The T Cell Epitope Landscape of SARS-CoV-2 Variants of Concern
Abstract
:1. Introduction
2. Results
2.1. A Resource of Mutated Epitopes in VOCs from the Perspective of Their Antigen Presentation Potential
2.2. The AP Profile (A Proxy of T-Cell Immunity) Is Robust across All SARS-CoV-2 VOCs
2.3. The Antigen Presentation Potential across Different VOCs Does Not Significantly Differ for Most of the HLA Alleles Considered in the Analysis
2.4. A Mutation-Centric Perspective of the T Cell Epitope Landscape of VOCs
3. Methods
3.1. Shared Mutation Profile of VOCs
3.2. Predicted Probability of SARS-CoV-2 Mutated Peptides Being HLA-Presented on the Surface of Host Infected Cells
3.3. Statistical Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Callaway, E. Beyond Omicron: What’s next for COVID’s viral evolution. Nature 2021, 600, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradnik, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.E.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484.e15. [Google Scholar] [CrossRef] [PubMed]
- Katzourakis, A. COVID-19: Endemic doesn’t mean harmless. Nature 2022, 601, 485. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Universal Coronavirus Vaccines—An Urgent Need. N. Engl. J. Med. 2022, 386, 297–299. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Kidd, C.K.; Dan, J.M.; Ramirez, S.I.; Yu, E.D.; Mateus, J.; da Silva Antunes, R.; Moore, E.; Rubiro, P.; et al. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep. Med. 2021, 2, 100204. [Google Scholar] [CrossRef]
- Sigal, A. Milder disease with Omicron: Is it the virus or the pre-existing immunity? Nat. Rev. Immunol. 2022, 22, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Nelde, A.; Bilich, T.; Heitmann, J.S.; Maringer, Y.; Salih, H.R.; Roerden, M.; Lubke, M.; Bauer, J.; Rieth, J.; Wacker, M.; et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol. 2021, 22, 74–85. [Google Scholar] [CrossRef]
