Peptide-Based Vaccines in Clinical Phases and New Potential Therapeutic Targets as a New Approach for Breast Cancer: A Review
Abstract
1. Introduction
2. Breast Cancer
2.1. Types of Breast Cancer
2.2. Treatment of Breast Cancer
3. Vaccines
3.1. Vaccines against Cancer
3.2. Types of Vaccines against Breast Cancer
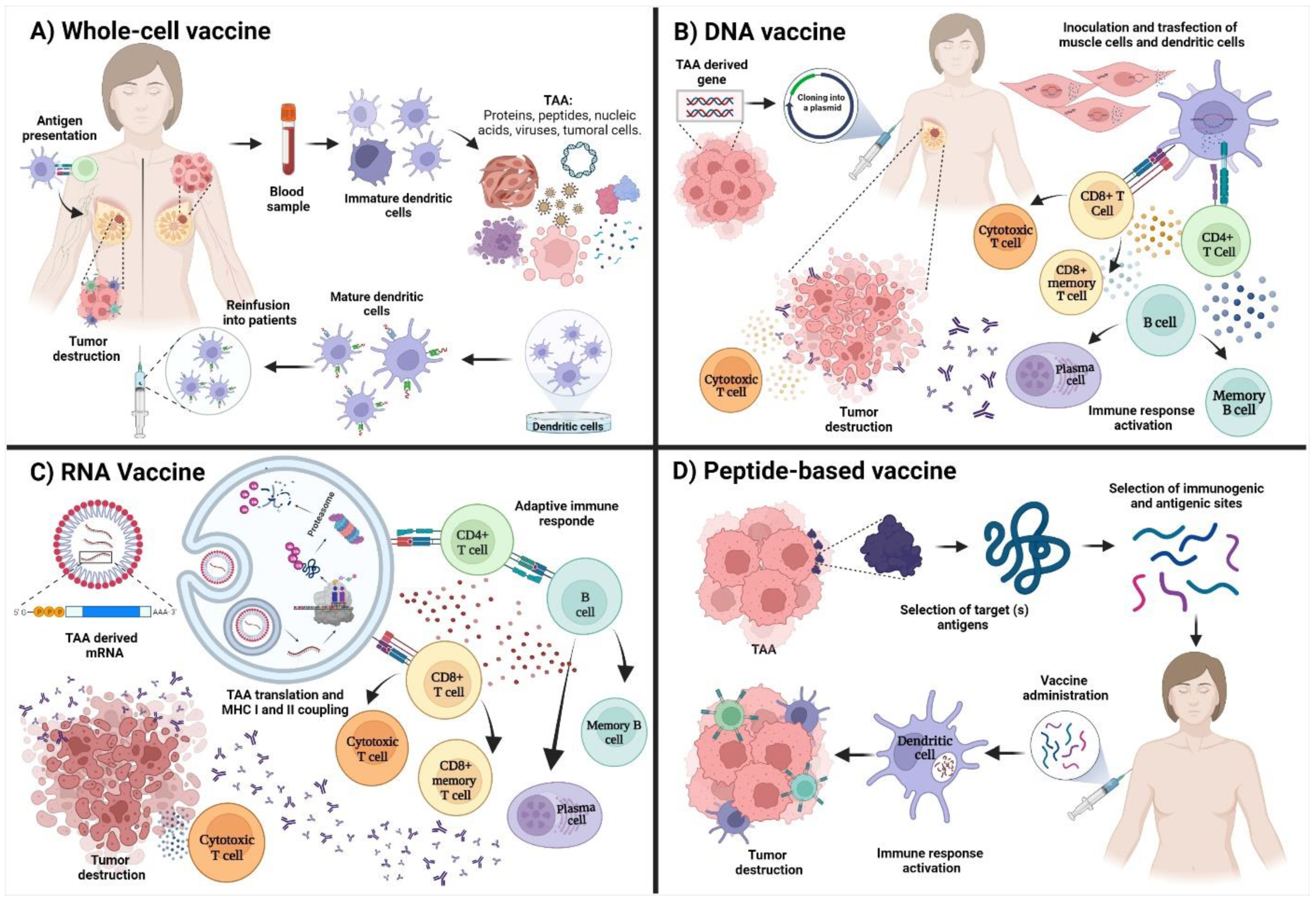
3.2.1. Whole-Cell Vaccines
3.2.2. DNA Vaccines
3.2.3. RNA Vaccines
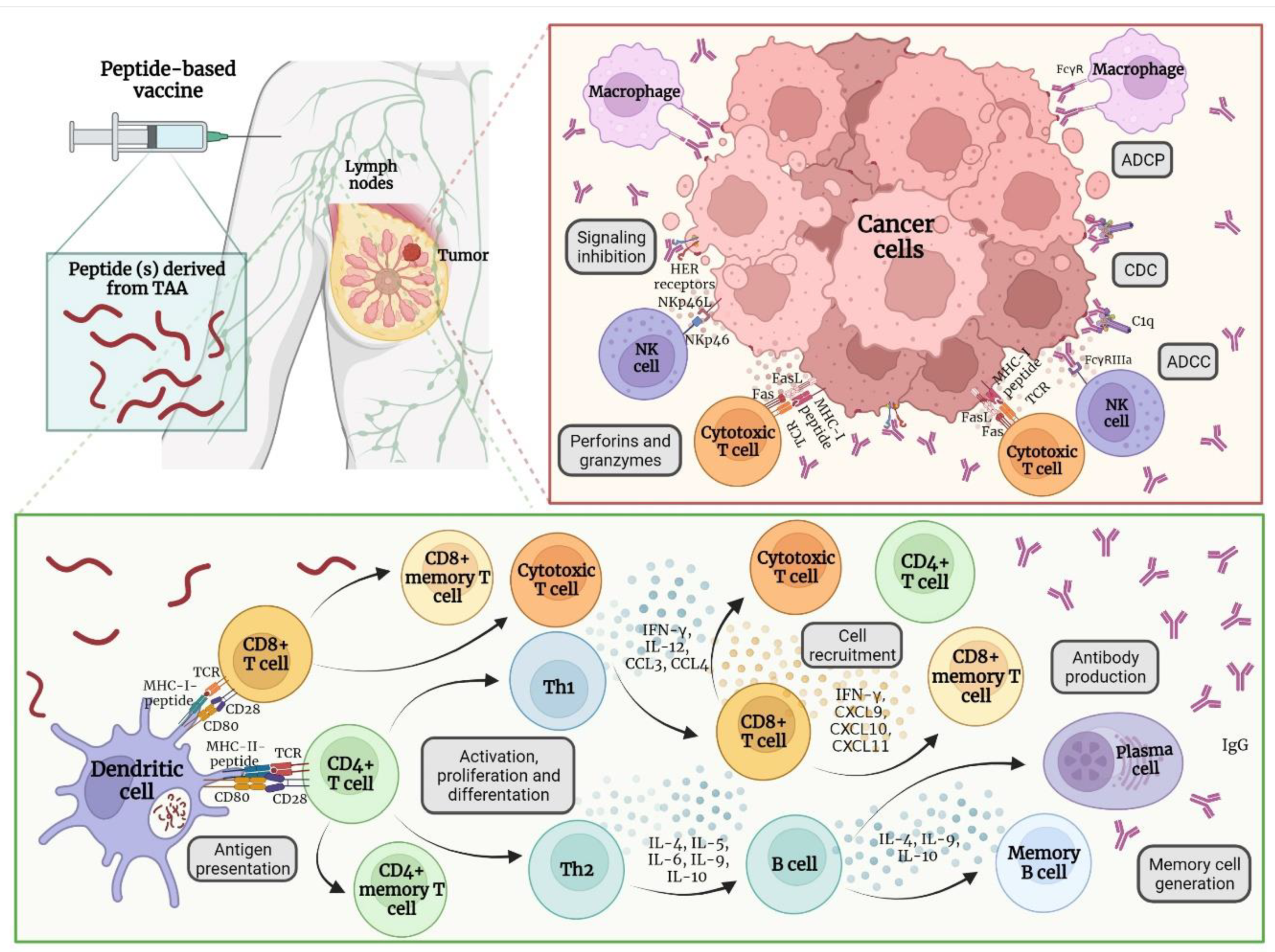
4. Peptide-Based Vaccines for Breast Cancer
4.1. Immune Response after Vaccine Administration
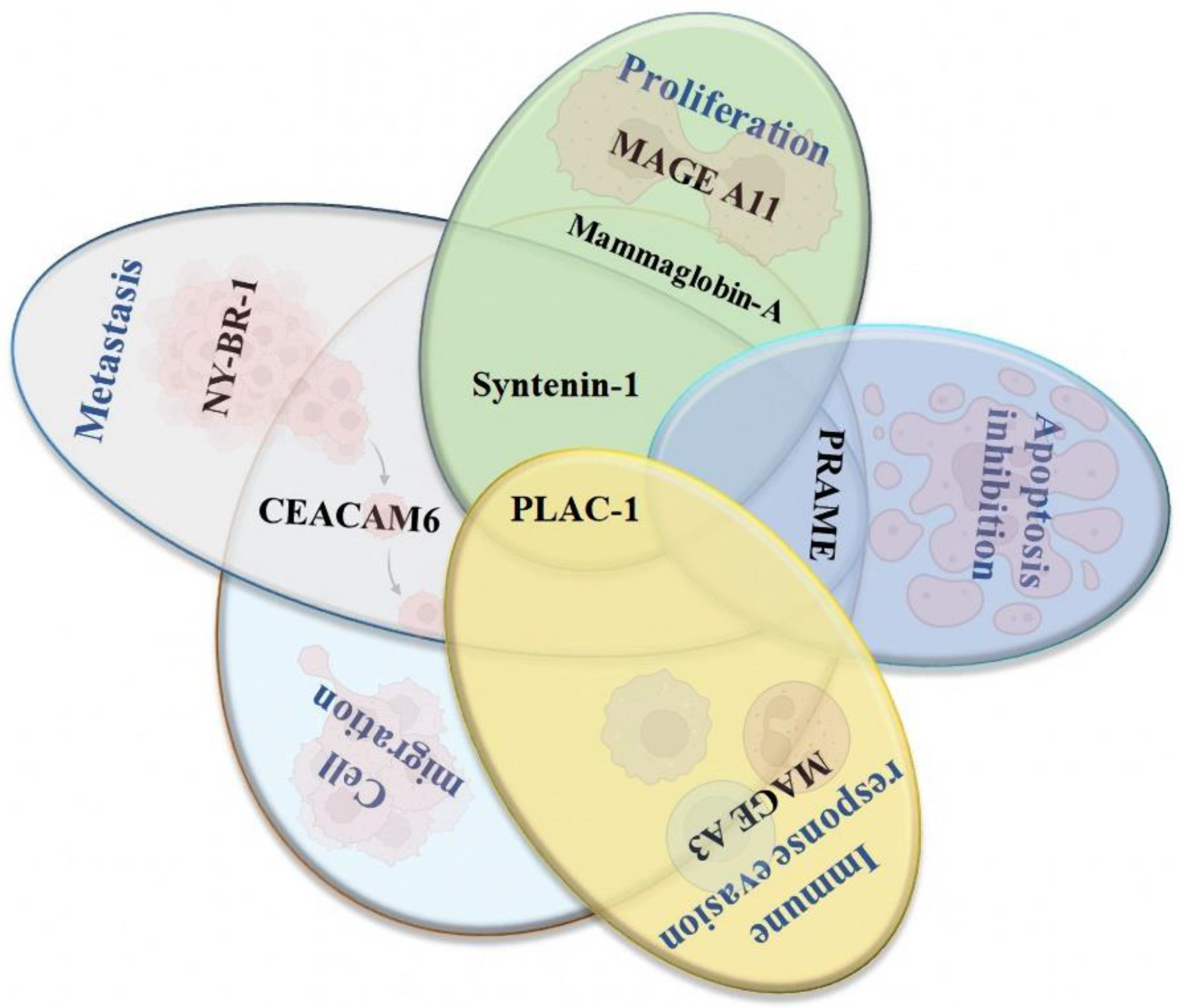
4.2. New Potential Therapeutic Targets for Developing Peptide-Based Vaccines for Breast Cancer
4.2.1. Syntenin-1
4.2.2. PLAC-1
4.2.3. Mammaglobin-α
4.2.4. NY-BR-1
4.2.5. PRAME
4.2.6. MAGE A3 and A11
4.2.7. CEACAM6
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, L.Y.; Tang, J.; Zhang, C.M.; Zeng, W.J.; Yan, H.; Li, M.P.; Chen, X.-P. New Immunotherapy Strategies in Breast Cancer. Int. J. Environ. Res. Public Health 2017, 14, 68. [Google Scholar] [CrossRef]
- Miliotou, A.N.; Papadopoulou, L.C. CAR T-cell Therapy: A New Era in Cancer Immunotherapy. Curr. Pharm. Biotechnol. 2018, 19, 5–18. [Google Scholar] [CrossRef]
- Arab, A.; Yazdian-Robati, R.; Behravan, J. HER2-Positive Breast Cancer Immunotherapy: A Focus on Vaccine Development. Arch. Immunol. Ther. Exp. 2020, 68, 2. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Rapsomaniki, M.A.; Chevrier, S.; Anzeneder, T.; Langwieder, C.; Dykgers, A.; Rees, M.; Ramaswamy, A.; Muenst, S.; Soysal, S.D.; et al. A Single-Cell Atlas of the Tumor and Immune Ecosystem of Human Breast Cancer. Cell. 2019, 177, 1330–1345. [Google Scholar] [CrossRef]
- Williams, A.D.; Payne, K.K.; Posey, A.D.; Hill, C.; Conejo-Garcia, J.; June, C.H.; Tchou, J. Immunotherapy for Breast Cancer: Current and Future Strategies. Curr. Surg. Rep. 2017, 5, 31. [Google Scholar] [CrossRef]
- Krasniqi, E.; Barchiesi, G.; Pizzuti, L.; Mazzotta, M.; Venuti, A.; Maugeri-Saccà, M.; Sanguineti, G.; Massimiani, G.; Sergi, D.; Carpano, S.; et al. Immunotherapy in HER2-positive breast cancer: State of the art and future perspectives. J. Hematol. Oncol. 2019, 12, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Viale, G.; Curigliano, G. Peptide vaccines in early breast cancer. Breast Edinb. Scotl. 2019, 44, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.Y.; Yu, K.D. Breast Cancer Vaccines: Disappointing or Promising? Front. Immunol. 2022, 13, 828386. [Google Scholar] [CrossRef]
