Immunogenicity and Safety of BNT162b2 Homologous Booster Vaccination in People Living with HIV under Effective cART
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Immunization Schedule
2.3. Serological Test for SARS-CoV-2 S1/S2 IgG
2.4. Safety
2.5. Statistical Analysis
3. Results
3.1. Study Population
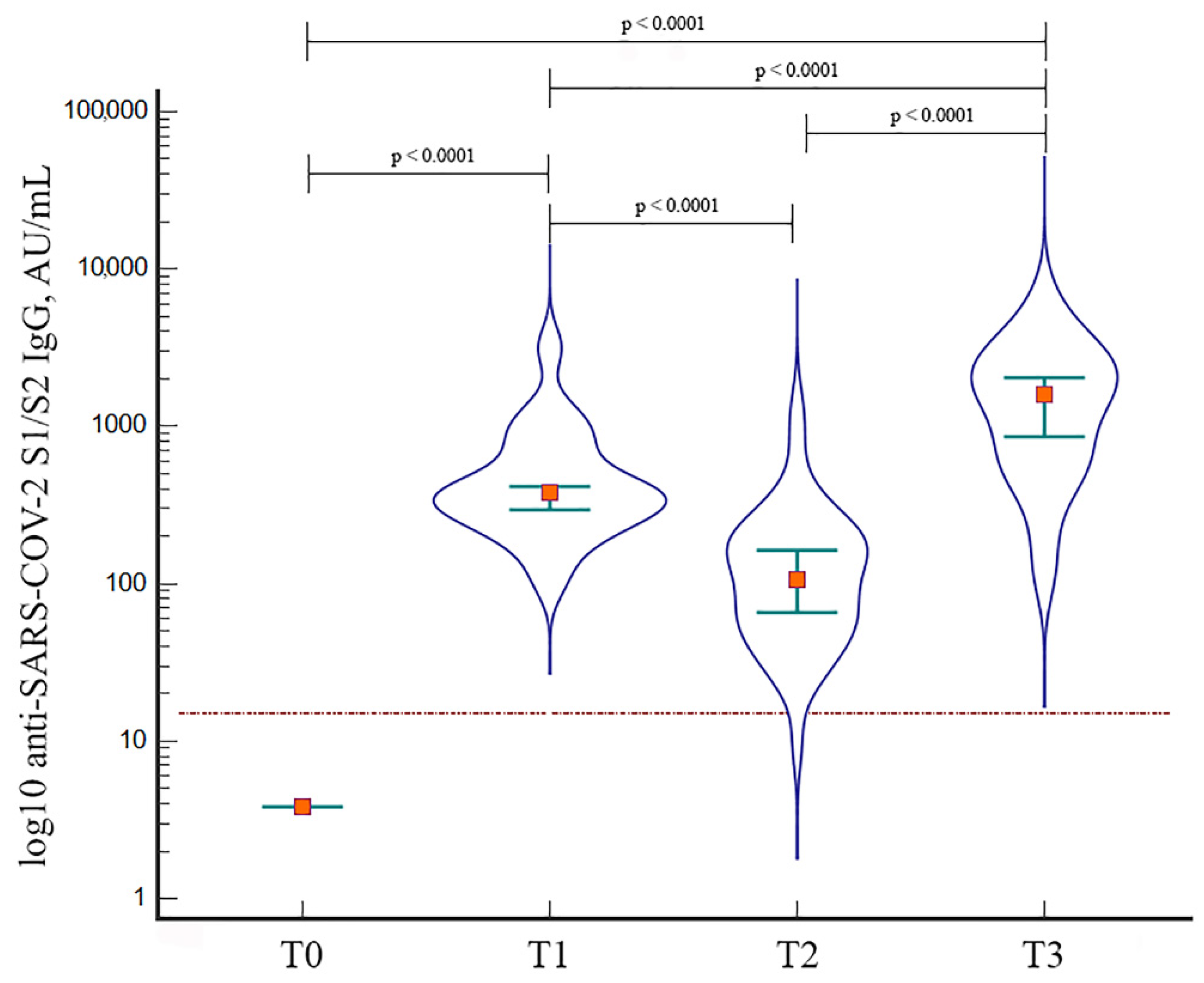
3.2. Immunogenicity of BNT162b2 Booster Dose
3.3. HIV-Related Parameters
3.4. Safety of BNT162b2 Booster Dose
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministero della Salute. Report Vaccini Anti COVID-19. Available online: https://www.governo.it/it/cscovid19/report-vaccini (accessed on 20 June 2022).