- da Silva Antunes, R.; Pallikkuth, S.; Williams, E.; Dawen Yu, E.; Mateus, J.; Quiambao, L.; Wang, E.; Rawlings, S.A.; Stadlbauer, D.; Jiang, K.; et al. Differential T-Cell Reactivity to Endemic Coronaviruses and SARS-CoV-2 in Community and Health Care Workers. J. Infect. Dis. 2021, 224, 70–80. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Grifoni, A.; Sidney, J.; Vita, R.; Peters, B.; Crotty, S.; Weiskopf, D.; Sette, A. SARS-CoV-2 human T cell epitopes: Adaptive immune response against COVID-19. Cell Host. Microbe 2021, 29, 1076–1092. [Google Scholar] [CrossRef]
- Su, Y.; Chen, D.; Yuan, D.; Lausted, C.; Choi, J.; Dai, C.L.; Voillet, V.; Duvvuri, V.R.; Scherler, K.; Troisch, P.; et al. Multi-Omics Resolves a Sharp Disease-State Shift between Mild and Moderate COVID-19. Cell 2020, 183, 1479–1495.e20. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, C.I.; Galloway, J.; Chu, H.Y.; Shipley, M.M.; Sung, K.; Itell, H.L.; Wolf, C.R.; Logue, J.K.; Magedson, A.; Garrett, M.E.; et al. Epitope profiling reveals binding signatures of SARS-CoV-2 immune response in natural infection and cross-reactivity with endemic human CoVs. Cell Rep. 2021, 35, 109164. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Pre-existing immunity to SARS-CoV-2: The knowns and unknowns. Nat. Rev. Immunol. 2020, 20, 457–458. [Google Scholar] [CrossRef]
- Ogbe, A.; Kronsteiner, B.; Skelly, D.T.; Pace, M.; Brown, A.; Adland, E.; Adair, K.; Akhter, H.D.; Ali, M.; Ali, S.E.; et al. T cell assays differentiate clinical and subclinical SARS-CoV-2 infections from cross-reactive antiviral responses. Nat. Commun. 2021, 12, 2055. [Google Scholar] [CrossRef]
- Lineburg, K.E.; Grant, E.J.; Swaminathan, S.; Chatzileontiadou, D.S.M.; Szeto, C.; Sloane, H.; Panikkar, A.; Raju, J.; Crooks, P.; Rehan, S.; et al. CD8(+) T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope cross-react with selective seasonal coronaviruses. Immunity 2021, 54, 1055–1065.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Alshukairi, A.N.; Baharoon, S.A.; Ahmed, W.A.; Bokhari, A.A.; Nehdi, A.M.; Layqah, L.A.; Alghamdi, M.G.; Al Gethamy, M.M.; Dada, A.M.; et al. Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci. Immunol. 2017, 2, eaan5393. [Google Scholar] [CrossRef] [Green Version]
