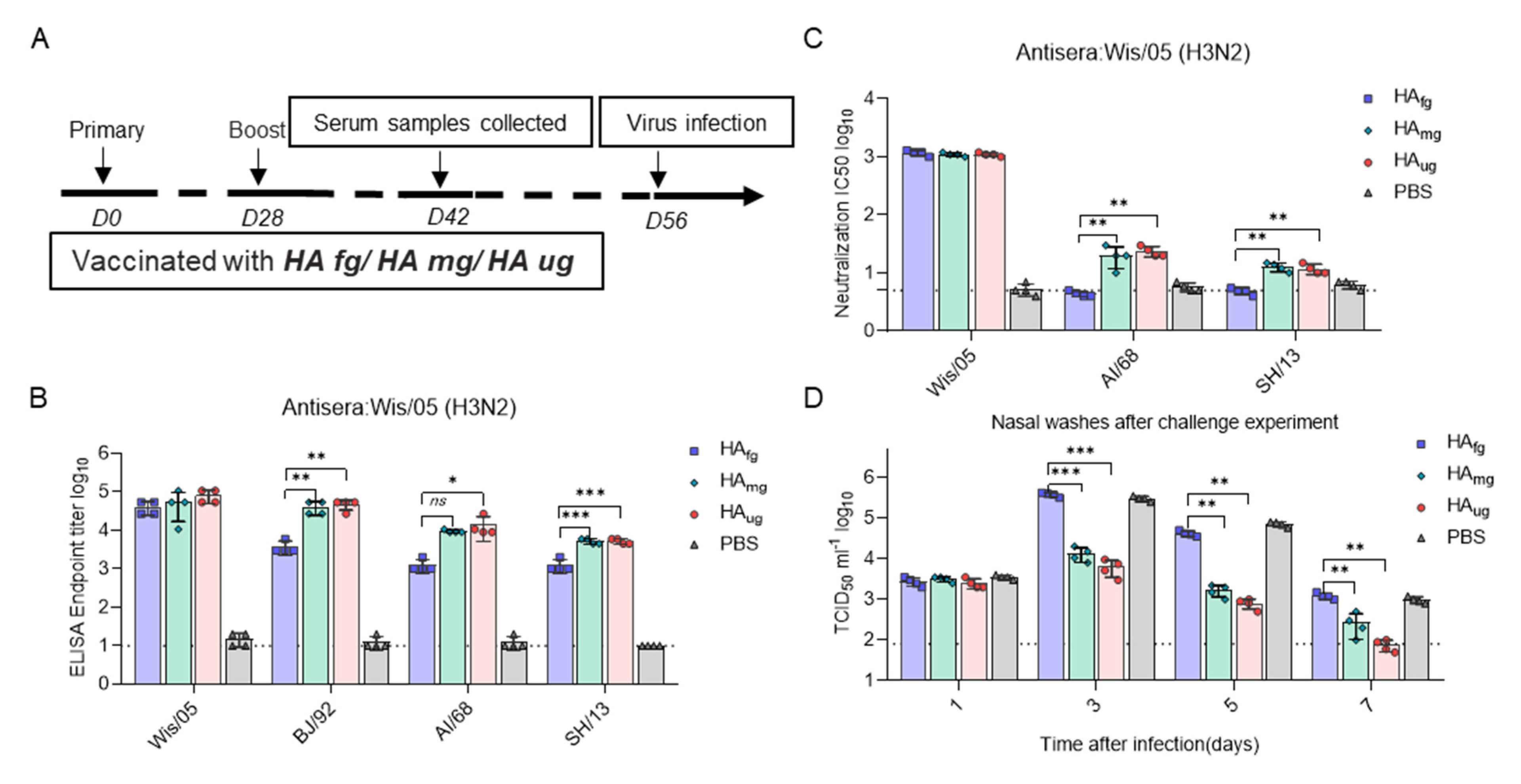
3.3. Deglycosylated Modified HAs as Vaccine Candidates Have Broad-Spectrum Antiviral Activity against Homotypic and Heterosubtypic Influenza A Virus in Mice
3.4. Glycosidase-Treated HA Vaccines Induce Protective Immunity against Influenza Virus Infection in Ferrets
4. Discussion
5. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belongia, E.A.; Simpson, M.D.; King, J.P.; Sundaram, M.E.; Kelley, N.S.; Osterholm, M.T.; Mclean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Stavros, S.; Asimakopoulos, G.; Pergialiotis, V.; Raftopoulos, V.; Talias, M.A.; Pavli, A.; Daskalakis, G.; Sindos, M.; Koutroumanis, P.; et al. Effectiveness of maternal vaccination with quadrivalent inactivated influenza vaccine in pregnant women and their infants in 2019–2020. Expert Rev. Vaccines 2022, 21, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.; Pleschka, S. Influenza H3N2 Vaccines: Recent Challenges. Trends Microbiol. 2018, 26, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Ma, C.; Wong, C.H. Vaccine design of hemagglutinin glycoprotein against influenza. Trends Biotechnol. 2011, 29, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Vanovic, T.; Harrison, S.C. Distinct functional determinants of influenza hemagglutinin-mediated membrane fusion. Elife 2015, 4, e11009. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Zhang, H.; Liu, G.D.; Xue, C.; Cao, Y. Targeting Hemagglutinin: Approaches for Broad Protection against the Influenza A Virus. Viruses 2019, 11, 405. [Google Scholar] [CrossRef]
- Shen, C.; Chen, J.; Li, R.; Zhang, M.; Wang, G.; Stegalkina, S.; Zhang, L.; Chen, J.; Cao, J.; Bi, X.; et al. A multimechanistic antibody targeting the receptor binding site potently cross-protects against influenza B viruses. Sci. Transl. Med. 2017, 9, eaam5752. [Google Scholar] [CrossRef]
- Altman, M.O.; Angel, M.; Kosik, I.; Trovão, N.S.; Zost, S.J.; Gibbs, J.S.; Casalino, L.; Amaro, R.E.; Hensley, S.E.; Nelson, M.I.; et al. Human Influenza A Virus Hemagglutinin Glycan Evolution Follows a Temporal Pattern to a Glycan Limit. Mbio 2019, 10, e00204-19. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Palese, P. Is a Universal Influenza Virus Vaccine Possible? Annu. Rev. Med. 2020, 71, 315–327. [Google Scholar] [CrossRef]
- Saito, M.; Itoh, Y.; Yasui, F.; Munakata, T.; Yamane, D.; Ozawa, M.; Ito, R.; Katoh, T.; Ishigaki, H.; Nakayama, M.; et al. Macrocyclic peptides exhibit antiviral effects against influenza virus HA and prevent pneumonia in animal models. Nat. Commun. 2021, 12, 2654. [Google Scholar] [CrossRef]
