Association between Recent Usage of Antibiotics and Immunogenicity within Six Months after COVID-19 Vaccination
Abstract
1. Introduction
2. Methods
Study Design and Participants
3. Outcomes of Interest
4. Exposure of Interest and Covariates
5. Statistical Analyses
6. Results
6.1. Patient Characteristics
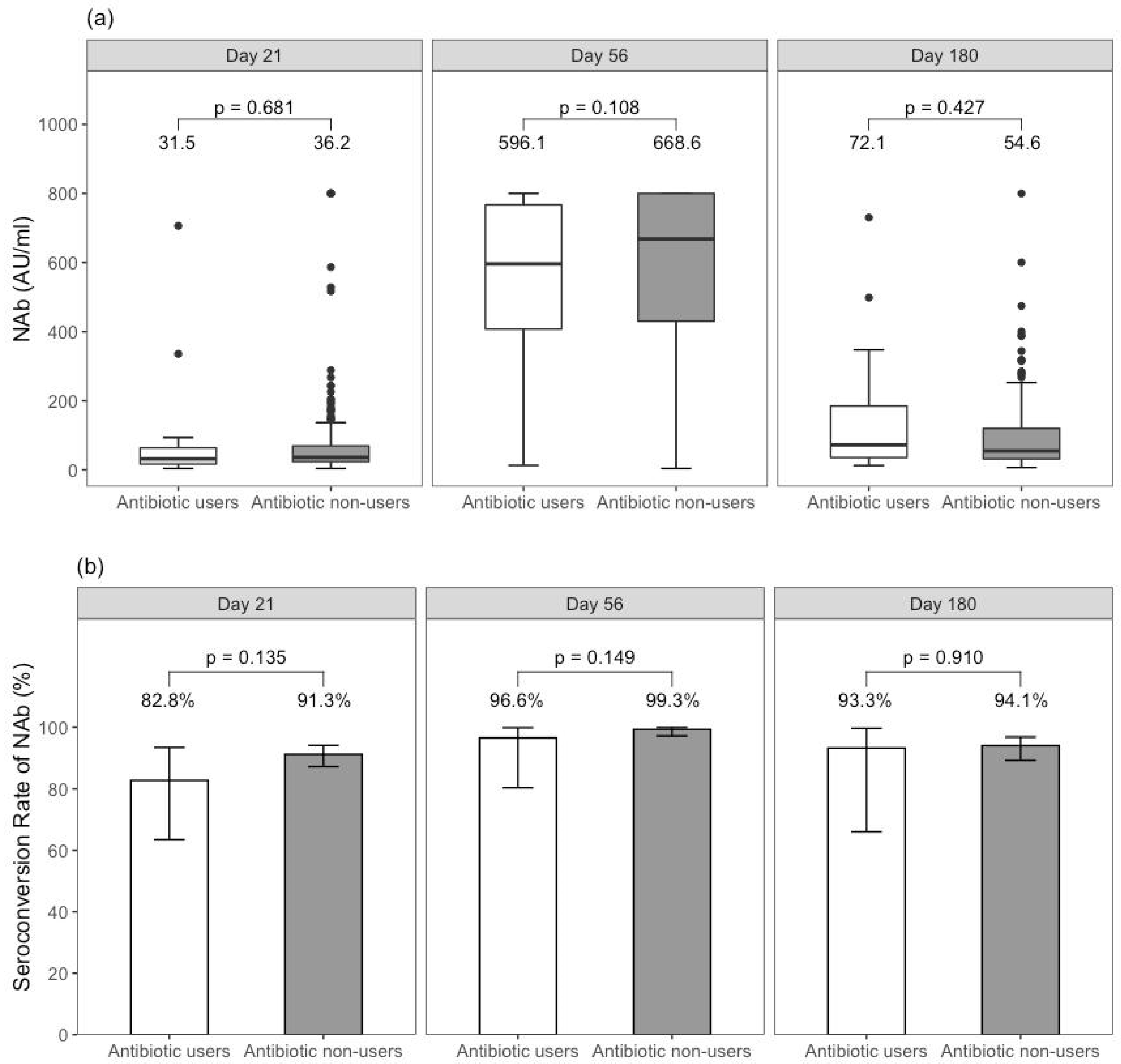
6.2. Humoral Immune Response among BNT162b2 Recipients
6.3. Reactogenicity among BNT162b2 Recipients
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Marcus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020, 11, 585354. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.W.; Mak, L.; Leung, G.M.; Cowling, B.J.; Peiris, M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe 2021, 2, e423. [Google Scholar] [CrossRef]
- Dhakal, S.; Klein, S.L. Host Factors Impact Vaccine Efficacy: Implications for Seasonal and Universal Influenza Vaccine Programs. J. Virol. 2019, 93, e00797-19. [Google Scholar] [CrossRef]
- Painter, S.D.; Ovsyannikova, I.G.; Poland, G.A. The weight of obesity on the human immune response to vaccination. Vaccine 2015, 33, 4422–4429. [Google Scholar] [CrossRef]
- Lynn, D.J.; Benson, S.C.; Lynn, M.A.; Pulendran, B. Modulation of immune responses to vaccination by the microbiota: Implications and potential mechanisms. Nat. Rev. Immunol. 2021, 22, 33–46. [Google Scholar] [CrossRef]
- Hagan, T.; Cortese, M.; Rouphael, N.; Boudreau, C.; Linde, C.; Maddur, M.S.; Das, J.; Wang, H.; Guthmiller, J.; Zheng, N.-Y.; et al. Antibiotics-Driven Gut Microbiome Perturbation Alters Immunity to Vaccines in Humans. Cell 2019, 178, 1313–1328.e13. [Google Scholar] [CrossRef]
- Mok, C.K.P.; Cohen, C.A.; Cheng, S.M.S.; Chen, C.; Kwok, K.-O.; Yiu, K.; Chan, T.-O.; Bull, M.; Ling, K.C.; Dai, Z.; et al. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong. Respirology 2021, 27, 301–310. [Google Scholar] [CrossRef]
- Davidson, L.E.; Fiorino, A.M.; Snydman, D.R.; Hibberd, P.L. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: A randomized double-blind placebo-controlled trial. Eur. J. Clin. Nutr. 2011, 65, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Rizzardini, G.; Eskesen, D.; Calder, P.C.; Capetti, A.; Jespersen, L.; Clerici, M. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2012, 107, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Kanaani, Z.A.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef]
- Chan, K.H.; Leung, K.Y.; Zhang, R.R.; Liu, D.; Fan, Y.; Chen, H.; Yuen, K.-Y.; Hung, I.F.-N. Performance of a Surrogate SARS-CoV-2-Neutralizing Antibody Assay in Natural Infection and Vaccination Samples. Diagnostics 2021, 11, 1757. [Google Scholar] [CrossRef]