- Januškevičienė, I.; Petrikaitė, V. Heterogeneity of breast cancer: The importance of interaction between different tumor cell populations. Life Sci. 2019, 239, 117009. [Google Scholar] [CrossRef]
- WHO. Cáncer de Mama. 2021. Available online: https://www.who.int/es/news-room/fact-sheets/detail/breast-cancer (accessed on 1 July 2022).
- SEOM. Cancer de Mama—SEOM: Sociedad Española de Oncología Médica. 2020. Available online: https://seom.org/info-sobre-el-cancer/cancer-de-mama (accessed on 1 July 2022).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Xu, T.; Long, T.; Zuo, H. Association Between BRCA Status and P53 Status in Breast Cancer: A Meta-Analysis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Casadei, S.; Coats, K.H.; Swisher, E.; Stray, S.M.; Higgins, J.; Roach, K.C.; Mandell, J.; Lee, M.K.; Ciernikova, S.; et al. Spectrum of mutations in BRCA1, BRCA2, CHEK2, and TP53 in families at high risk of breast cancer. JAMA 2006, 295, 1379–1388. [Google Scholar] [CrossRef]
- Ferrini, K.; Ghelfi, F.; Mannucci, R.; Titta, L. Lifestyle, nutrition and breast cancer: Facts and presumptions for consideration. Ecancermedicalscience 2015, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Hardefeldt, P.J.; Edirimanne, S.; Eslick, G.D. Diabetes increases the risk of breast cancer: A meta-analysis. Endocr. Relat. Cancer 2012, 19, 793–803. [Google Scholar] [CrossRef]
- Chiang, S.K.; Chang, W.C.; Chen, S.E.; Chang, L.C. DOCK1 Regulates Growth and Motility through the RRP1B-Claudin-1 Pathway in Claudin-Low Breast Cancer Cells. Cancers 2019, 11, 1762. [Google Scholar] [CrossRef]
- Parker, J.S.; Mullins, M.; Cheang, M.C.U.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef]
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef]
- De Silva, S.; Tennekoon, K.H.; Karunanayake, E.H. Overview of the genetic basis toward early detection of breast cancer. Breast Cancer Targets Ther. 2019, 11, 71–80. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef] [PubMed]
- ACS. Breast Cancer|Breast Cancer Information & Overview. 2020. Available online: https://www.cancer.org/cancer/breast-cancer.html (accessed on 1 July 2022).