- Ministero della Salute; Commissario Straordinario per l’Emergenza; Istituto Superiore di Sanità; Agenas; Aifa. Vaccinazione Anti-SARS-CoV-2/COVID-19. Raccomandazioni Ad Interim Sui Gruppi Target Della Vaccinazione Anti SARS-CoV-2/COVID-19. Gazzetta Ufficiale Serie Generale n.72. 24 March 2021. [Google Scholar]
- Mellor, M.M.; Bast, A.C.; Jones, N.R.; Roberts, N.W.; Ordóñez-Mena, J.M.; Reith, A.J.M.; Butler, C.C.; Matthews, P.C.; Dorward, J. Risk of Adverse Coronavirus Disease 2019 Outcomes for People Living with HIV. AIDS 2021, 35, F1–F10. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Rosalind, J.; Christian, K.; Kurniawan, A. Human Immunodeficiency Virus and Mortality from Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. S. Afr. J. HIV Med. 2021, 22, 1220. [Google Scholar] [CrossRef]
- Ssentongo, P.; Heilbrunn, E.S.; Ssentongo, A.E.; Advani, S.; Chinchilli, V.M.; Nunez, J.J.; Du, P. Epidemiology and Outcomes of COVID-19 in HIV-Infected Individuals: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 6283. [Google Scholar] [CrossRef] [PubMed]
- Bertagnolio, S.; Thwin, S.S.; Silva, R.; Nagarajan, S.; Jassat, W.; Fowler, R.; Haniffa, R.; Reveiz, L.; Ford, N.; Doherty, M.; et al. Clinical Features of, and Risk Factors for, Severe or Fatal COVID-19 among People Living with HIV Admitted to Hospital: Analysis of Data from the WHO Global Clinical Platform of COVID-19. Lancet HIV 2022, 9, e486–e495. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine Over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of Effectiveness of Vaccines Against SARS-CoV-2 Infection and COVID-19 Disease: Results of a Systematic Review and Meta-Regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Falsey, A.R.; Frenck, R.W.; Walsh, E.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Bailey, R.; Swanson, K.A.; Xu, X.; et al. SARS-CoV-2 Neutralization with BNT162b2 Vaccine Dose 3. N. Engl. J. Med. 2021, 385, 1627–1629. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.D.; Kitchin, N.; Xu, X.; Dychter, S.S.; Lockhart, S.; Gurtman, A.; Perez, J.L.; Zerbini, C.; Dever, M.E.; Jennings, T.W.; et al. Safety and Efficacy of a Third Dose of BNT162b2 Covid-19 Vaccine. N. Engl. J. Med. 2022, 386, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zou, S.; Ming, F.; Zhang, Z.; Xing, Z.; Wu, S.; Guo, W.; Tang, W.; Liang, K. Early Efficacy and Safety of the Third Dose Inactivated COVID-19 Vaccine among People Living with HIV. J. Acquir. Immune Defic. Syndr. 2022, 90, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, H.R.; Mwimanzi, F.; Cheung, P.K.; Sang, Y.; Yaseen, F.; Umviligihozo, G.; Kalikawe, R.; Speckmaier, S.; Moran-Garcia, N.; Datwani, S.; et al. People with HIV Receiving Suppressive Antiretroviral Therapy show Typical Antibody Durability After Dual COVID-19 Vaccination, and Strong Third Dose Responses. J. Infect. Dis 2022, 7, jiac229. [Google Scholar] [CrossRef] [PubMed]
- Gianserra, L.; Donà, M.G.; Giuliani, E.; Stingone, C.; Pontone, M.; Buonomini, A.R.; Giuliani, M.; Pimpinelli, F.; Morrone, A.; Latini, A. Correspondence to “High Seroconversion Rate After Vaccination with mRNA BNT162b2 Vaccine Against SARS-CoV-2 among People with HIV—But HIV Viremia Matters?”. AIDS, 2022; 36, 1319–1320. [Google Scholar]
- Jedicke, N.; Stankov, M.V.; Cossmann, A.; Dopfer-Jablonka, A.; Knuth, C.; Ahrenstorf, G.; Ramos, G.M.; Behrens, G.M.N. Humoral Immune Response Following Prime and Boost BNT162b2 Vaccination in People Living with HIV on Antiretroviral Therapy. HIV Med. 2022, 23, 558–563. [Google Scholar] [CrossRef]
- Antinori, A.; Cozzi-Lepri, A.; Vergori, A.; Tavelli, A.; Giannella, M.; Cicalini, S.; Marconi, L.; Yellenki, V.; Meschi, S.; Pellicanò, G.; et al. Humoral Immunogenicity to SARS-CoV-2 mRNA Vaccine Third Additional/Booster Shot in People Living with HIV (PLWH) by Current CD4 Count; Oral Communication; ICAR: Bergamo, Italy, 2022. [Google Scholar]