- Corbett, K.S.; Moin, S.M.; Yassine, H.M.; Cagigi, A.; Kanekiyo, M.; Boyoglu-Barnum, S.; Myers, I.S.; Tsybovsky, Y.; Wheatley, K.A.; Schramm, A.C.; et al. Design of Nanoparticulate Group 2 Influenza Virus Hemagglutinin Stem Antigens That Activate Unmutated Ancestor B Cell Receptors of Broadly Neutralizing Antibody Lineages. Mbio 2019, 10, e02810–e02818. [Google Scholar] [CrossRef]
- Liu, W.C.; Jan, J.T.; Huang, Y.J.; Chen, T.H.; Wu, A.C. Unmasking Stem-Specific Neutralizing Epitopes by Abolishing N-Linked Glycosylation Sites of Influenza Virus Hemagglutinin Proteins for Vaccine Design. J. Virol. 2016, 90, 8496–8508. [Google Scholar] [CrossRef] [PubMed]
- Pica, N.; Hai, R.; Krammer, F.; Wang, T.T.; Maamary, J.; Eggink, D.; Tan, G.S.; Krause, J.C.; Moran, T.; Stein, C.R.; et al. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc. Natl. Acad. Sci. USA 2012, 109, 2573–2578. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.C.; Wilson, I.A. Structural Biology of Influenza Hemagglutinin: An Amaranthine Adventure. Viruses 2020, 12, 1053. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.G.; Crocker, P.R. Glycoimmunology: Ignore at your peril! Immunol. Rev. 2009, 230, 5–8. [Google Scholar] [CrossRef]
- Alymova, I.V.; York, I.A.; Air, G.M.; Cipollo, J.F.; Gulati, S.; Baranovich, T.; Kumar, A.; Zeng, H.; Gansebom, H.; McCullers, J.A. Glycosylation changes in the globular head of H3N2 influenza hemagglutinin modulate receptor binding without affecting virus virulence Irina. Sci. Rep. 2016, 6, 36216. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Suzuki, Y. Evidence for N-Glycan Shielding of Antigenic Sites during Evolution of Human Influenza A Virus Hemagglutinin. J. Virol. 2012, 86, 3446–3451. [Google Scholar] [CrossRef]
- Thornlow, D.N.; Macintyre, A.N.; Oguin, T.H.; Karlsson, A.B.; Stover, E.L.; Lynch, H.E.; Sempowski, G.D.; Schmidt, A.G. Altering the Immunogenicity of Hemagglutinin Immunogens by Hyperglycosylation and Disulfide Stabilization. Front. Immunol. 2021, 12, 737973. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, J.R.; Tseng, Y.C.; Wong, C.H. Glycans on influenza hemagglutinin affect receptor binding and immune response. Proc. Natl. Acad. Sci. USA 2009, 106, 18137–18142. [Google Scholar] [CrossRef]
