Biological Therapy of Severe Asthma with Dupilumab, a Dual Receptor Antagonist of Interleukins 4 and 13
Abstract
1. Introduction
2. Pathophysiologic Functions of IL-4 and IL-13 in Type 2 Asthma
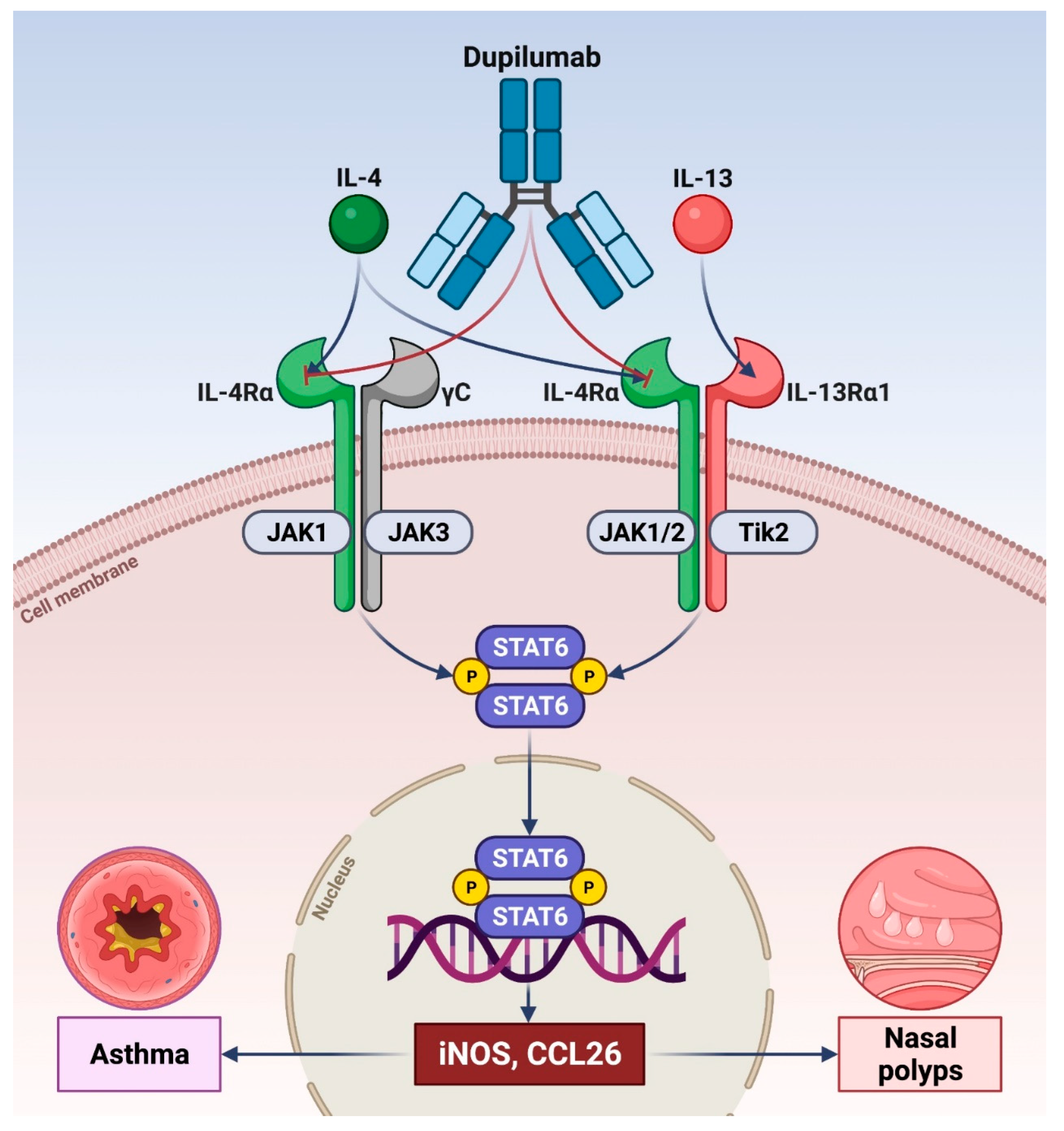
3. Role of Dupilumab as Add-On Biological Therapy of Severe Asthma
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Primers 2015, 1, 15025. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, K.; Paoletti, G.; Puggioni, F.; Racca, F.; De Luca, F.; Giorgis, V.; Canonica, G.W.; Heffler, E. Asthma from immune pathogenesis to precision medicine. Semin. Immunol. 2019, 46, 101294. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.; Pier, J.; Litonjua, A.A. Asthma epidemiology and risk factors. Semin. Immunopathol. 2020, 42, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.; Lee, G.B. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Thomsen, S.F. The contribution of twin studies to the understanding of the aetiology of asthma and atopic diseases. Eur. Clin. Respir. J. 2015, 2, 27803. [Google Scholar] [CrossRef]
- Komlósi, Z.I.; van de Veen, W.; Kovács, N.; Szűcs, G.; Sokolowska, M.; O’Mahony, L.; Akdis, M.; Akdis, C.A. Cellular and molecular mechanisms of allergic asthma. Mol. Asp. Med. 2022, 85, 100995. [Google Scholar] [CrossRef]
- Wenzel, S.E. Severe adult asthmas integrating clinical features, biology, and therapeutics to improve outcomes. Am. J. Respir. Crit. Care Med. 2021, 203, 809–821. [Google Scholar] [CrossRef]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Suraya, R.; Nagano, T.; Katsurada, M.; Sekiya, R.; Kobayashi, K.; Nishimura, Y. Molecular mechanism of asthma and its novel molecular target therapeutic agent. Respir. Investig. 2021, 59, 291–301. [Google Scholar] [CrossRef]
- Carr, T.F.; Zeki, A.A.; Kraft, M. Eosinophilic and noneosinophilic asthma. Am. J. Respir. Crit. Care Med. 2018, 197, 22–37. [Google Scholar] [CrossRef]
- Tliba, O.; Panettieri, R.A., Jr. Paucigranulocytic asthma, uncoupling of airway obstruction from inflammation. J. Allergy Clin. Immunol. 2019, 143, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.K.; Bush, A.; Stokes, J.; Nair, P.; Akuthota, P. Eosinophilic asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, N.; Gogoi, M.; McKenzie, A.N.J. Group 2 innate lymphoid cells: Team players in regulating asthma. Annu. Rev. Immunol. 2021, 39, 167–198. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef]
- Matucci, A.; Bormioli, S.; Nencini, F.; Maggi, E.; Vultaggio, A. The emerging role of type 2 inflammation in asthma. Expert Rev. Clin. Immunol. 2021, 17, 63–71. [Google Scholar] [CrossRef]
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the pathophysiology of severe asthma. Front. Physiol. 2019, 10, 1514. [Google Scholar] [CrossRef]
- Calven, J.; Ax, E.; Radinger, M. The airway epithelium—A central player in asthma pathogenesis. Int. J. Mol. Sci. 2020, 21, 8907. [Google Scholar] [CrossRef]
- Hong, H.; Liao, S.; Chen, F.; Yang, Q.; Wang, D.Y. Role of IL-25, IL-33, and TSLP in triggering united airway diseases toward type 2 inflammation. Allergy 2020, 75, 2794–2804. [Google Scholar] [CrossRef]