- Edridge, A.W.D.; Kaczorowska, J.; Hoste, A.C.R.; Bakker, M.; Klein, M.; Loens, K.; Jebbink, M.F.; Matser, A.; Kinsella, C.M.; Rueda, P.; et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020, 26, 1691–1693. [Google Scholar] [CrossRef]
- Tang, F.; Quan, Y.; Xin, Z.T.; Wrammert, J.; Ma, M.J.; Lv, H.; Wang, T.B.; Yang, H.; Richardus, J.H.; Liu, W.; et al. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: A six-year follow-up study. J. Immunol. 2011, 186, 7264–7268. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.P.; Wang, N.C.; Chang, Y.H.; Tian, X.Y.; Na, D.Y.; Zhang, L.Y.; Zheng, L.; Lan, T.; Wang, L.F.; Liang, G.D. Duration of antibody responses after severe acute respiratory syndrome. Emerg. Infect. Dis. 2007, 13, 1562–1564. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Oberhardt, V.; Luxenburger, H.; Kemming, J.; Schulien, I.; Ciminski, K.; Giese, S.; Csernalabics, B.; Lang-Meli, J.; Janowska, I.; Staniek, J.; et al. Rapid and stable mobilization of CD8(+) T cells by SARS-CoV-2 mRNA vaccine. Nature 2021, 597, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Skelly, D.T.; Harding, A.C.; Gilbert-Jaramillo, J.; Knight, M.L.; Longet, S.; Brown, A.; Adele, S.; Adland, E.; Brown, H.; Medawar Laboratory, T.; et al. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat. Commun. 2021, 12, 5061. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.A.; Kissler, S.M.; Fauver, J.R.; Mack, C.; Tai, C.G.; Samant, R.M.; Connelly, S.; Anderson, D.J.; Khullar, G.; MacKay, M.; et al. Viral dynamics and duration of PCR positivity of the SARS-CoV-2 Omicron variant. medRxiv 2022. [Google Scholar] [CrossRef]
- Puhach, O.; Adea, K.; Hulo, N.; Sattonnet, P.; Genecand, C.; Iten, A.; Bausch, F.J.; Kaiser, L.; Vetter, P.; Eckerle, I.; et al. Infectious viral load in unvaccinated and vaccinated patients infected with SARS-CoV-2 WT, Delta and Omicron. medRxiv 2022. [Google Scholar] [CrossRef]