- Chen, J.R.; Yu, Y.H.; Tseng, Y.C.; Chiang, W.L.; Chiang, M.F.; Ko, Y.A.; Chiu, Y.K.; Ma, H.H.; Wu, C.Y.; Jan, J.T.; et al. Vaccination of monoglycosylated hemagglutinin induces cross-strain protection against influenza virus infections. Proc. Natl. Acad. Sci. USA 2014, 111, 2476–2481. [Google Scholar] [CrossRef]
- Das, S.R.; Puigbo, P.; Hensley, S.E.; Hurt, D.E.; Bennink, J.R.; Yewdell, J.W. Glycosylation focuses sequence variation in the influenza A virus H1 hemagglutinin globular domain. PLoS Pathog. 2010, 6, e1001211. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Heuer, D.; Wolff, T.; Herwig, A.; Klenk, H.D. N-Glycans attached to the stem domain of haemagglutinin efficiently regulate influenza A virus replication. J. Gen. Virol. 2002, 83 Pt 3, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Wan, Y.M.; Qiu, C.L.; Quiñones-Parra, S.; Zhu, Z.; Loh, L.; Tian, D.; Ren, Y.; Hu, Y.; Zhang, X.; et al. Recovery from severe H7N9 disease is associated with diverse response mechanisms dominated by CD8(+) T cells. Nat. Commun. 2015, 6, 6833. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.M.; Li, C.K.; Chui, C.S.; Huang, A.Y.K.; Perkins, M.; Liebner, J.C.; Lambkin-Williams, R.; Gilbert, A.; Oxford, J.; Nicholas, B.; et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012, 18, 274–280. [Google Scholar] [CrossRef]
- Farrukee, R.; Tai, C.M.K.; Oh, D.Y.; Anderson, D.E.; Gunalan, V.; Hibberd, M.; Lau, G.Y.K.; Barr, I.G.; von Messling, V.; Maurer-Stroh, S.; et al. Utilising animal models to evaluate oseltamivir efficacy against influenza A and B viruses with reduced in vitro susceptibility. PLoS Pathog. 2020, 16, e1008592. [Google Scholar] [CrossRef]
- Maher, J.A.; DeStefano, J. The ferret: An animal model to study influenza virus. Lab Anim. 2004, 33, 50–53. [Google Scholar] [CrossRef]
- O’Donnell, C.D.; Subbarao, K. The contribution of animal models to the understanding of the host range and virulence of influenza A viruses. Microbes Infect. 2011, 13, 502–515. [Google Scholar] [CrossRef]
- Boni, M.F. Vaccination and antigenic drift in influenza. Vaccine 2008, 26 (Suppl. S3), C8–C14. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Kondor, R.J.G.; Chung, J.R.; Zimmerman, R.K.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; et al. Effect of Antigenic Drift on Influenza Vaccine Effectiveness in the United States-2019–2020. Clin. Infect. Dis. 2021, 73, e4244–e4250. [Google Scholar] [CrossRef]
- Schulze, I.T. Effects of glycosylation on the properties and functions of influenza virus hemagglutinin. J. Infect. Dis. 1997, 176 (Suppl. S1), S24–S28. [Google Scholar] [CrossRef]