- Christianson, C.A.; Goplen, N.P.; Zafar, I.; Irvin, C.; Good, J.T., Jr.; Rollins, D.R.; Gorentla, B.; Liu, W.; Gorska, M.M.; Chu, H.; et al. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J. Allergy Clin. Immunol. 2015, 136, 59–68. [Google Scholar] [CrossRef]
- Pelaia, C.; Crimi, C.; Vatrella, A.; Tinello, C.; Terracciano, R.; Pelaia, G. Molecular targets for biological therapies of severe asthma. Front. Immunol. 2020, 11, 603312. [Google Scholar] [CrossRef]
- Pelaia, C.; Pelaia, G.; Crimi, C.; Longhini, F.; Lombardo, N.; Savino, R.; Sciacqua, A.; Vatrella, A. Biologics in severe asthma. Minerva. Med. 2022, 113, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Kraft, M.; Rabe, K.F.; Deniz, Y.; Rowe, P.J.; Ruddy, M.; Castro, M. Understanding the key issues in the treatment of uncontrolled persistent asthma with type 2 inflammation. Eur. Respir. J. 2021, 58, 2003393. [Google Scholar] [CrossRef] [PubMed]
- Porsbjerg, C.M.; Sverrild, A.; Lloyd, C.M.; Menzies-Gow, A.N.; Bel, E.H. Anti-alarmins in asthma: Targeting the airway epithelium with next-generation biologics. Eur. Respir. J. 2020, 56, 2000260. [Google Scholar] [CrossRef]
- Albrecht, M. Turning off the alarm—Targeting alarmins and other epithelial mediators of allergic inflammation with biologics. Allergol. Select. 2021, 5, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Vatrella, A.; Gallelli, L.; Terracciano, R.; Navalesi, P.; Maselli, R.; Pelaia, G. Dupilumab for the treatment of asthma. Expert. Opin. Biol. Ther. 2017, 17, 1565–1572. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Bertolini, F.; Carriero, V. The role of dupilumab in severe asthma. Biomedicines 2021, 9, 1096. [Google Scholar] [CrossRef]
- Pelaia, C.; Heffler, E.; Crimi, C.; Maglio, A.; Vatrella, A.; Pelaia, G.; Canonica, G.W. Interleukins 4 and 13 in asthma: Key pathophysiologic cytokines and druggable molecular targets. Fron. Pharmacol. 2022, 13, 851940. [Google Scholar] [CrossRef]
- Steinke, J.W.; Borish, L. Th2 cytokines and asthma. Interleukin 4: Its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir. Res. 2001, 2, 66–70. [Google Scholar] [CrossRef]
- Corren, J. Role of interleukin-13 in asthma. Curr. Allergy Asthma Rep. 2013, 13, 415–420. [Google Scholar] [CrossRef]
- Maggi, L.; Montaini, G.; Mazzoni, A.; Rossettini, B.; Capone, M.; Rossi, M.C.; Santarlasci, V.; Liotta, F.; Rossi, O.; Gallo, O.; et al. Human circulating group 2 innate lymphoid cells can express CD154 and promote IgE production. J. Allergy Clin. Immunol. 2017, 139, 964–976. [Google Scholar] [CrossRef]
- Vijayanand, P.; Seumois, G.; Simpson, L.J.; Abdul-Wajid, S.; Baumjohann, D.; Panduro, M.; Huang, X.; Interlandi, J.; Djuretic, I.M.; Brown, D.R.; et al. Interleukin-4 production by follicular helper T cells requires the conserved IL-4 enhancer hypersensitivity site V. Immunity 2012, 36, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, U.; Chen, J.S.; Zhang, B.; Flynn, W.F.; Lu, Y.; Song, W.; Joseph, J.; Gertie, J.A.; Xu, L.; Collet, M.A.; et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 2019, 365, 6456. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, G.E.; Horvat, J.C.; Beagley, K.W.; Hansbro, P.M. Immunological decision making: How does the immune system decide to mount a helper T-cell response? Immunology 2008, 123, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Sokol, C.L.; Barton, G.M.; Farr, A.G.; Medzhitov, R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nature Immunol. 2008, 9, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Chen, J.; Zhang, H.; Duan, L. Interleukin-4 inhibits regulatory T cell differentiation through regulating CD103+ dendritic cells. Front. Immunol. 2017, 8, 214. [Google Scholar] [CrossRef]
- Palomares, O.; Akdis, M.; Martin-Fontecha, M.; Akdis, C.A. Mechanisms of immune regulation in allergic diseases: The role of regulatory T and B cells. Immunol. Rev. 2017, 278, 219–236. [Google Scholar] [CrossRef]
- Finkelman, F.D.; Katona, I.M.; Urban, J.F., Jr.; Holmes, J.; Ohara, J.; Tung, A.S.; Sample, J.V.; Paul, W.E. IL-4 is required to generate and sustain in vivo IgE responses. J. Immunol. 1988, 141, 2335–2341. [Google Scholar]
- Grünig, G.; Warnock, M.; Wakil, A.E.; Venkayya, R.; Brombacher, F.; Rennick, D.M.; Sheppard, D.; Mohrs, M.; Donaldson, D.D.; Locksley, R.M.; et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998, 282, 2261–2263. [Google Scholar] [CrossRef]
- Novosad, J.; Krcmova, I. Evaluation of our view on the IgE molecule role in bronchial asthma and the clinical effect of its modulation by omalizumab: Where do we stand today? Int. J. Immunopathol. Pharmacol. 2020, 34, 1–15. [Google Scholar] [CrossRef]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef]
- Marone, G.; Granata, F.; Pucino, V.; Pecoraro, A.; Heffler, E.; Loffredo, S.; Scadding, G.W.; Varricchi, G. The intriguing role of interleukin 13 in the pathophysiology of asthma. Front. Pharmacol. 2019, 10, 1387. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.H.; Abdelwahab, S.F.; Wan, J.; Cai, W.; Huixuan, W.; Jianjun, C.; Kumar, K.D.; Vasudevan, A.; Sadek, A.; Su, Z.; et al. Alternatively activated macrophages, a double-edged sword in allergic asthma. J. Transl. Med. 2020, 18, 58. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Díaz, M.; Lerner, A.D.; Yu, D.H.; Thiboutot, J.P.; Liu, M.C.; Yarmus, L.B.; Bose, S.; Heller, N.M. Sex differences in M2 polarization, chemokine and IL-4 receptors in monocytes and macrophages from asthmatics. Cell Immunol. 2021, 360, 104252. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, H.F.; Phipps, S.; Foster, P.S. Eosinophil trafficking in allergy and asthma. J. Allergy Clin. Immunol. 2007, 119, 1303–1310. [Google Scholar] [CrossRef]
- Komiya, A.; Nagase, H.; Yamada, H.; Sekiya, T.; Yamaguchi, M.; Sano, Y.; Hanai, N.; Furuya, A.; Ohta, K.; Matsushima, K.; et al. Concerted expression of eotaxin-1, eotaxin-2, and eotaxin-3 in human bronchial epithelial cells. Cell Immunol. 2003, 225, 91–100. [Google Scholar] [CrossRef]
- Sweerus, K.; Lachowicz-Scroggins, M.; Gordon, E.; LaFemina, M.; Huang, X.; Parikh, M.; Kanegai, C.; Fahy, J.V.; Frank, J.A. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J. Allergy Clin. Immunol. 2017, 139, 72–81.e71. [Google Scholar] [CrossRef]