- Kozlov, M. How does Omicron spread so fast? A high viral load isn’t the answer. Nature 2022. [Google Scholar] [CrossRef]
- Carreno, J.M.; Alshammary, H.; Tcheou, J.; Singh, G.; Raskin, A.J.; Kawabata, H.; Sominsky, L.A.; Clark, J.J.; Adelsberg, D.C.; Bielak, D.A.; et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature 2022, 602, 682–688. [Google Scholar] [CrossRef]
- VanBlargan, L.A.; Errico, J.M.; Halfmann, P.J.; Zost, S.J.; Crowe, J.E., Jr.; Purcell, L.A.; Kawaoka, Y.; Corti, D.; Fremont, D.H.; Diamond, M.S. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat. Med. 2022, 28, 490–495. [Google Scholar]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef]
- Collie, S.; Champion, J.; Moultrie, H.; Bekker, L.G.; Gray, G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 494–496. [Google Scholar] [CrossRef]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. SARS-CoV-2 T Cell Responses Elicited by COVID-19 Vaccines or Infection Are Expected to Remain Robust against Omicron. Viruses 2022, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Keeton, R.; Tincho, M.B.; Ngomti, A.; Baguma, R.; Benede, N.; Suzuki, A.; Khan, K.; Cele, S.; Bernstein, M.; Karim, F.; et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 2022, 603, 488–492. [Google Scholar] [CrossRef]
- GeurtsvanKessel, C.H.; Geers, D.; Schmitz, K.S.; Mykytyn, A.Z.; Lamers, M.M.; Bogers, S.; Scherbeijn, S.; Gommers, L.; Sablerolles, R.S.G.; Nieuwkoop, N.N.; et al. Divergent SARS CoV-2 Omicron-reactive T- and B cell responses in COVID-19 vaccine recipients. Sci. Immunol. 2022, 7, eabo2202. [Google Scholar] [CrossRef]
- Liu, J.; Chandrashekar, A.; Sellers, D.; Barrett, J.; Lifton, M.; McMahan, K.; Sciacca, M.; VanWyk, H.; Wu, C.; Yu, J.; et al. Vaccines Elicit Highly Cross-Reactive Cellular Immunity to the SARS-CoV-2 Omicron Variant. medRxiv 2022. [Google Scholar] [CrossRef]