- Eggink, D.; Goff, P.H.; Palese, P. Guiding the immune response against influenza virus hemagglutini toward the conserved stalk domain by hyperglycosylation of the globular head domain. J. Virol. 2014, 88, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, V.; Kane, R.S. Glycosylation as a tool for rational vaccine design. Biotechnol. Bioeng. 2020, 117, 2556–2570. [Google Scholar] [CrossRef]
- Chen, J.; Wang, P.; Yuan, L.; Zhang, L.; Zhao, H.; Chen, C.; Wang, X.; Han, J.; Chen, Y.; Jia, J.; et al. A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2. Sci. Bull. 2022, 67, 1372–1387. [Google Scholar] [CrossRef]
- De Vries, R.D.; Nieuwkoop, N.J.; Krammer, F.; Hu, B.; Rimmelzwaan, G.F. Analysis of the vaccine-induced influenza B virus hemagglutinin-specific antibody dependent cellular cytotoxicity response. Virus Res. 2020, 277, 197839. [Google Scholar] [CrossRef] [PubMed]
- De Taeye, S.W.; Bentlage, A.E.H.; Mebius, M.M.; Meesters, J.I.; Lissenberg-Thunnissen, S.; Falck, D.; Sénard, T.; Saleh, N.; Wuhrer, M.; Schuurman, J.; et al. FcgammaR Binding and ADCC Activity of Human IgG Allotypes. Front. Immunol. 2020, 11, 740. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Chen, J.; Shen, C.; Wang, G.; Lu, Z.; Zeng, D.; Gao, Y.; Chen, H.; Xia, N.; Chen, Y. Vaccination with Deglycosylated Modified Hemagglutinin Broadly Protects against Influenza Virus Infection in Mice and Ferrets. Vaccines 2022, 10, 1304. https://doi.org/10.3390/vaccines10081304
Zhang L, Chen J, Shen C, Wang G, Lu Z, Zeng D, Gao Y, Chen H, Xia N, Chen Y. Vaccination with Deglycosylated Modified Hemagglutinin Broadly Protects against Influenza Virus Infection in Mice and Ferrets. Vaccines. 2022; 10(8):1304. https://doi.org/10.3390/vaccines10081304
Chicago/Turabian StyleZhang, Limin, Junyu Chen, Chenguang Shen, Guosong Wang, Zhen Lu, Dian Zeng, Ying Gao, Huiqing Chen, Ningshao Xia, and Yixin Chen. 2022. "Vaccination with Deglycosylated Modified Hemagglutinin Broadly Protects against Influenza Virus Infection in Mice and Ferrets" Vaccines 10, no. 8: 1304. https://doi.org/10.3390/vaccines10081304
APA StyleZhang, L., Chen, J., Shen, C., Wang, G., Lu, Z., Zeng, D., Gao, Y., Chen, H., Xia, N., & Chen, Y. (2022). Vaccination with Deglycosylated Modified Hemagglutinin Broadly Protects against Influenza Virus Infection in Mice and Ferrets. Vaccines, 10(8), 1304. https://doi.org/10.3390/vaccines10081304