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Peart, O. Breast intervention and breast cancer treatment options. Radiol. Technol. 2015, 86, 535M–558M. [Google Scholar]
- De Vita, V.T.; Chu, E. A History of Cancer Chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef]
- Jin, K.-T.; Lu, Z.-B.; Chen, J.-Y.; Liu, Y.-Y.; Lan, H.-R.; Dong, H.-Y.; Yang, F.; Zhao, Y.-Y.; Chen, X.-Y. Recent Trends in Nanocarrier-Based Targeted Chemotherapy: Selective Delivery of Anticancer Drugs for Effective Lung, Colon, Cervical, and Breast Cancer Treatment. J. Nanomater. 2020, 2020, e9184284. [Google Scholar] [CrossRef]
- Meiliana, A.; Dewi, N.M.; Wijaya, A. Cancer Immunotherapy: A Review. Indones. Biomed. J. 2016, 8, 1–20. [Google Scholar] [CrossRef][Green Version]
- Bustos Fiore, A.; Gutiérrez, A.B.; Acosta, L.G.; Segura Cros, C.; Ramos de la Rosa, R. Immunotherapy in oncology: A new challenge for radiologists. Radiologia 2019, 61, 134–142. [Google Scholar] [CrossRef]
- Page, D.B.; Bourla, A.B.; Daniyan, A.; Naidoo, J.; Smith, E.; Smith, M.; Friedman, C.; Khalil, D.N.; Funt, S.; Shoushtari, A.N.; et al. Tumor immunology and cancer immunotherapy: Summary of the 2014 SITC primer. J. Immunother. Cancer 2015, 3, 25. [Google Scholar] [CrossRef][Green Version]
- Donnelly, R.F. Vaccine delivery systems. Hum. Vaccines Immunother. 2017, 13, 17–18. [Google Scholar] [CrossRef]
- Oli, A.N.; Obialor, W.O.; Ifeanyichukwu, M.O.; Odimegwu, D.C.; Okoyeh, J.N.; Emechebe, G.O.; Adejumo, S.A.; Ibeanu, G.C. Immunoinformatics and Vaccine Development: An Overview. ImmunoTargets Ther. 2020, 9, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. Personalized vaccinology: A review. Vaccine 2018, 36, 5350–5357. [Google Scholar] [CrossRef]
- Di Pasquale, A.; Preiss, S.; Tavares, D.a.; Silva, F.; Garçon, N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef]
- Martínez-Mateo, P.; Bustos-Fonseca, M.J.; Gil-Díaz, M.J. Actualización en vacunas. Teoría, realidades y mitos (I). Med. Fam. SEMERGEN 2012, 38, 160–166. [Google Scholar] [CrossRef]
- WHO. Distintos Tipos de Vacunas que Existen. 2021. Available online: https://www.who.int/es/news-room/feature-stories/detail/the-race-for-a-covid-19-vaccine-explained (accessed on 5 July 2022).