- Norquist, J.M.; Khawaja, S.S.; Kurian, C.; Mast, T.C.; Liaw, K.-L.; Robertson, M.N.; Evans, B.; Gutsch, D.; Saddier, P. Adaptation of a previously validated vaccination report card for use in adult vaccine clinical trials to align with the 2007 FDA Toxicity Grading Scale Guidance. Hum. Vaccines Immunother. 2012, 8, 1208–1212. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Hou, Z.; Liu, J.; Gu, Y.; Wu, Y.; Chen, Z.; Ji, J.; Diao, S.; Qiu, Y.; Zou, S.; et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): A multicenter study. J. Hepatol. 2021, 75, 439–441. [Google Scholar] [CrossRef]
- Cheung, K.S.; Lam, L.K.; Seto, W.K.; Leung, W.K. Use of Antibiotics during Immune Checkpoint Inhibitor Treatment Is Associated with Lower Survival in Hepatocellular Carcinoma. Liver Cancer 2021, 10, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaum, V.; Mandelboim, M.; Doolman, R.; Amit, S.; et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021, 9, 999–1009. [Google Scholar] [CrossRef]
- Soffer, S.; Glicksberg, B.S.; Zimlichman, E.; Efros, O.; Levin, M.A.; Freeman, R.; Reich, D.L.; Klang, E. The association between obesity and peak antibody titer response in COVID-19 infection. Obesity 2021, 29, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Abt, M.C.; Osborne, L.C.; Monticelli, L.A.; Doering, T.A.; Alenghat, T.; Sonnenberg, G.F.; Paley, M.A.; Antenus, M.; Williams, K.L.; Erikson, J.; et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012, 37, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef]
- Wu, S.; Jiang, Z.-Y.; Sun, Y.-F.; Yu, B.; Chen, J.; Dai, C.-Q.; Wu, X.-L.; Tang, X.-L.; Chen, X.-Y. Microbiota Regulates the TLR7 Signaling Pathway Against Respiratory Tract Influenza A Virus Infection. Curr. Microbiol. 2013, 67, 414–422. [Google Scholar] [CrossRef]
- Oh, J.Z.; Ravindran, R.; Chassaing, B.; Carvalho, F.A.; Maddur, M.S.; Bower, M.; Hakimpour, P.; Gill, K.P.; Nakaya, H.I.; Yarovinsky, F.; et al. TLR5-Mediated Sensing of Gut Microbiota Is Necessary for Antibody Responses to Seasonal Influenza Vaccination. Immunity 2014, 41, 478–492. [Google Scholar] [CrossRef]
- Schaupp, L.; Muth, S.; Rogell, L.; Kofoed-Branzk, M.; Melchior, F.; Lienenklaus, S.; Ganal-Vonarburg, S.C.; Klein, M.; Guendel, F.; Hain, T.; et al. Microbiota-Induced Type I Interferons Instruct a Poised Basal State of Dendritic Cells. Cell 2020, 181, 1080–1096.e19. [Google Scholar] [CrossRef]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef]
- Williams, W.B.; Liao, H.-X.; Moody, M.A.; Kepler, T.B.; Alam, S.M.; Gao, F.; Wiehe, K.; Trama, A.M.; Jones, K.; Zhang, R.; et al. Diversion of HIV-1 vaccine–induced immunity by gp41-microbiota cross-reactive antibodies. Science 2015, 349, aab1253. [Google Scholar] [CrossRef] [PubMed]
- Carrasco Pro, S.; Lindestam Arlehamn, C.S.; Dhanda, S.K.; Carpenter, C.; Lindvall, M.; Faruqi, A.A.; Santee, C.A.; Renz, H.; Sidney, J.; Peters, B.; et al. Microbiota epitope similarity either dampens or enhances the immunogenicity of disease-associated antigenic epitopes. PLoS ONE 2018, 13, e0196551. [Google Scholar] [CrossRef] [PubMed]