- Steelant, B.; Wawrzyniac, P.; Martens, K.; Jonckheere, A.C.; Pugin, B.; Schrijvers, R.; Bullens, D.M.; Vanoirbeek, J.A.; Krawczyk, K.; Dreher, A.; et al. Blocking histone deacetylase activity as a novel target for epithelial barrier defects in patients with allergic rhinitis. J. Allergy Clin. Immunol. 2019, 144, 1242–1253.e1247. [Google Scholar] [CrossRef]
- Dickinson, J.D.; Alevy, Y.; Malvin, N.P.; Patel, K.K.; Gunsten, S.P.; Holtzman, M.J.; Stappenbeck, T.S.; Brody, S.L. IL-13 activates autophagy to regulate secretion in airway epithelial cells. Autophagy 2016, 12, 397–409. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Silkoff, P.E. Perspectives on exhaled nitric oxide. J. Breath Res. 2017, 11, 047104. [Google Scholar] [CrossRef]
- Lee, C.G.; Homer, R.J.; Zhu, Z.; Lanone, S.; Wang, X.; Koteliansky, V.; Shipley, J.M.; Gotwals, P.; Noble, P.; Chen, Q.; et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor-β1. J. Exp. Med. 2001, 194, 809–821. [Google Scholar] [CrossRef]
- Firszt, R.; Francisco, D.; Church, T.D.; Thomas, J.M.; Ingram, J.L.; Kraft, M. Interleukin-13 induces collagen type-1 expression through matrix metalloproteinase-2 and transforming growth factor-β1 in airway fibroblasts in asthma. Eur. Respir. J. 2014, 43, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Maes, T.; Joos, G.F.; Brusselle, G.G. Targeting IL-4 in asthma: Lost in translation? Am. J. Respir. Cell Mol. Biol. 2012, 47, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Berry, M.A.; Parker, D.; Siddiqui, S.; Morgan, A.; May, R.; Monk, P.; Bradding, P.; Wardlaw, A.J.; Pavord, I.D.; et al. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J. Allergy Clin. Immunol. 2008, 121, 685–691. [Google Scholar] [CrossRef]
- Prieto, J.; Lensmar, C.; Roquet, A.; van der Ploeg, I.; Gigliotti, D.; Eklund, A.; Grunewald, J. Increased interleukin-13 mRNA expression in bronchoalveolar lavage cells of atopic patients with mild asthma after repeated low-dose allergen provocations. Respir. Med. 2000, 94, 806–814. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Howard, T.D.; Koppelman, G.H.; Xu, J.; Zheng, S.L.; Postma, D.S.; Meyers, D.A.; Bleecker, E.R. Gene-gene interaction in asthma: IL4RA and IL-13 in a Dutch population with asthma. Am. J. Hum. Genet. 2002, 70, 230–236. [Google Scholar] [CrossRef]
- Li, X.; Howard, T.D.; Zheng, S.L.; Haselkorn, T.; Peters, S.P.; Meyers, D.A.; Bleecker, E.R. Genome-wide association study of asthma identifies RAD50-IL13 and HLA-DR/DQ regions. J. Allergy Clin. Immunol. 2010, 125, 328–335. [Google Scholar] [CrossRef]
- Coyle, A.J.; Le Gros, G.; Bertrand, C.; Tsuyuki, S.; Heusser, C.H.; Kopf, M.; Anderson, G.P. Interleukin-4 is required for the induction of lung Th2 mucosal immunity. Am. J. Respir. Cell Mol. Biol. 1995, 13, 54–59. [Google Scholar] [CrossRef]
- Wills-Karp, M.; Luyimbazi, J.; Xu, X.; Schofield, B.; Neben, T.Y.; Karp, C.L.; Donaldson, D.D. Interleukin-13: Central mediator of allergic asthma. Science 1998, 282, 2258–2261. [Google Scholar] [CrossRef]
- Munitz, A.; Brandt, E.B.; Mingler, M.; Finkelman, F.D.; Rothenberg, M.E. Distinct roles for IL-13 and IL-4 via IL-13 receptor α1 and the type II IL-4 receptor in asthma pathogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 7240–7245. [Google Scholar] [CrossRef]