- Vaserstein, L. Markov Processes over Denumerable Products of Spaces, Describing Large Systems of Automata. Probl. Peredachi Inf. 1969, 5, 64–72. [Google Scholar]
- Gonzalez-Galarza, F.F.; McCabe, A.; Santos, E.; Jones, J.; Takeshita, L.; Ortega-Rivera, N.D.; Cid-Pavon, G.M.D.; Ramsbottom, K.; Ghattaoraya, G.; Alfirevic, A.; et al. Allele frequency net database (AFND) 2020 update: Gold-standard data classification, open access genotype data and new query tools. Nucleic Acids Res. 2020, 48, D783–D788. [Google Scholar] [CrossRef]
- Malone, B.; Simovski, B.; Moline, C.; Cheng, J.; Gheorghe, M.; Fontenelle, H.; Vardaxis, I.; Tennoe, S.; Malmberg, J.A.; Stratford, R.; et al. Artificial intelligence predicts the immunogenic landscape of SARS-CoV-2 leading to universal blueprints for vaccine designs. Sci. Rep. 2020, 10, 22375. [Google Scholar] [CrossRef]
- Wilks, S.H.; Mühlemann, B.; Shen, X.; Türeli, S.; LeGresley, E.B.; Netzl, A.; Caniza, M.A.; Chacaltana-Huarcaya, J.N.; Daniell, X.; Datto, M.B.; et al. Mapping SARS-CoV-2 antigenic relationships and serological responses. bioRxiv 2022. [Google Scholar] [CrossRef]
- Lavine, J.S.; Bjornstad, O.N.; Antia, R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science 2021, 371, 741–745. [Google Scholar] [CrossRef]
- Shaman, J.; Galanti, M. Will SARS-CoV-2 become endemic? Science 2020, 370, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Katzourakis, A.; Stilianakis, N.I. Antigenic evolution will lead to new SARS-CoV-2 variants with unpredictable severity. Nat. Rev. Microbiol. 2022, 20, 251–252. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; COVID-19 Genomics UK (COG-UK) Consortium; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Nathan, A.; Kaseke, C.; Berrios, C.; Khatri, A.; Choi, S.; Getz, M.A.; Tano-Menka, R.; Ofoman, O.; Gayton, A.; et al. T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals. Cell 2022, 185, 1041–1051.e6. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. T-cell vaccines could top up immunity to COVID, as variants loom large. Nat. Biotechnol. 2022, 40, 3–4. [Google Scholar] [CrossRef] [PubMed]

