Cellular and Humoral Immunity after the Third Vaccination against SARS-CoV-2 in Hematopoietic Stem-Cell Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Volunteers
2.2. CoV-iSpot for Interferon-γ and Interleukin-2
2.3. In-House ELISpot Assay
2.4. Assessment of Neutralizing Antibodies by Competitive Immunofluorescence
2.5. Assessment of Neutralizating Antibodies by Cytopathic Effects
2.6. Antibody ELISA
2.7. Statistical Analysis
3. Results
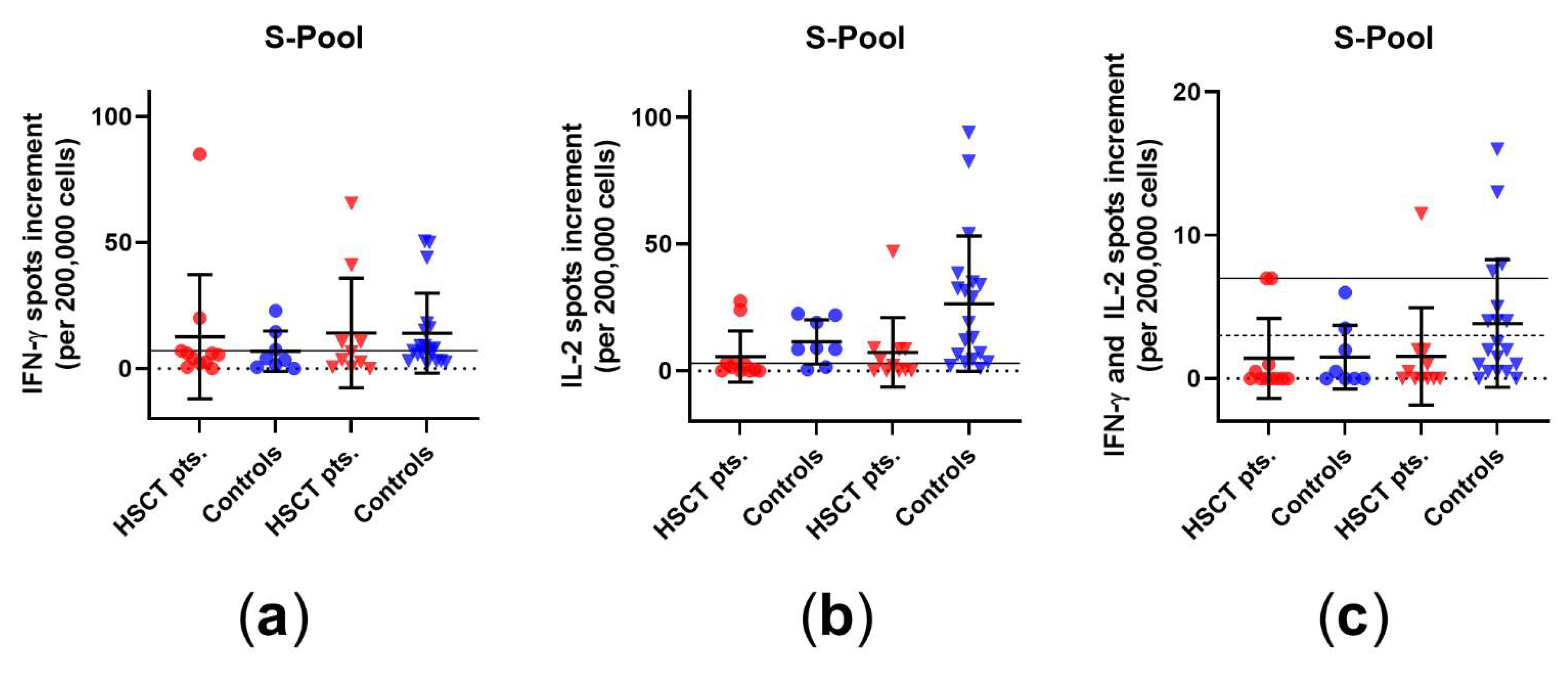
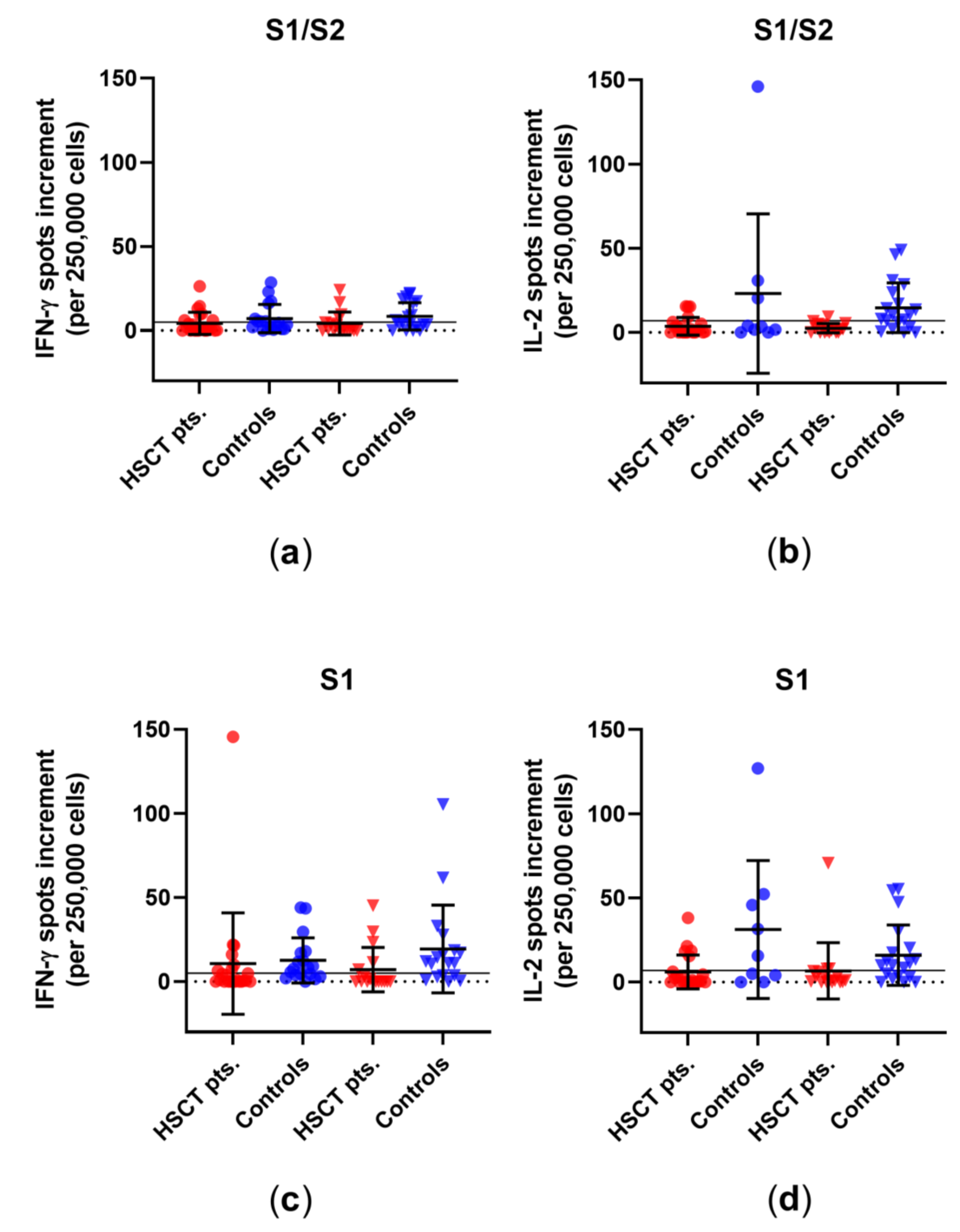