- Kotsimbos, T.C.; Ernst, P.; Hamid, Q.A. Interleukin-13 and interleukin-4 are coexpressed in atopic asthma. Proc. Assoc. Am. Physicians. 1996, 108, 368–373. [Google Scholar]
- Nelms, K.; Keegan, A.D.; Zamorano, J.; Ryan, J.J.; Paul, W.E. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999, 17, 701–738. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Tanaka, T.; Shi, W.; Matsumoto, M.; Minami, M.; Kashiwamura, S.; Nakanishi, K.; Yoshida, N.; Kishimoto, T.; Akira, S. Essential role of STAT6 in IL-4 signalling. Nature 1996, 380, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Chatila, T.A. Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends Mol. Med. 2004, 10, 493–499. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.M.; Heller, N.M. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine 2015, 75, 38–50. [Google Scholar] [CrossRef]
- Wills-Karp, M.; Finkelman, F.D. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci. Signal. 2008, 1, pe55. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Welch, A.E.; Hanson, E.M.; Boothby, M.R.; Keegan, A.D. Interleukin-4 and interleukin-13 signaling connections maps. Science 2003, 300, 1527–1528. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.H.; Schindler, U.; Smiley, S.T.; Grusby, M.J. STAT-6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 1996, 4, 313–319. [Google Scholar] [CrossRef]
- Harb, H.; Chatila, T. Mechanisms of dupilumab. Clin. Exp. Allergy 2020, 50, 5–14. [Google Scholar] [CrossRef]
- Gour, N.; Wills-Karp, M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine 2015, 75, 68–78. [Google Scholar] [CrossRef]
- Massey, O.; Suphioglu, C. Recent advances in the inhibition of the IL-4 cytokine pathway for the treatment of allergen-induced asthma. Int. J. Mol. Sci. 2021, 22, 13655. [Google Scholar] [CrossRef]
- Zeng, W.P. ‘All things considered’: Transcriptional regulation of T helper type 2 cell differentiation from precursor to effector activation. Immunology 2013, 140, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Tindemans, I.; Serafini, N.; Di Santo, J.P.; Hendriks, R.W. GATA-3 function in innate and adaptive immunity. Immunity 2014, 41, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.; Rosa, L.; Daines, M.; Khurana Hershey, G. Reconstitution of a functional human type II IL-4/IL-13 receptor in mouse B cells: Demonstration of species specificity. J. Immunol. 2001, 166, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Goto, K.; Misawa, M. Interleukin-13-induced activation of signal transducer and activator of transcription 6 is mediated by an activation of Janus kinase 1 in cultured human bronchial smooth muscle cells. Pharmacol. Rep. 2012, 64, 454–458. [Google Scholar]
- Zheng, T.; Liu, W.; Oh, S.Y.; Zhu, Z.; Hu, B.; Homer, R.J.; Cohn, L.; Grusby, M.J.; Elias, J.A. IL-13 receptor α2 selectively inhibits IL-13-induced responses in the murine lung. J. Immunol. 2008, 180, 522–529. [Google Scholar] [CrossRef]
- Shirley, M. Dupilumab: First global approval. Drugs 2017, 77, 1115–1121. [Google Scholar] [CrossRef]
- Santini, G.; Mores, N.; Malerba, M.; Mondino, C.; Anzivino, R.; Macis, G.; Montuschi, P. Dupilumab for the treatment of asthma. Expert Opin. Investig. Drugs 2017, 26, 357–366. [Google Scholar] [CrossRef]