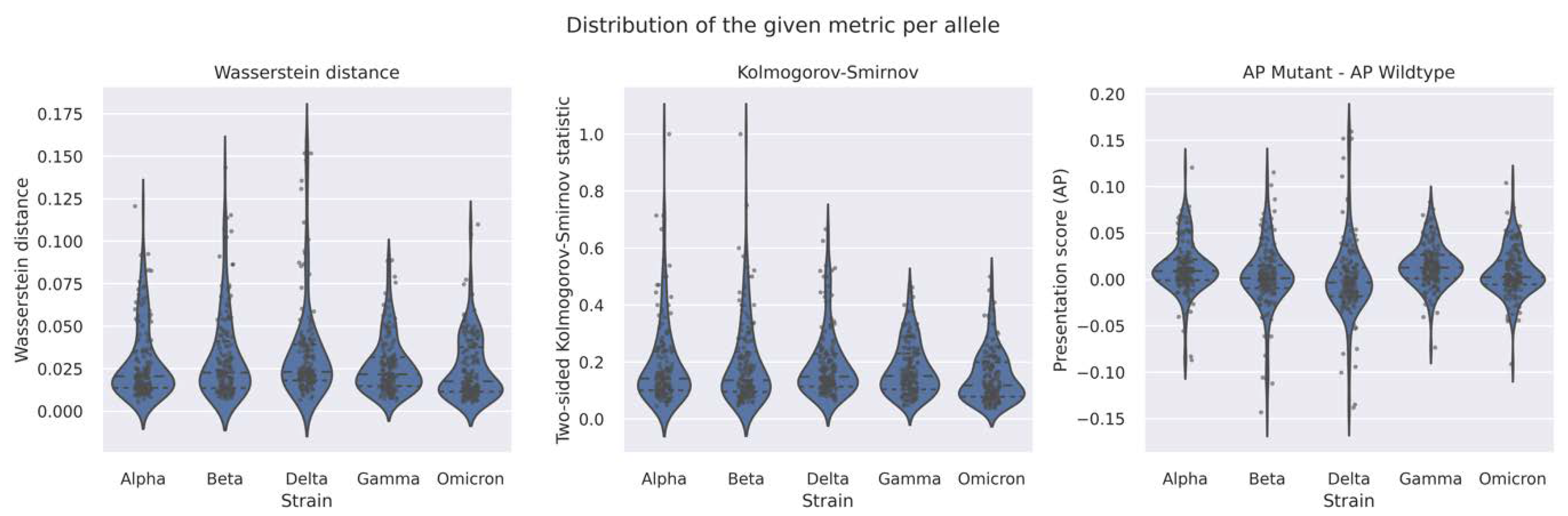

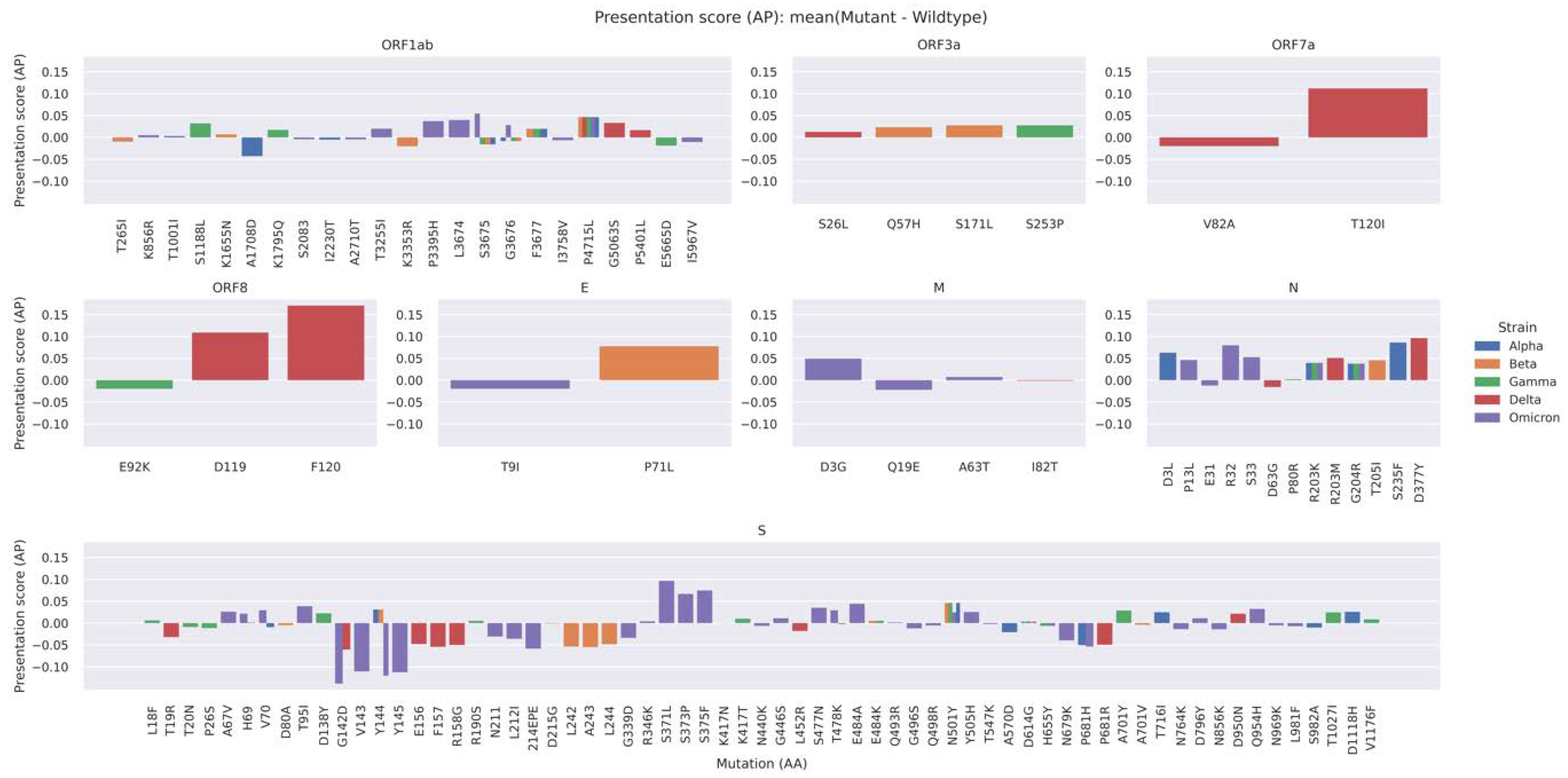
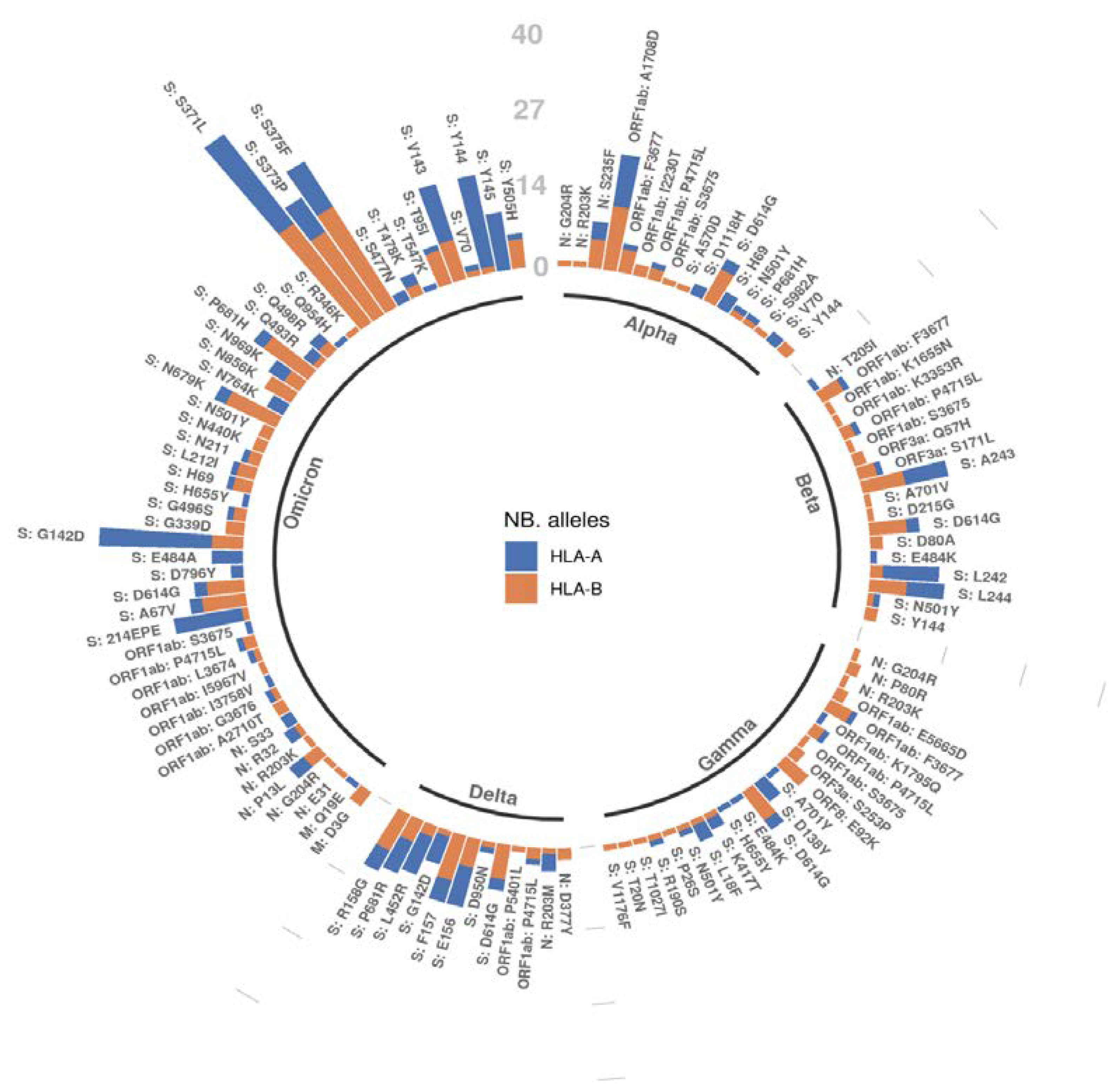
| VOC | Avg. AP Score Diff. (Mutant–Wildtype) | Two-Sample Kolmogorov–Smirnov Test | Wasserstein Distance | |
|---|---|---|---|---|
| Statistic | p-Value | |||
| Alpha | 0.0092 | 0.0381 | 1.4631 × 10−9 | 0.0092 |
| Beta | 0.0034 | 0.0147 | 0.0798 | 0.0037 |
| Gamma | 0.0134 | 0.06 | 3.3352 × 10−27 | 0.0134 |
| Delta | −0.0008 | 0.0305 | 4.7707 × 10−6 | 0.0089 |
| Omicron | 0.0069 | 0.0292 | 4.0882 × 10−16 | 0.0071 |
| VOC | Nb. of Non-Synonymous Mutations | Nb. of Mutated Peptides | Fraction of All Wildtype Peptides in the Original Wuhan Strain |
|---|---|---|---|
| Alpha | 21 | 14,485 | 0.0173 |
| Beta | 22 | 14,890 | 0.0178 |
| Gamma | 25 | 17,106 | 0.0204 |
| Delta | 22 | 13,952 | 0.0167 |
| Omicron | 59 | 42,356 | 0.0506 |
| Wildtype (Wuhan) | NA | 837,211 | 1 |
| VOC | Avg. AP Score Diff. (Mutant–Wildtype) | Two-Sample Kolmogorov–Smirnov Test Statistic | Wasserstein Distance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Percentile | Mean | Percentile | Mean | Percentile | ||||||||
| 5th | 25th | 75th | 95th | 25th | 75th | 95th | 25th | 75th | 95th | ||||
| Alpha | 0.0127 | −0.0224 | −0.0006 | 0.0217 | 0.065 | 0.1898 | 0.1011 | 0.2391 | 0.4779 | 0.0285 | 0.0138 | 0.0355 | 0.0729 |
| Beta | 0.0028 | −0.0478 | −0.0094 | 0.0152 | 0.0596 | 0.1935 | 0.0956 | 0.2362 | 0.475 | 0.0316 | 0.0136 | 0.0412 | 0.0863 |
| Gamma | 0.0151 | −0.0522 | 0.0012 | 0.0266 | 0.0622 | 0.1738 | 0.1042 | 0.2301 | 0.475 | 0.0268 | 0.0148 | 0.0318 | 0.0959 |
| Delta | 0.0006 | −0.017 | −0.018 | 0.0129 | 0.0564 | 0.1919 | 0.114 | 0.2287 | 0.3333 | 0.0343 | 0.0181 | 0.0393 | 0.0595 |
| Omicron | 0.0074 | −0.0302 | −0.0054 | 0.0204 | 0.05 | 0.1473 | 0.0781 | 0.2 | 0.3333 | 0.0251 | 0.0115 | 0.0377 | 0.0556 |
| VOC | HLA Allele | Wasserstein Distance | Populations Highest Allele Frequency (AFND) |