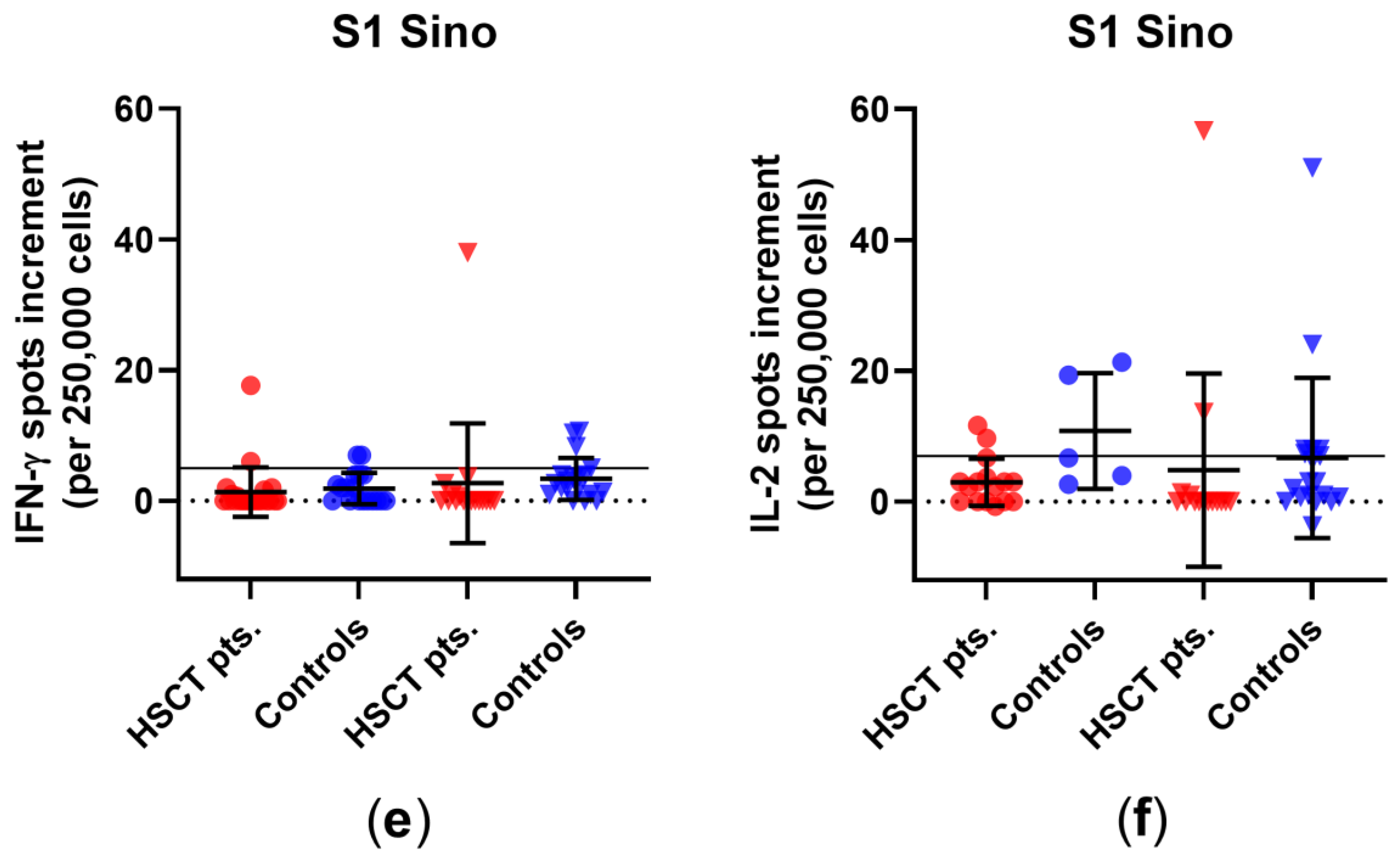
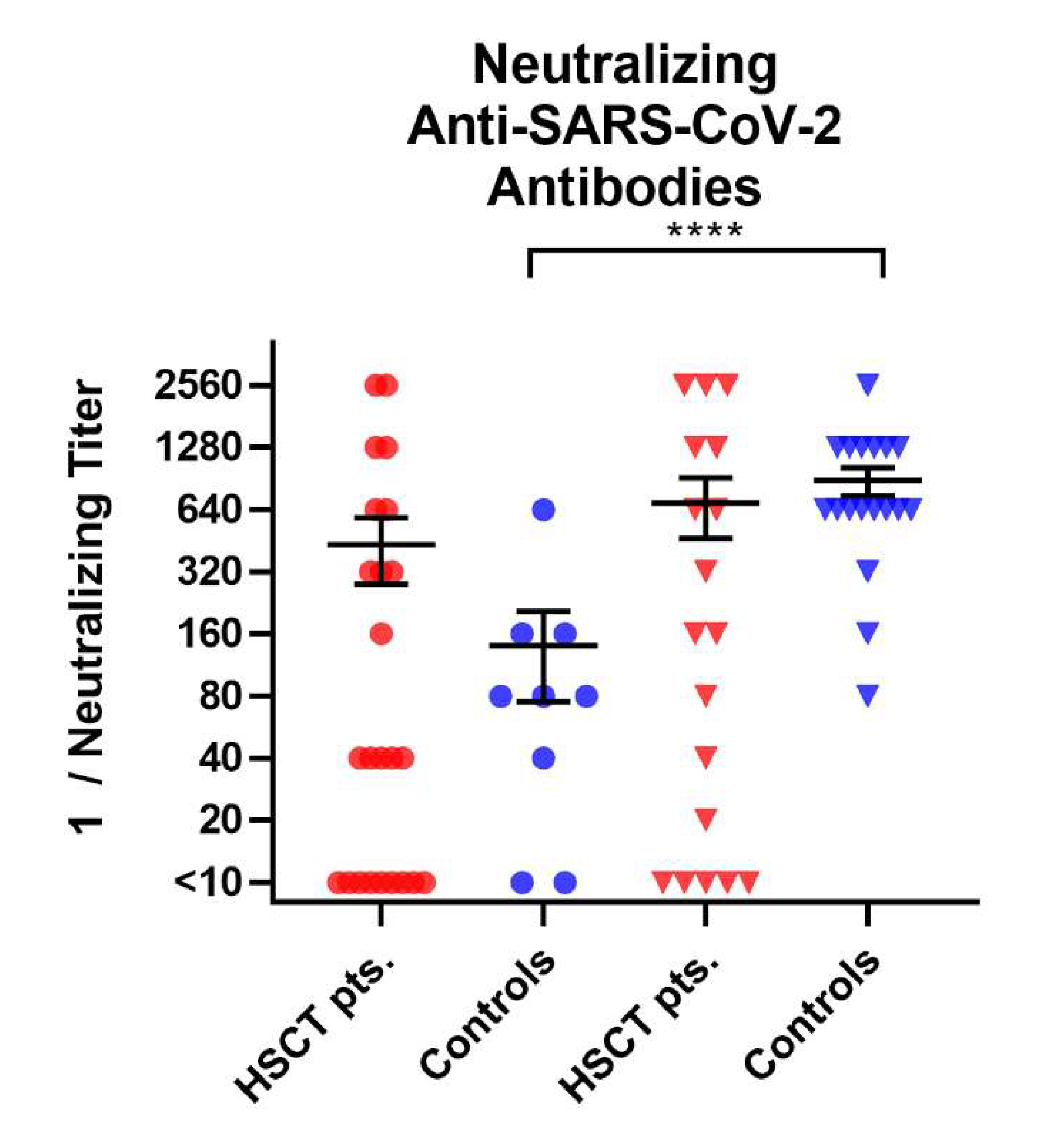
3.1. Comparison of Cellular Immunity in HSCT Patients and Healthy