- Vatrella, A.; Fabozzi, I.; Calabrese, C.; Maselli, R.; Pelaia, G. Dupilumab: A novel treatment for asthma. J. Asthma. Allergy 2014, 7, 123–130. [Google Scholar] [CrossRef]
- Kovalenko, P.; DiCioccio, A.T.; Davis, J.D.; Li, M.; Ardeleanu, M.; Graham, N.; Soltys, R. Exploratory population PK analysis of dupilumab, a fully human monoclonal antibody against IL-4Rα, in atopic dermatitis patients and normal volunteers. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 617–624. [Google Scholar] [CrossRef]
- Wenzel, S.; Ford, L.; Pearlman, D.; Spector, S.; Sher, L.; Skobieranda, F.; Wang, L.; Kirkesseli, S.; Rocklin, R.; Bock, B.; et al. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 2013, 368, 2455–2466. [Google Scholar] [CrossRef]
- Wechsler, M.E. Inhibiting IL-4 and IL-13 in difficult-to-control asthma. N. Engl. J. Med. 2013, 368, 2511–2513. [Google Scholar] [CrossRef]
- Wenzel, S.; Castro, M.; Corren, J.; Maspero, J.; Wang, L.; Zhang, B.; Pirozzi, G.; Sutherland, E.R.; Evans, R.R.; Joish, V.N.; et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016, 388, 31–44. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Castro, M.; O’Riordan, T.; Hanania, N.A.; Pavord, I.D.; Quirce, S.; Chipps, B.E.; Wenzel, S.E.; Thangavelu, K.; Rice, M.S.; et al. Dupilumab efficacy in patients with uncontrolled, moderate-to-severe allergic asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 516–526. [Google Scholar] [CrossRef]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. New Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Ford, L.B.; Maspero, J.F.; Pavord, I.D.; Papi, A.; Bourdin, A.; Watz, H.; Castro, M.; Nenasheva, N.M.; Tohda, Y.; et al. Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): An open-label extension study. Lancet Respir. Med. 2022, 10, 11–25. [Google Scholar] [CrossRef]
- Bacharier, L.B.; Maspero, J.F.; Katelaris, C.H.; Fiocchi, A.G.; Gagnon, R.; de Mir, I.; Jain, N.; Sher, L.D.; Mao, X.; Liu, D.; et al. Dupilumab in children with uncontrolled moderate-to-severe asthma. New Engl. J. Med. 2021, 385, 2230–2240. [Google Scholar] [CrossRef]
- Dupin, C.; Belhadi, D.; Guilleminault, L.; Gamez, A.S.; Berger, P.; De Blay, F.; Bonniaud, P.; Leroyer, C.; Mahay, G.; Girodet, P.O.; et al. Effectiveness and safety of dupilumab for the treatment of severe asthma in a real-life French multi-centre adult cohort. Clin. Exp. Allergy 2020, 50, 789–798. [Google Scholar] [CrossRef]
- Campisi, R.; Crimi, C.; Nolasco, S.; Beghè, B.; Antonicelli, L.; Guarnieri, G.; Scichilone, N.; Porto, M.; Macchia, L.; Scioscia, G.; et al. Real-world experience with dupilumab in severe asthma: One-year data from an Italian Named Patient Program. J. Asthma Allergy 2021, 14, 575–583. [Google Scholar] [CrossRef]
- Pelaia, C.; Lombardo, N.; Busceti, M.T.; Piazzetta, G.; Crimi, C.; Calabrese, C.; Vatrella, A.; Pelaia, G. Short-term evaluation of dupilumab effects in patients with severe asthma and nasal polyposis. J. Asthma Allergy 2021, 14, 1165–1172. [Google Scholar] [CrossRef]