- Wang, Y.; Song, X.; Zheng, Y.; Liu, Z.; Li, Y.; Qian, X.; Pang, X.; Zhang, Y.; Yin, Y. Cancer/testis Antigen MAGEA3 Interacts with STAT1 and Remodels the Tumor Microenvironment. Int. J. Med. Sci. 2018, 15, 1702–1712. [Google Scholar] [CrossRef]
- Thomas, S.; Prendergast, G.C. Cancer Vaccines: A Brief Overview. Methods Mol. Biol. 2016, 1403, 755–761. [Google Scholar] [PubMed]
- Vermaelen, K. Vaccine Strategies to Improve Anti-cancer Cellular Immune Responses. Front. Immunol. 2019, 10, 8. [Google Scholar] [CrossRef]
- Emens, L.A. Cancer vaccines: On the threshold of success. Expert Opin. Emerg. Drugs 2008, 13, 295–308. [Google Scholar] [CrossRef]
- Chiang, C.L.L.; Coukos, G.; Kandalaft, L.E. Whole Tumor Antigen Vaccines: Where Are We? Vaccines 2015, 3, 344–372. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Peoples, G.E.; Singletary, S.E. Breast cancer vaccines. Cancer 2007, 110, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- De Paula Peres, L.; da Luz, F.A.C.; dos Anjos Pultz, B.; Brígido, P.C.; de Araújo, R.A.; Goulart, L.R.; Silva, M.J.B. Peptide vaccines in breast cancer: The immunological basis for clinical response. Biotechnol. Adv. 2015, 33, 1868–1877. [Google Scholar] [CrossRef]
- Geng, F.; Bao, X.; Dong, L.; Guo, Q.-Q.; Guo, J.; Xie, Y.; Zhou, Y.; Yu, B.; Wu, H.; Wu, J.-X.; et al. Doxorubicin pretreatment enhances FAPα/survivin co-targeting DNA vaccine anti-tumor activity primarily through decreasing peripheral MDSCs in the 4T1 murine breast cancer model. Oncoimmunology 2020, 9, 1747350. [Google Scholar] [CrossRef]
- Heery, C.R.; Ibrahim, N.K.; Arlen, P.M.; Mohebtash, M.; Murray, J.L.; Koenig, K.; Madan, R.A.; McMahon, S.; Marté, J.L.; Steinberg, S.M.; et al. A Phase 2 Randomized Trial of Docetaxel Alone or in Combination with a Therapeutic Cancer Vaccine (PANVAC) in Patients with Metastatic Breast Cancer. JAMA Oncol. 2015, 1, 1087–1095. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Miao, L.; Liu, Q.; Musetti, S.; Li, J.; Huang, L. Combination Immunotherapy of MUC1 mRNA Nano-vaccine and CTLA-4 Blockade Effectively Inhibits Growth of Triple Negative Breast Cancer. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 45–55. [Google Scholar] [CrossRef]
- Morse, M.A.; Gwin, W.R.; Mitchell, D.A. Vaccine Therapies for Cancer: Then and Now. Target Oncol. 2021, 16, 121–152. [Google Scholar] [CrossRef]
- Emens, L.A. Breast cancer immunobiology driving immunotherapy: Vaccines and immune checkpoint blockade. Expert Rev. Anticancer Ther. 2012, 12, 1597–1611. [Google Scholar] [CrossRef]
- Maeng, H.M.; Berzofsky, J.A. Strategies for developing and optimizing cancer vaccines. F1000Research 2019, 8, 654. [Google Scholar] [CrossRef] [PubMed]
- Burke, E.E.; Kodumudi, K.; Ramamoorthi, G.; Czerniecki, B.J. Vaccine Therapies for Breast Cancer. Surg. Oncol. Clin. N. Am. 2019, 28, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Crosby, E.J.; Gwin, W.R.; Blackwell, K.; Marcom, P.K.; Chang, S.; Maecker, H.T.; Broadwater, G.; Hyslop, T.M.; Kim, S.; Rogatko, A.; et al. Vaccine-Induced Memory CD8+ T Cells Provide Clinical Benefit in HER2 Expressing Breast Cancer: A Mouse to Human Translational Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 2725–2736. [Google Scholar] [CrossRef]
- Perez, C.R.; De Palma, M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat. Commun. 2019, 10, 5408. [Google Scholar] [CrossRef]
- Chopra, A.; Kim, T.S.; O-Sullivan, I.; Martinez, D.; Cohen, E.P. Combined therapy of an established, highly aggressive breast cancer in mice with paclitaxel and a unique DNA-based cell vaccine. Int. J. Cancer 2006, 118, 2888–2898. [Google Scholar] [CrossRef]
- De Zoeten, E.; Carr-Brendel, V.; Markovic, D.; Taylor-Papadimitriou, J.; Cohen, E.P. Treatment of breast cancer with fibroblasts transfected with DNA from breast cancer cells. J. Immunol. 1999, 162, 6934–6941. [Google Scholar] [PubMed]
- Koski, G.K.; Koldovsky, U.; Xu, S.; Mick, R.; Sharma, A.; Fitzpatrick, E.; Weinstein, S.; Nisenbaum, H.; Levine, B.L.; Fox, K.; et al. A novel dendritic cell-based immunization approach for the induction of durable Th1-polarized anti-HER-2/neu responses in women with early breast cancer. J. Immunother. 2012, 35, 54–65. [Google Scholar] [CrossRef]
- Sharma, A.; Koldovsky, U.; Xu, S.; Mick, R.; Roses, R.; Fitzpatrick, E.; Weinstein, S.; Nisenbaum, H.; Levine, B.L.; Fox, K.; et al. HER-2 Pulsed Dendritic Cell Vaccine Can Eliminate HER-2 Expression and Impact DCIS. Cancer 2012, 118, 4354–4362. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jeang, J.; Yang, A.; Wu, T.C.; Hung, C.F. DNA vaccine for cancer immunotherapy. Hum. Vaccines Immunother. 2015, 10, 3153–3164. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Petrovsky, N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev. Vaccines 2016, 15, 313–329. [Google Scholar] [CrossRef]