- Amirthalingam, G.; Bernal, J.L.; Andrews, N.J.; Whitaker, H.; Gower, C.; Stowe, J.; Tessier, E.; Subbarao, S.; Ireland, G.; Baawuah, F.; et al. Serological responses and vaccine effectiveness for extended COVID-19 vaccine schedules in England. Nat. Commun. 2021, 12, 7217. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, A.; Marchevsky, N.G.; Jenkin, D.; Aboagye, J.; Aley, P.K.; Angus, B.; Belij-Rammerstorfer, S.; Bibi, S.; Bittaye, M.; Cappuccini, F.; et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: A substudy of two randomised controlled trials (COV001 and COV002). Lancet 2021, 398, 981–990. [Google Scholar] [CrossRef]
- Ai, J.; Wang, J.; Liu, D.; Xiang, H.; Guo, Y.; Lv, J.; Zhang, Q.; Li, J.; Zhang, X.; Li, Q.; et al. Safety and Immunogenicity of SARS-CoV-2 Vaccines in Patients With Chronic Liver Diseases (CHESS-NMCID 2101): A Multicenter Study. Clin Gastroenterol Hepatol 2022, 20, 1516–1524.e2. [Google Scholar] [CrossRef]
- Furman, D.; Hejblum, B.P.; Simon, N.; Jojic, V.; Dekker, C.L.; Thiébaut, R.; Tibshirani, R.J.; Davis, M.M. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 869–874. [Google Scholar] [CrossRef]
- Zhang, R.; Khong, K.W.; Leung, K.Y.; Liu, D.; Fan, Y.; Lu, L.; Chan, P.C.; Chen, L.; To, K.K.-W.; Chen, H.; et al. Antibody Response of BNT162b2 and CoronaVac Platforms in Recovered Individuals Previously Infected by COVID-19 against SARS-CoV-2 Wild Type and Delta Variant. Vaccines 2021, 9, 1442. [Google Scholar] [CrossRef]
- Lu, L.; Mok, B.W.; Chen, L.L.; Chan, J.M.-C.; Tsang, O.T.-Y.; Lam, B.H.-S.; Chuang, V.W.-M.; Chu, A.W.-H.; Chan, W.-M.; Ip, J.D.; et al. Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin. Infect. Dis. 2021, preprint, ciab1041. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Jin, P.; Li, J.; Pan, H.; Wu, Y.; Zhu, F. Immunological surrogate endpoints of COVID-2019 vaccines: The evidence we have versus the evidence we need. Signal Transduct. Target. Ther. 2021, 6, 48. [Google Scholar] [CrossRef]
- De Araujo Torres, D.; do Carmo Bueno Ribeiro, L.; de Freitas Linhares Riello, A.P.; Horovitz, D.D.G.; Ribeiro Pinto, L.F.; Croda, J. Reinfection of COVID-19 after 3 months with a distinct and more aggressive clinical presentation: Case report. J. Med. Virol. 2020, 93, 1857–1859. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Hung, I.F.; Chan, K.H.; Yuan, S.; To, W.-K.; Tsang, D.N.-C.; Cheng, V.C.-C.; Chen, Z.; Kok, K.-H.; Yuen, K.-Y. Serum antibody profile of a patient with COVID-19 reinfection. Clin. Infect. Dis. 2021, 72, e659–e662. [Google Scholar] [CrossRef] [PubMed]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: A retrospective, total population cohort study in Sweden. Lancet 2022, 399, 814–823. [Google Scholar] [CrossRef]
| All (n = 316) | Antibiotic Users (n = 29) | Antibiotic Non-Users (n = 287) | p-Value | |