- Jarjour, N.N.; Erzurum, S.C.; Bleecker, E.R.; Calhoun, W.J.; Castro, M.; Comhair, S.A.; Chung, K.F.; Curran-Everett, D.; Dweik, R.A.; Fain, S.B.; et al. Severe asthma—Lessons learned from the National Heart, Lung, and Blood Institute severe asthma research program. Am. J. Respir. Crit. Care Med. 2012, 185, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef]
- Benzecry, V.; Pravettoni, V.; Segatto, G.; Marzano, A.V.; Ferrucci, S. Type 2 inflammation: Atopic dermatitis, asthma, and hypereosinophilia successfully treated with dupilumab. J. Investig. Allergol. Clin. Immunol. 2021, 31, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Dellon, E.S.; Hamilton, J.D.; Collins, M.H.; Peterson, K.; Chehade, M.; Schoepfer, A.M.; Safroneeva, E.; Rothenberg, M.E.; Falk, G.W.; et al. Efficacy of dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology 2020, 158, 111–122.e10. [Google Scholar] [CrossRef]
- Moran, A.; Pavord, I.D. Anti-IL-4/IL-13 for the treatment of asthma: The story so far. Expert Opin. Biol. Ther. 2020, 20, 283–294. [Google Scholar] [CrossRef]
| Cytokine | Cellular Target | Main Effects |
|---|---|---|
| IL-4 | Th cells | Commitment to Th2 cell lineage |
| IL-4 and IL-13 | B cells | IgE isotype switch |
| IL-4 and IL-13 | Eosinophils | Cell trafficking towards inflammatory sites |
| IL-4 and IL-13 | Airway epithelial cells | Disruption of epithelial layer, up-regulation of iNOS |
| IL-13 | Goblet cells | Mucus production |
| IL-13 | Airway fibroblasts | Cell proliferation |
| IL-13 | Airway smooth muscle cells | Increased contractility and proliferation |
| Trial Name | Duration | Main Results or Endpoints |
|---|---|---|
| LIBERTY ASTHMA QUEST [83] | 52 weeks | Fewer asthma exacerbations, better ACQ score, FEV1 increase, lower FeNO levels. |
| LIBERTY ASTHMA VENTURE [85] | 24 weeks | Decreased OCS intake, fewer asthma exacerbations, FEV1 increase. |
| TRAVERSE [86] | 96 weeks | Long-term safety and efficacy. |
| LIBERTY ASTHMA VOYAGE [87] | 52 weeks | In children: fewer asthma exacerbations, better ACQ score, FEV1 increase. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelaia, C.; Pelaia, G.; Crimi, C.; Maglio, A.; Armentaro, G.; Calabrese, C.; Sciacqua, A.; Gallelli, L.; Vatrella, A. Biological Therapy of Severe Asthma with Dupilumab, a Dual Receptor Antagonist of Interleukins 4 and 13. Vaccines 2022, 10, 974. https://doi.org/10.3390/vaccines10060974
Pelaia C, Pelaia G, Crimi C, Maglio A, Armentaro G, Calabrese C, Sciacqua A, Gallelli L, Vatrella A. Biological Therapy of Severe Asthma with Dupilumab, a Dual Receptor Antagonist of Interleukins 4 and 13. Vaccines. 2022; 10(6):974. https://doi.org/10.3390/vaccines10060974
Chicago/Turabian StylePelaia, Corrado, Giulia Pelaia, Claudia Crimi, Angelantonio Maglio, Giuseppe Armentaro, Cecilia Calabrese, Angela Sciacqua, Luca Gallelli, and Alessandro Vatrella. 2022. "Biological Therapy of Severe Asthma with Dupilumab, a Dual Receptor Antagonist of Interleukins 4 and 13" Vaccines 10, no. 6: 974. https://doi.org/10.3390/vaccines10060974
APA StylePelaia, C., Pelaia, G., Crimi, C., Maglio, A., Armentaro, G., Calabrese, C., Sciacqua, A., Gallelli, L., & Vatrella, A. (2022). Biological Therapy of Severe Asthma with Dupilumab, a Dual Receptor Antagonist of Interleukins 4 and 13. Vaccines, 10(6), 974. https://doi.org/10.3390/vaccines10060974