- Levy, I.; Wieder-Finesod, A.; Litchevsky, V.; Biber, A.; Indenbaum, V.; Olmer, L.; Huppert, A.; Mor, O.; Goldstein, M.; Levin, E.G.; et al. Immunogenicity and Safety of the BNT162b2 mRNA COVID-19 Vaccine in People Living with HIV-1. Clin. Microbiol. Infect. 2021, 27, 1851–1855. [Google Scholar] [CrossRef]
- Bozzi, G.; Lombardi, A.; Ludovisi, S.; Muscatello, A.; Manganaro, L.; Cattaneo, D.; Gori, A.; Bandera, A. Transient Increase in Plasma HIV RNA After COVID-19 Vaccination with mRNA-1272. Int. J. Infect. Dis. 2021, 113, 125–126. [Google Scholar] [CrossRef]
- Menni, C.; May, A.; Polidori, L.; Louca, P.; Wolf, J.; Capdevila, J.; Hu, C.; Ourselin, S.; Steves, C.J.; Valdes, A.M.; et al. COVID-19 Vaccine Waning and Effectiveness and Side-Effects of Boosters: A Prospective Community Study from the ZOE COVID Study. Lancet Infect. Dis 2022, 22, 1002–1010. [Google Scholar] [CrossRef]
- Formeister, E.J.; Chien, W.; Agrawal, Y.; Carey, J.P.; Stewart, C.M.; Sun, D.Q. Preliminary Analysis of Association between COVID-19 Vaccination and Sudden Hearing Loss using US Centers for Disease Control and Prevention Vaccine Adverse Events Reporting System Data. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 674–676. [Google Scholar] [CrossRef]
- Tuekprakhon, A.; Nutalai, R.; Dijokaite-Guraliuc, A.; Zhou, D.; Ginn, H.M.; Selvaraj, M.; Liu, C.; Mentzer, A.J.; Supasa, P.; Duyvesteyn, H.M.E.; et al. Antibody Escape of SARS-CoV-2 Omicron BA.4 and BA.5 from Vaccine and BA.1 Serum. Cell 2022, 185, 2422.e13–2433.e13. [Google Scholar] [PubMed]
| Variable | N | Median Anti-S1/S2 IgG (AU/mL) | 25th–75th Percentile | p-Value |
|---|---|---|---|---|
| Sex | 0.74 | |||
| men | 37 | 1530 | 636 to 2543 | |
| women | 5 | 1670 | 474 to 4160 | |
| Age, years | 0.67 | |||
| up to 53 | 22 | 1580 | 659 to 2590 | |
| >53 | 20 | 1590 | 519 to 2545 | |
| BMI | 0.74 | |||
| normal weight | 15 | 1770 | 860 to 2183 | |
| overweight | 19 | 1370 | 495 to 2763 | |
| obese | 8 | 963 | 519 to 2680 | |
| Nadir CD4+, cells/mm3 | 0.81 | |||
| up to 200 | 25 | 1630 | 600 to 2618 | |
| >200 | 17 | 1530 | 612 to 2298 | |
| Baseline CD4+, cells/mm3 | 0.91 | |||
| up to 500 | 12 | 1375 | 736 to 2890 | |
| >500 | 30 | 1600 | 586 to 2550 | |
| Dual therapy | 0.09 | |||
| no | 27 | 1270 | 514 to 2455 | |
| yes | 15 | 1960 | 1105 to 2763 | |
| INSTI-based regimen | 0.07 | |||
| no | 24 | 967 | 519 to 2120 | |
| yes | 18 | 1820 | 1270 to 3240 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianserra, L.; Donà, M.G.; Giuliani, E.; Stingone, C.; Pontone, M.; Buonomini, A.R.; Giuliani, M.; Pimpinelli, F.; Morrone, A.; Latini, A. Immunogenicity and Safety of BNT162b2 Homologous Booster Vaccination in People Living with HIV under Effective cART. Vaccines 2022, 10, 1243. https://doi.org/10.3390/vaccines10081243
Gianserra L, Donà MG, Giuliani E, Stingone C, Pontone M, Buonomini AR, Giuliani M, Pimpinelli F, Morrone A, Latini A. Immunogenicity and Safety of BNT162b2 Homologous Booster Vaccination in People Living with HIV under Effective cART. Vaccines. 2022; 10(8):1243. https://doi.org/10.3390/vaccines10081243
Chicago/Turabian StyleGianserra, Laura, Maria Gabriella Donà, Eugenia Giuliani, Christof Stingone, Martina Pontone, Anna Rita Buonomini, Massimo Giuliani, Fulvia Pimpinelli, Aldo Morrone, and Alessandra Latini. 2022. "Immunogenicity and Safety of BNT162b2 Homologous Booster Vaccination in People Living with HIV under Effective cART" Vaccines 10, no. 8: 1243. https://doi.org/10.3390/vaccines10081243
APA StyleGianserra, L., Donà, M. G., Giuliani, E., Stingone, C., Pontone, M., Buonomini, A. R., Giuliani, M., Pimpinelli, F., Morrone, A., & Latini, A. (2022). Immunogenicity and Safety of BNT162b2 Homologous Booster Vaccination in People Living with HIV under Effective cART. Vaccines, 10(8), 1243. https://doi.org/10.3390/vaccines10081243