|---|---|---|---|---|
| Age ≥ 60 years (n, %) | 47 (14.9%) | 3 (10.3) | 44 (15.3) | 0.472 |
| Male sex (n, %) | 100 (31.6%) | 6 (20.7) | 94 (32.8) | 0.183 |
| DM (n, %) | 23 (7.3%) | 4 (13.8) | 19 (6.6) | 0.157 |
| Overweight/obesity (n, %) | 156 (49.4%) | 14 (48.3) | 142 (49.5) | 0.902 |
| Hypertension (n, %) | 98 (31.0%) | 6 (20.7) | 92 (32.1) | 0.207 |
| Raised LDL (≥3.4 mmol/L) (n,%) | 57 (18.0%) | 9 (31.0) | 48 (16.7) | 0.056 |
| Smoking (n, %) | 14 (4.4%) | 0 (0) | 14 (4.9) | 0.224 |
| Alcohol use (n, %) | 18 (5.7%) | 1 (3.4) | 17 (5.9) | 0.584 |
| Moderate/severe hepatic steatosis (CAP ≥ 268 dB/M) (n, %) | 70 (22.2%) | 8 (27.6) | 62 (21.6) | 0.460 |
| Patient | Indication of Antibiotic Use | Type of Antibiotics | Dosage | Total Duration | PPI Use |
|---|---|---|---|---|---|
| 1 | Skin and soft tissue infection | Amoxycillin/clavulanic acid | 1 g BD | 14 days | 0 |
| 2 | Skin and soft tissue infection | Amoxycillin/clavulanic acid | 1 g BD | 7 days | 0 |
| 3 | Skin and soft tissue infection | Amoxycillin/clavulanic acid | 1 g BD | 12 days | 0 |
| 4 | Skin and soft tissue infection | Amoxycillin/clavulanic acid | 375 mg tds | 7 days | 14 days |
| 5 | Skin and soft tissue infection | Amoxycillin/clavulanic acid | 375 mg tds | 7 days | 0 |
| 6 | Skin and soft tissue infection | Amoxycillin/clavulanic acid | 1 g BD | 19 days | 0 |
| 7 | Skin and soft tissue infection | Amoxycillin/clavulanic acid | 1 g BD | 7 days | 42 days |
| 8 | Skin and soft tissue infection | Amoxycillin/clavulanic acid | 1 g BD | 13 days | 0 |
| 9 | Skin and soft tissue infection | Amoxycillin/clavulanic acid | 1 g BD | 7 days | 0 |
| 10 | Skin and soft tissue infection | Amoxycillin/clavulanic acid | 375 mg tds | 7 days | 0 |
| 11 | Skin and soft tissue infection | Amoxycillin/clavulanic acid | 1 g BD | 15 days | 0 |
| 12 | Skin and soft tissue infection | Doxycycline | 100 mg daily | 91 days | 0 |
| 13 | Skin and soft tissue infection | Amoxycillin/clavulanic acid | 375 mg tds | 7 days | 0 |
| 14 | Skin and soft tissue infection | Amoxycillin/clavulanic acid | 375 mg tds | 5 days | 35 days |
| 15 | Skin and soft tissue infection | Amoxycillin/clavulanic acid | 375 mg tds | 7 days | 0 |
| 16 | Dental infection | Amoxycillin | NA | 3 days | 0 |
| 17 | Dental infection | Amoxycillin + Metronidazole | 500 mg tds 400 mg tds | 15 days | 0 |
| 18 | Dental infection | Amoxycillin/clavulanic acid | 375 mg tds | 7 days | 14 days |
| 19 | Dental infection | Amoxicillin + metronidazole | 250 mg tds 400 mg tds | 7 days | 0 |
| 20 | Dental infection | Amoxycillin/clavulanic acid + metronidazole | 375 mg tds 200 mg tds | 4 days | 0 |
| 21 | Dental infection | Metronidazole | 200 mg tds | 7 days | 0 |
| 22 | Genitourinary tract infection | Amoxycillin/clavulanic acid | 1 g BD | 7 days | 0 |