- Lee, J.; Arun Kumar, S.; Jhan, Y.Y.; Bishop, C.J. Engineering DNA vaccines against infectious diseases. Acta Biomater. 2018, 80, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Mandl, S.J.; Rountree, R.B.; Dalpozzo, K.; Do, L.; Lombardo, J.R.; Schoonmaker, P.L.; Dirmeier, U.; Steigerwald, R.; Giffon, T.; Laus, R.; et al. Immunotherapy with MVA-BN®-HER2 induces HER-2-specific Th1 immunity and alters the intratumoral balance of effector and regulatory T cells. Cancer Immunol. Immunother. 2012, 61, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Foy, S.P.; Mandl, S.J.; Cruz, T.D.; Cote, J.J.; Gordon, E.J.; Trent, E.; Delcayre, A.; Breitmeyer, J.; Franzusoff, A.; Rountree, R.B. Poxvirus-based active immunotherapy synergizes with CTLA-4 blockade to increase survival in a murine tumor model by improving the magnitude and quality of cytotoxic T cells. Cancer Immunol. Immunother. 2016, 65, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.; Jaramillo, A.; Benshoff, N.D.; Campbell, L.G.; Fleming, T.P.; Dietz, J.R.; Mohanakumar, T. Response of established human breast tumors to vaccination with mammaglobin-A cDNA. J. Natl. Cancer Inst. 2004, 96, 1388–1396. [Google Scholar] [CrossRef]
- Childs, J.; Higgins, D.; DeShong, K.; Heckman-Stoddard, B.; Wojtowicz, M.; Stanton, S.; Bailey, H.; Wisinski, K.; Disis, M. Abstract OT3-01-03: A phase I trial of the safety and immunogenicity of a DNA plasmid based vaccine (WOKVAC) encoding epitopes derived from three breast cancer antigens (IGFBP2, HER2, and IGF1R) in patients with breast cancer. Cancer Res. 2017, 77 (Suppl. S4), OT3-01-3. [Google Scholar] [CrossRef]
- Duperret, E.K.; Trautz, A.; Ammons, D.; Perales-Puchalt, A.; Wise, M.C.; Yan, J.; Reed, C.; Weiner, D.B. Alteration of the tumor stroma using a consensus DNA vaccine targeting Fibroblast Activation Protein (FAP) synergizes with anti-tumor vaccine therapy in mice. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Guo, J.; Guo, Q.-Q.; Xie, Y.; Dong, L.; Zhou, Y.; Liu, C.-L.; Yu, B.; Wu, H.; Wu, J.-X.; et al. A DNA vaccine expressing an optimized secreted FAPα induces enhanced anti-tumor activity by altering the tumor microenvironment in a murine model of breast cancer. Vaccine 2019, 37, 4382–4391. [Google Scholar] [CrossRef]
- Fiedler, K.; Lazzaro, S.; Lutz, J.; Rauch, S.; Heidenreich, R. mRNA Cancer Vaccines. Recent Results Cancer Res. 2016, 209, 61–85. [Google Scholar] [PubMed]
- Geall, A.J.; Mandl, C.W.; Ulmer, J.B. RNA: The new revolution in nucleic acid vaccines. Semin. Immunol. 2013, 25, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, Y.; Zhang, Y.; Wang, Y.; Alotaibi, F.; Qiu, L.; Wang, H.; Peng, S.; Liu, Y.; Li, Q.; et al. miRNA-5119 regulates immune checkpoints in dendritic cells to enhance breast cancer immunotherapy. Cancer Immunol. Immunother. 2020, 69, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide Vaccine: Progress and Challenges. Vaccines 2014, 2, 515–536. [Google Scholar] [CrossRef]
- Dillon, P.M.; Petroni, G.R.; Smolkin, M.E.; Brenin, D.R.; Chianese-Bullock, K.A.; Smith, K.T.; Olson, W.C.; Fanous, I.S.; Nail, C.J.; Brenin, C.M.; et al. A pilot study of the immunogenicity of a 9-peptide breast cancer vaccine plus poly-ICLC in early stage breast cancer. J. Immunother. Cancer 2017, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, P.; Artaud, C.; Bay, S.; Ganneau, C.; Campone, M.; Delaloge, S.; Gourmelon, C.; Loirat, D.; Medioni, J.; Pein, F.; et al. The fully synthetic glycopeptide MAG-Tn3 therapeutic vaccine induces tumor-specific cytotoxic antibodies in breast cancer patients. Cancer Immunol. Immunother. 2020, 69, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Kalli, K.R.; Block, M.S.; Kasi, P.M.; Erskine, C.L.; Hobday, T.J.; Dietz, A.; Padley, D.; Gustafson, M.P.; Shreeder, B.; Puglisi-Knutson, D.; et al. Folate Receptor Alpha Peptide Vaccine Generates Immunity in Breast and Ovarian Cancer Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 3014–3025. [Google Scholar] [CrossRef]
- Toh, U.; Sakurai, S.; Saku, S.; Takao, Y.; Okabe, M.; Iwakuma, N.; Shichijo, S.; Yamada, A.; Itoh, K.; Akagi, Y. Early phase II study of mixed 19-peptide vaccine monotherapy for refractory triple-negative breast cancer. Cancer Sci. 2020, 111, 2760–2769. [Google Scholar] [CrossRef] [PubMed]
- Antonilli, M.; Rahimi, H.; Visconti, V.; Napoletano, C.; Ruscito, I.; Zizzari, I.G.; Caponnetto, S.; Barchiesi, G.; Iadarola, R.; Pierelli, L.; et al. Triple peptide vaccination as consolidation treatment in women affected by ovarian and breast cancer: Clinical and immunological data of a phase I/II clinical trial. Int. J. Oncol. 2016, 48, 1369–1378. [Google Scholar] [CrossRef]