|---|---|---|---|
| Alpha | HLA-A*01:02 | 0.121 | Brazil, Australia, Azores |
| HLA-A*25:01 | 0.092 | ||
| HLA-B*27:05 | 0.092 | ||
| Beta | HLA-B*27:06 | 0.143 | Australia, Brazil |
| HLA-B*48:01 | 0.115 | ||
| HLA-A*25:01 | 0.113 | ||
| Gamma | HLA-A*25:01 | 0.089 | Australia, Austria, Azores (results based only on HLA-A*25:01) * |
| HLA-B*18:02 | 0.088 | ||
| HLA-A*33:03 | 0.079 | ||
| Delta | HLA-B*46:01 | 0.159 | China, Hong Kong, Malaysia (results based only on HLA-B*46:01) * |
| HLA-B*27:03 | 0.152 | ||
| HLA-B*27:05 | 0.152 | ||
| Omicron | HLA-B*27:02 | 0.110 | Bulgaria, Germany, Israel (results based only on HLA-B*27:02) |
| HLA-B*18:02 | 0.104 | ||
| HLA-A*24:02 | 0.077 |
| VOC | HLA Type | Avg. AP Score Difference (Mutant–Wildtype) | Wasserstein Distance |
|---|---|---|---|
| Alpha | HLA-A | 0.0126 | 0.0126 |
| HLA-B | 0.0064 | 0.0065 | |
| Beta | HLA-A | 0.0038 | 0.0039 |
| HLA-B | 0.0031 | 0.0045 | |
| Gamma | HLA-A | 0.0185 | 0.0185 |
| HLA-B | 0.0093 | 0.0093 | |
| Delta | HLA-A | −0.0004 | 0.0118 |
| HLA-B | −0.0011 | 0.0071 | |
| Omicron | HLA-A | 0.0132 | 0.0133 |
| HLA-B | 0.0017 | 0.0025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tennøe, S.; Gheorghe, M.; Stratford, R.; Clancy, T. The T Cell Epitope Landscape of SARS-CoV-2 Variants of Concern. Vaccines 2022, 10, 1123. https://doi.org/10.3390/vaccines10071123
Tennøe S, Gheorghe M, Stratford R, Clancy T. The T Cell Epitope Landscape of SARS-CoV-2 Variants of Concern. Vaccines. 2022; 10(7):1123. https://doi.org/10.3390/vaccines10071123
Chicago/Turabian StyleTennøe, Simen, Marius Gheorghe, Richard Stratford, and Trevor Clancy. 2022. "The T Cell Epitope Landscape of SARS-CoV-2 Variants of Concern" Vaccines 10, no. 7: 1123. https://doi.org/10.3390/vaccines10071123
APA StyleTennøe, S., Gheorghe, M., Stratford, R., & Clancy, T. (2022). The T Cell Epitope Landscape of SARS-CoV-2 Variants of Concern. Vaccines, 10(7), 1123. https://doi.org/10.3390/vaccines10071123