Controls before and after Third Vaccination
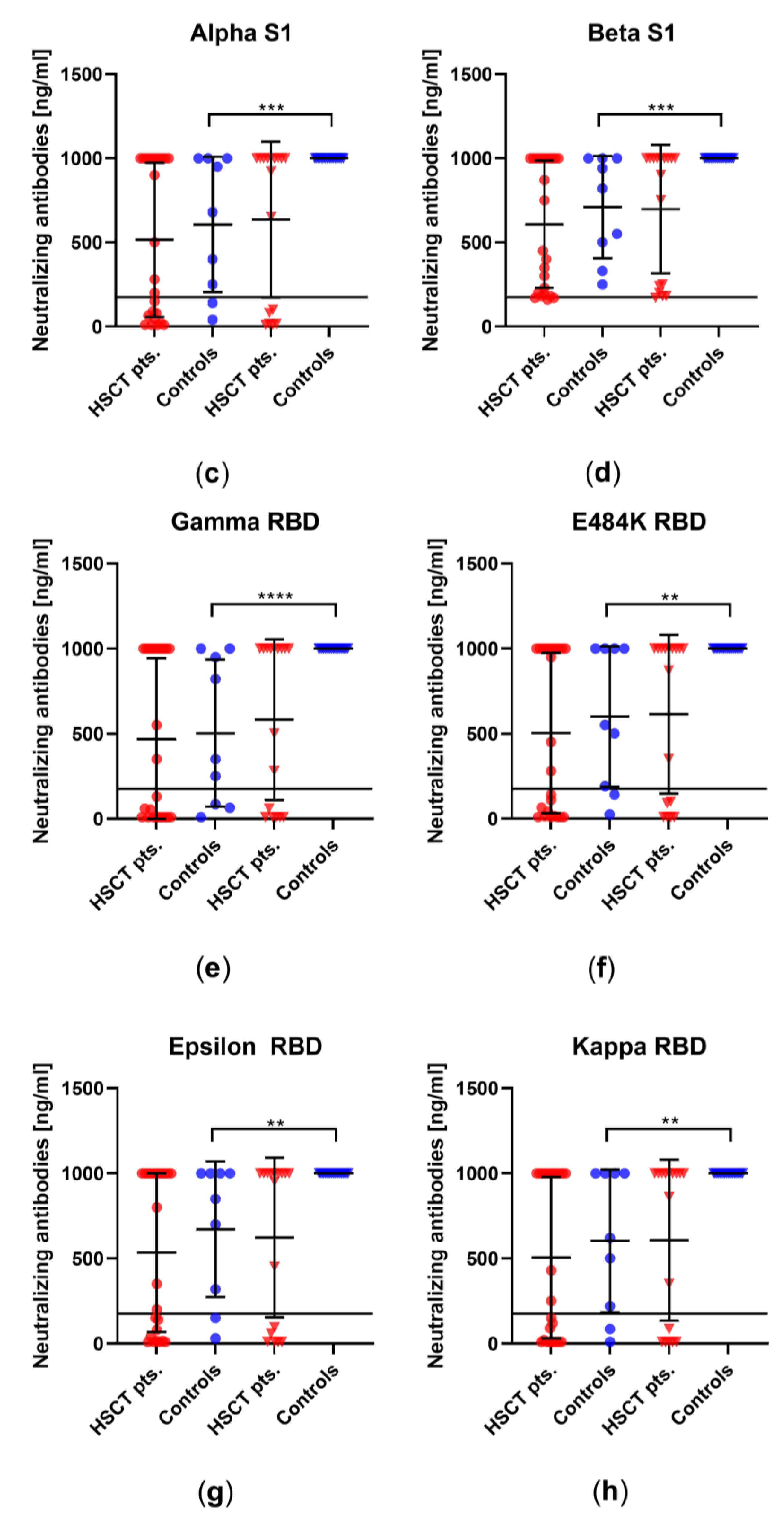
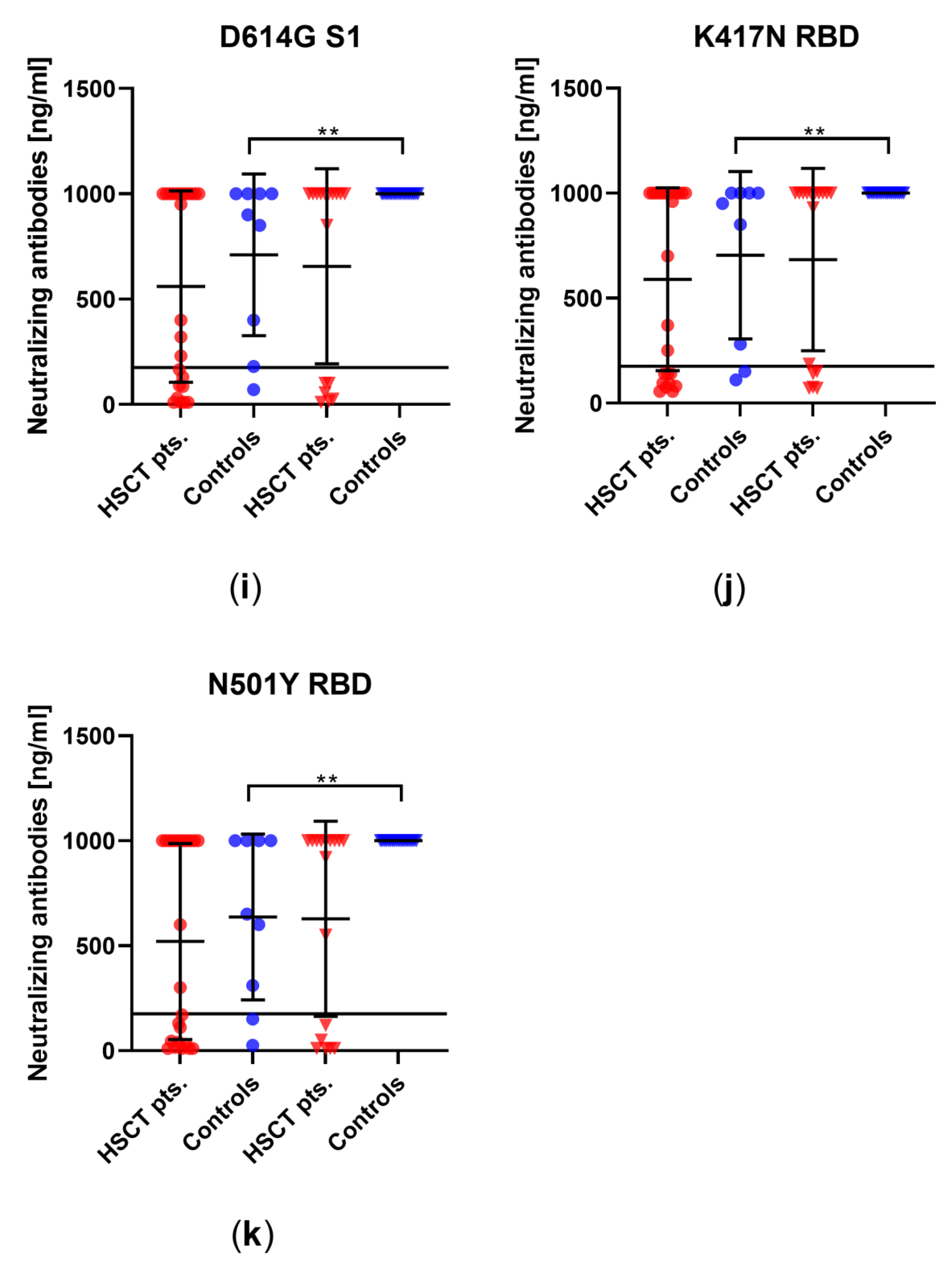