- Hutchins, L.F.; Makhoul, I.; Emanuel, P.D.; Pennisi, A.; Siegel, E.R.; Jousheghany, F.; Guo, X.; Pashov, A.D.; Monzavi-Karbassi, B.; Kieber-Emmons, T. Targeting tumor-associated carbohydrate antigens: A phase I study of a carbohydrate mimetic-peptide vaccine in stage IV breast cancer subjects. Oncotarget 2017, 8, 99161–99178. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, M.G.; Benavides, L.C.; Holmes, J.P.; Gates, J.D.; Mittendorf, E.A.; Ponniah, S.; Peoples, G.E. Results of the first phase 1 clinical trial of the HER-2/neu peptide (GP2) vaccine in disease-free breast cancer patients: United States Military Cancer Institute Clinical Trials Group Study I-04. Cancer 2010, 116, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Ardavanis, A.; Litton, J.K.; Shumway, N.M.; Hale, D.F.; Murray, J.L.; Perez, S.A.; Ponniah, S.; Baxevanis, C.N.; Papamichail, M.; et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget 2016, 7, 66192–66201. [Google Scholar] [CrossRef] [PubMed]
- Peoples, G.E.; Gurney, J.M.; Hueman, M.T.; Woll, M.M.; Ryan, G.B.; Storrer, C.E.; Fisher, C.; Shriver, C.D.; Ioannides, C.G.; Ponniah, S. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 7536–7545. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Clifton, G.T.; Holmes, J.P.; Schneble, E.; van Echo, D.; Ponniah, S.; Peoples, G.E. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann. Oncol. 2014, 25, 1735–1742. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Lu, B.; Melisko, M.; Hiller, J.P.; Bondarenko, I.; Brunt, A.M.; Sergii, G.; Petrakova, K.; Peoples, G.E. Efficacy and Safety Analysis of Nelipepimut-S Vaccine to Prevent Breast Cancer Recurrence: A Randomized, Multicenter, Phase III Clinical Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 4248–4254. [Google Scholar] [CrossRef]
- Brown, T.A.; Mittendorf, E.A.; Hale, D.F.; Myers, J.W.; Peace, K.M.; Jackson, D.O.; Greene, J.M.; Vreeland, T.J.; Clifton, G.T.; Ardavanis, A.; et al. Prospective, randomized, single-blinded, multi-center phase II trial of two HER2 peptide vaccines, GP2 and AE37, in breast cancer patients to prevent recurrence. Breast Cancer Res. Treat. 2020, 181, 391–401. [Google Scholar] [CrossRef]
- Clifton, G.T.; Peace, K.M.; Holmes, J.P.; Vreeland, T.J.; Hale, D.F.; Herbert, G.S.; Litton, J.K.; Murthy, R.K.; Lukas, J.; Peoples, G.E.; et al. Initial safety analysis of a randomized phase II trial of nelipepimut-S + GM-CSF and trastuzumab compared to trastuzumab alone to prevent recurrence in breast cancer patients with HER2 low-expressing tumors. Clin. Immunol. 2019, 201, 48–54. [Google Scholar] [CrossRef]
- Takahashi, R.; Toh, U.; Iwakuma, N.; Takenaka, M.; Otsuka, H.; Furukawa, M.; Fujii, T.; Seki, N.; Kawahara, A.; Kage, M.; et al. Feasibility study of personalized peptide vaccination for metastatic recurrent triple-negative breast cancer patients. Breast Cancer Res. 2014, 16, R70. [Google Scholar] [CrossRef] [PubMed]
- Laubreton, D.; Bay, S.; Sedlik, C.; Artaud, C.; Ganneau, C.; Dériaud, E.; Viel, S.; Puaux, A.-L.; Amigorena, S.; Gérard, C.; et al. The fully synthetic MAG-Tn3 therapeutic vaccine containing the tetanus toxoid-derived TT830-844 universal epitope provides anti-tumor immunity. Cancer Immunol. Immunother. 2016, 65, 315–325. [Google Scholar] [CrossRef]
- Islam, M.A.; Rice, J.; Reesor, E.; Zope, H.; Tao, W.; Lim, M.; Ding, J.; Chen, Y.; Aduluso, D.; Zetter, B.R.; et al. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials 2021, 266, 120431. [Google Scholar] [CrossRef] [PubMed]
- Nelde, A.; Rammensee, H.G.; Walz, J.S. The Peptide Vaccine of the Future. Mol. Cell Proteomics. 2021, 20, 100022. [Google Scholar] [CrossRef]
- Owen, J.A.; Punt, J.; Stranford, S.A.; Jones, P.P.; Kuby, J. Kuby Inmunología: Séptima Edición, 7th ed.; McGraw Hill Educacion: Mexico City, México, 2014. [Google Scholar]
- Qin, F.; Xia, F.; Chen, H.; Cui, B.; Feng, Y.; Zhang, P.; Chen, J.; Luo, M. A Guide to Nucleic Acid Vaccines in the Prevention and Treatment of Infectious Diseases and Cancers: From Basic Principles to Current Applications. Front. Cell Dev. Biol. 2021, 9, 633776. [Google Scholar] [CrossRef] [PubMed]
- Caballero, O.L.; Shousha, S.; Zhao, Q.; Simpson, A.J.G.; Coombes, R.C.; Neville, A.M. Expression of Cancer/Testis genes in ductal carcinoma in situ and benign lesions of the breast. Oncoscience 2014, 1, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Dyrskjøt, L.; Zieger, K.; Kissow Lildal, T.; Reinert, T.; Gruselle, O.; Coche, T.; Borre, M.; Ørntoft, T.F. Expression of MAGE-A3, NY-ESO-1, LAGE-1 and PRAME in urothelial carcinoma. Br. J. Cancer 2012, 107, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Janelle, V.; Rulleau, C.; Del Testa, S.; Carli, C.; Delisle, J.S. T-Cell Immunotherapies Targeting Histocompatibility and Tumor Antigens in Hematological Malignancies. Front. Immunol. 2020, 11, 276. [Google Scholar] [CrossRef]