| 23 | Genitourinary tract infection | Amoxycillin/clavulanic acid | 1 g BD | 7 days | 0 |
| 24 | Genitourinary tract infection | Amoxycillin/clavulanic acid | 1 g BD | 7 days | 0 |
| 25 | Genitourinary tract infection | Amoxycillin/clavulanic acid | 1 g BD | 7 days | 0 |
| 26 | Gastroenteritis | Amoxycillin/clavulanic acid + metronidazole | NA | 10 days | 0 |
| 27 | Helicobacter pylori infection | Amoxycillin + Clarithromycin | 1 g BD 500 mg BD | 14 days | 14 days |
| 28 | Upper respiratory tract infection | Amoxycillin | NA | 14 days | 49 days |
| 29 | Not documented | Levofloxacin | 500 mg daily | 7 days | 0 |
| Adjusted OR * | 95% CI | |
|---|---|---|
| BNT162b2 (one dose) Day 21 | ||
| Antibiotic usage | 0.26 | 0.08–0.96 |
| Age ≥ 60 years | 0.34 | 0.13–0.95 |
| Male sex | 0.14 | 0.05–0.34 |
| DM | 0.42 | 0.12–1.66 |
| Overweight/obesity | 0.92 | 0.37–2.29 |
| Hypertension | 1.07 | 0.44–2.82 |
| Raised LDL (≥3.4 mmol/L) | 3.82 | 1.01–25.4 |
| Smoking | 1.14 | 0.22–9.40 |
| Alcohol use | 1.02 | 0.26–5.20 |
| Moderate/severe hepatic steatosis (CAP ≥ 268 dB/M) | 2.48 | 0.80–8.83 |
| BNT162b2 (two doses) Day 56 | ||
| Antibiotic usage | 0.03 | 0.001–1.15 |
| Age ≥ 60 years | 0.38 | 0.006–69.18 |
| Male sex | NA * | NA * |
| DM | 0.02 | 0.0002–2.78 |
| Overweight/obesity | 0.58 | 0.008–27.85 |
| Hypertension | 0.40 | 0.008–25.06 |
| Raised LDL (≥3.4 mmol/L) | 0.56 | 0.01–101.48 |
| Smoking | NA * | NA * |
| Alcohol use | NA * | NA * |
| Moderate/severe hepatic steatosis (CAP ≥ 268 dB/M) | NA * | NA * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheung, K.-S.; Lam, L.-K.; Zhang, R.; Ooi, P.-H.; Tan, J.-T.; To, W.-P.; Hui, C.-H.; Chan, K.-H.; Seto, W.-K.; Hung, I.F.N.; et al. Association between Recent Usage of Antibiotics and Immunogenicity within Six Months after COVID-19 Vaccination. Vaccines 2022, 10, 1122. https://doi.org/10.3390/vaccines10071122
Cheung K-S, Lam L-K, Zhang R, Ooi P-H, Tan J-T, To W-P, Hui C-H, Chan K-H, Seto W-K, Hung IFN, et al. Association between Recent Usage of Antibiotics and Immunogenicity within Six Months after COVID-19 Vaccination. Vaccines. 2022; 10(7):1122. https://doi.org/10.3390/vaccines10071122
Chicago/Turabian StyleCheung, Ka-Shing, Lok-Ka Lam, Ruiqi Zhang, Poh-Hwa Ooi, Jing-Tong Tan, Wai-Pan To, Chun-Him Hui, Kwok-Hung Chan, Wai-Kay Seto, Ivan F. N. Hung, and et al. 2022. "Association between Recent Usage of Antibiotics and Immunogenicity within Six Months after COVID-19 Vaccination" Vaccines 10, no. 7: 1122. https://doi.org/10.3390/vaccines10071122
APA StyleCheung, K.-S., Lam, L.-K., Zhang, R., Ooi, P.-H., Tan, J.-T., To, W.-P., Hui, C.-H., Chan, K.-H., Seto, W.-K., Hung, I. F. N., & Leung, W. K. (2022). Association between Recent Usage of Antibiotics and Immunogenicity within Six Months after COVID-19 Vaccination. Vaccines, 10(7), 1122. https://doi.org/10.3390/vaccines10071122