- Das, S.K.; Bhutia, S.K.; Azab, B.; Kegelman, T.P.; Peachy, L.; Santhekadur, P.K.; Dasgupta, S.; Dash, R.; Dent, P.; Grant, S.; et al. MDA-9/Syntenin and IGFBP-2 Promote Angiogenesis in Human Melanoma. Cancer Res. 2013, 73, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.E.; Shen, X.N.; Das, S.K.; Emdad, L.; Sarkar, D.; Fisher, P.B. MDA-9/Syntenin (SDCBP) modulates small GTPases RhoA and Cdc42 via transforming growth factor β1 to enhance epithelial-mesenchymal transition in breast cancer. Oncotarget 2016, 7, 80175–80189. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Wang, H.; Wang, B.; Zhao, K.; Jiang, W.; Bai, W.; Liu, J.; Yin, J. Syntenin1/MDA-9 (SDCBP) induces immune evasion in triple-negative breast cancer by upregulating PD-L1. Breast Cancer Res. Treat. 2018, 171, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R.; Roucourt, B.; Lembo, F.; Fares, J.; Carcavilla, A.M.; Restouin, A.; Zimmermann, P.; Ghossoub, R. Syntenin controls migration, growth, proliferation, and cell cycle progression in cancer cells. Front. Pharmacol. 2015, 6, 241. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, X.; Shi, C.; Jin, L.; Hu, J.; Zhang, A.; Li, J.; Vijayendra, N.; Doodala, V.; Weiss, S.; et al. Plac1 Is a Key Regulator of the Inflammatory Response and Immune Tolerance In Mammary Tumorigenesis. Sci. Rep. 2018, 8, 5717. [Google Scholar] [CrossRef]
- Li, Y.; Chu, J.; Li, J.; Feng, W.; Yang, F.; Wang, Y.; Zhang, Y.; Sun, C.; Yang, M.; Vasilatos, S.N.; et al. Cancer/testis antigen-Plac1 promotes invasion and metastasis of breast cancer through Furin/NICD/PTEN signaling pathway. Mol. Oncol. 2018, 12, 1233–1248. [Google Scholar] [CrossRef] [PubMed]
- Roldán, D.B.; Grimmler, M.; Hartmann, C.; Hubich-Rau, S.; Beißert, T.; Paret, C.; Cagna, G.; Rohde, C.; Wöll, S.; Koslowski, M.; et al. PLAC1 is essential for FGF7/FGFRIIIb-induced Akt-mediated cancer cell proliferation. Oncotarget 2020, 11, 1862–1875. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, M.; Wu, Z.; Qi, Y.; Wu, Y.; Dai, C.; Sun, M.; Li, L.; Gao, Y. Identification of a novel HLA-A2-restricted cytotoxic T lymphocyte epitope from cancer-testis antigen PLAC1 in breast cancer. Amino Acids 2012, 42, 2257–2265. [Google Scholar] [CrossRef]
- Oloomi, M.; Moazzezy, N.; Bouzari, S. Comparing blood versus tissue-based biomarkers expression in breast cancer patients. Heliyon 2020, 6, e03728. [Google Scholar] [CrossRef]
- Wang, Z.; Spaulding, B.; Sienko, A.; Liang, Y.; Li, H.; Nielsen, G.; Gong, G.Y.; Ro, J.Y.; Zhai, Q.J. Mammaglobin, a valuable diagnostic marker for metastatic breast carcinoma. Int. J. Clin. Exp. Pathol. 2009, 2, 384–389. [Google Scholar]
- Hu, Y.; Liu, P.; Wu, D.; Jiang, Y. Prognostic role of plasma mammaglobin A expression in breast carcinoma patients: A meta-analysis. OncoTargets Ther. 2018, 11, 3245–3255. [Google Scholar] [CrossRef]
- Kim, S.W.; Goedegebuure, P.; Gillanders, W.E. Mammaglobin-A is a target for breast cancer vaccination. Oncoimmunology 2016, 5, e1069940. [Google Scholar] [CrossRef] [PubMed]
- Galvis Jiménez, J.M.; Curtidor, H.; Patarroyo, M.A.; Monterrey, P.; Ramírez Clavijo, S.R. Mammaglobin peptide as a novel biomarker for breast cancer detection. Cancer Biol. Ther. 2013, 14, 327–332. [Google Scholar] [CrossRef]
- Kosaloglu, Z.; Bitzer, J.; Halama, N.; Huang, Z.; Zapatka, M.; Schneeweiss, A.; Jäger, D.; Zörnig, I. In silico SNP analysis of the breast cancer antigen NY-BR-1. BMC Cancer 2016, 16, 901. [Google Scholar] [CrossRef]
- Theurillat, J.-P.; Ingold, F.; Frei, C.; Zippelius, A.; Varga, Z.; Seifert, B.; Chen, Y.-T.; Jäger, D.; Knuth, A.; Moch, H. NY-ESO-1 protein expression in primary breast carcinoma and metastases: Correlation with CD8+ T-cell and CD79a+ plasmacytic/B-cell infiltration. Int. J. Cancer. 2007, 120, 2411–2417. [Google Scholar] [CrossRef] [PubMed]
- Balafoutas, D.; Hausen, A.Z.; Mayer, S.; Hirschfeld, M.; Jaeger, M.; Denschlag, D.; Gitsch, G.; Jungbluth, A.; Stickeler, E. Cancer testis antigens and NY-BR-1 expression in primary breast cancer: Prognostic and therapeutic implications. BMC Cancer 2013, 13, 271. [Google Scholar] [CrossRef]
- Gardyan, A.; Osen, W.; Zörnig, I.; Podola, L.; Agarwal, M.; Aulmann, S.; Ruggiero, E.; Schmidt, M.; Halama, N.; Leuchs, B.; et al. Identification of NY-BR-1-specific CD4(+) T cell epitopes using HLA-transgenic mice. Int. J. Cancer 2015, 136, 2588–2597. [Google Scholar] [CrossRef]
- Epping, M.T.; Bernards, R. A causal role for the human tumor antigen preferentially expressed antigen of melanoma in cancer. Cancer Res. 2006, 66, 10639–10642. [Google Scholar] [CrossRef]
- Epping, M.T.; Hart, A.A.M.; Glas, A.M.; Krijgsman, O.; Bernards, R. PRAME expression and clinical outcome of breast cancer. Br. J. Cancer 2008, 99, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, J.; Zhong, J.; Yang, Z.; Tang, A.; Li, S. Clinicopathological and Prognostic Significance of PRAME Overexpression in Human Cancer: A Meta-Analysis. BioMed Res. Int. 2020, 2020, 8828579. [Google Scholar] [CrossRef]
- Al-Khadairi, G.; Naik, A.; Thomas, R.; Al-Sulaiti, B.; Rizly, S.; Decock, J. PRAME promotes epithelial-to-mesenchymal transition in triple negative breast cancer. J. Transl. Med. 2019, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Taheri Anganeh, M.; Savardashtaki, A.; Vafadar, A.; Movahedpour, A.; Shabaninejad, Z.; Maleksabet, A.; Amiri, A.; Ghasemi, Y.; Irajie, C. In Silico Design and Evaluation of PRAME+FliCΔD2D3 as a New Breast Cancer Vaccine Candidate. Iran J. Med. Sci. 2021, 46, 52–60. [Google Scholar]
- Chomez, P.; De Backer, O.; Bertrand, M.; De Plaen, E.; Boon, T.; Lucas, S. An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res. 2001, 61, 5544–5551. [Google Scholar]
- Dhodapkar, M.V.; Osman, K.; Teruya Feldstein, J.; Filippa, D.; Hedvat, C.V.; Iversen, K.; Kolb, D.; Geller, M.D.; Hassoun, H.; Kewalramani, T.; et al. Expression of cancer/testis (CT) antigens MAGE-A1, MAGE-A3, MAGE-A4, CT-7, and NY-ESO-1 in malignant gammopathies is heterogeneous and correlates with site, stage and risk status of disease. Cancer Immun. 2003, 3, 9. [Google Scholar]
- Xia, L.P.; Xu, M.; Chen, Y.; Shao, W.W. Expression of MAGE-A11 in breast cancer tissues and its effects on the proliferation of breast cancer cells. Mol. Med. Rep. 2013, 7, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sood, D.; Gupta, A.; Jha, N.K.; Jain, P.; Chandra, R. Cytotoxic T-lymphocyte elicited therapeutic vaccine candidate targeting cancer against MAGE-A11 carcinogenic protein. Biosci. Rep. 2020, 40, BSR20202349. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, R.D.; Leon, E.; Hansen, H.J.; Goldenberg, D.M. Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer 2007, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Poola, I.; Shokrani, B.; Bhatnagar, R.; DeWitty, R.L.; Yue, Q.; Bonney, G. Expression of carcinoembryonic antigen cell adhesion molecule 6 oncoprotein in atypical ductal hyperplastic tissues is associated with the development of invasive breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12, 4773–4783. [Google Scholar] [CrossRef]
- Johnson, B.; Mahadevan, D. Emerging Role and Targeting of Carcinoembryonic Antigen-related Cell Adhesion Molecule 6 (CEACAM6) in Human Malignancies. Clin. Cancer Drugs. 2015, 2, 100–111. [Google Scholar] [CrossRef]
| Vaccine | Description | Clinical Phase | Evidence |
|---|---|---|---|
| NeuVax™ Peptide derived from HER2. | Breast cancer with low or moderate expression of HER2. GM-CSF and water. | III | Register: NCT01479244 [81] |
| GP2 Peptide derived from HER2. | Breast cancer HLA-A2+ with positive lymph nodes in tumors with positive HER2 expression. | II | [78,82] |
| AE37 Peptide derived from HER2. | Breast cancer with positive and negative lymph nodes, with positive expression of HER2. | II | [78] |
| KRM-19 Mixed vaccine of 19 peptides derived from multiple AATs. | Metastatic triple-negative breast cancer with resistance to the conventional treatment. | II | Register: UMIN000014616 [74] |
| Nelipepimut-S peptide + GM-CSF + trastuzumab. | Breast cancer with low expression of HER2. | II | [83] |
| HLA-matched personalized peptide vaccine. | Recurrent metastatic breast cancer. | II | Register: UMIN000001844 [84] |
| P10s-PADRE | Triple-negative breast cancer (TNBC) in stages I, II, or III. | I/II | Register: NCT02938442 |
| Multipeptide MUC1/ErbB2/CEA | high-risk disease-free ovarian and breast cancer after completion of standard therapies. | I/II | [75] |
| FRα multi-epitope | Vaccine + cyclophosphamide + sargramostim in treating patients with stage II-III breast cancer. | II | Register: NCT03012100 |
| MAG-TN3+ AS15 | Breast neoplasms. | I | Register: NCT02364492 [72,85] |
| Mimotope P10s-PADRE/MONTANIDE ISA 51 VG | Peptide mimotope-based vaccine of tumor-associated carbohydrate antigens in patients with stage IV breast cancer. | I | Register: NCT01390064 [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolás-Morales, M.L.; Luisa-Sanjuan, A.; Gutiérrez-Torres, M.; Vences-Velázquez, A.; Ortuño-Pineda, C.; Espinoza-Rojo, M.; Navarro-Tito, N.; Cortés-Sarabia, K. Peptide-Based Vaccines in Clinical Phases and New Potential Therapeutic Targets as a New Approach for Breast Cancer: A Review. Vaccines 2022, 10, 1249. https://doi.org/10.3390/vaccines10081249
Nicolás-Morales ML, Luisa-Sanjuan A, Gutiérrez-Torres M, Vences-Velázquez A, Ortuño-Pineda C, Espinoza-Rojo M, Navarro-Tito N, Cortés-Sarabia K. Peptide-Based Vaccines in Clinical Phases and New Potential Therapeutic Targets as a New Approach for Breast Cancer: A Review. Vaccines. 2022; 10(8):1249. https://doi.org/10.3390/vaccines10081249
Chicago/Turabian StyleNicolás-Morales, María Lilia, Arianna Luisa-Sanjuan, Mayralina Gutiérrez-Torres, Amalia Vences-Velázquez, Carlos Ortuño-Pineda, Mónica Espinoza-Rojo, Napoleón Navarro-Tito, and Karen Cortés-Sarabia. 2022. "Peptide-Based Vaccines in Clinical Phases and New Potential Therapeutic Targets as a New Approach for Breast Cancer: A Review" Vaccines 10, no. 8: 1249. https://doi.org/10.3390/vaccines10081249
APA StyleNicolás-Morales, M. L., Luisa-Sanjuan, A., Gutiérrez-Torres, M., Vences-Velázquez, A., Ortuño-Pineda, C., Espinoza-Rojo, M., Navarro-Tito, N., & Cortés-Sarabia, K. (2022). Peptide-Based Vaccines in Clinical Phases and New Potential Therapeutic Targets as a New Approach for Breast Cancer: A Review. Vaccines, 10(8), 1249. https://doi.org/10.3390/vaccines10081249