3.2. Comparison of Humoral Immune Responses to Variants of SARS-CoV-2 in HSCT Patients and Healthy Controls
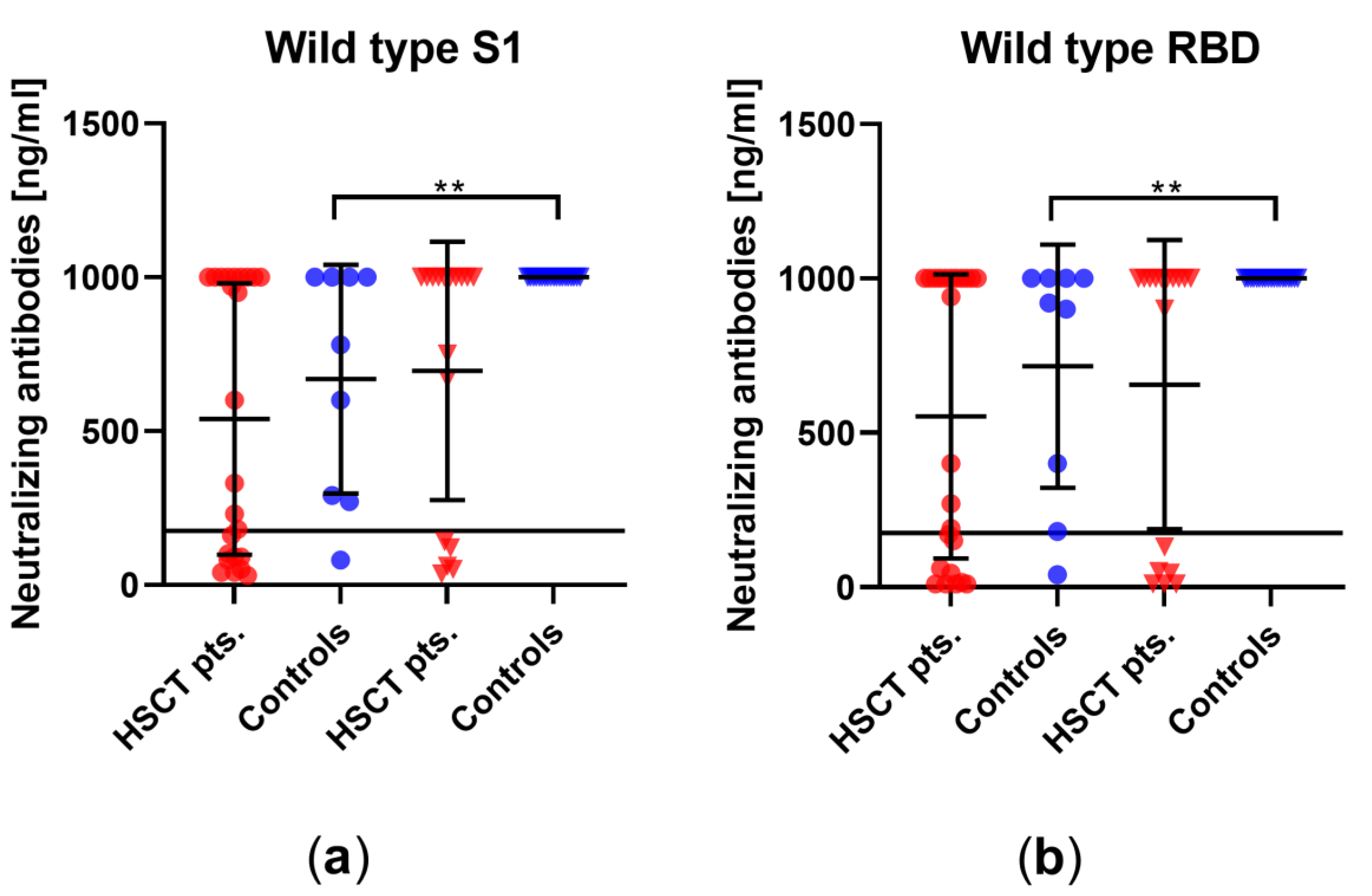
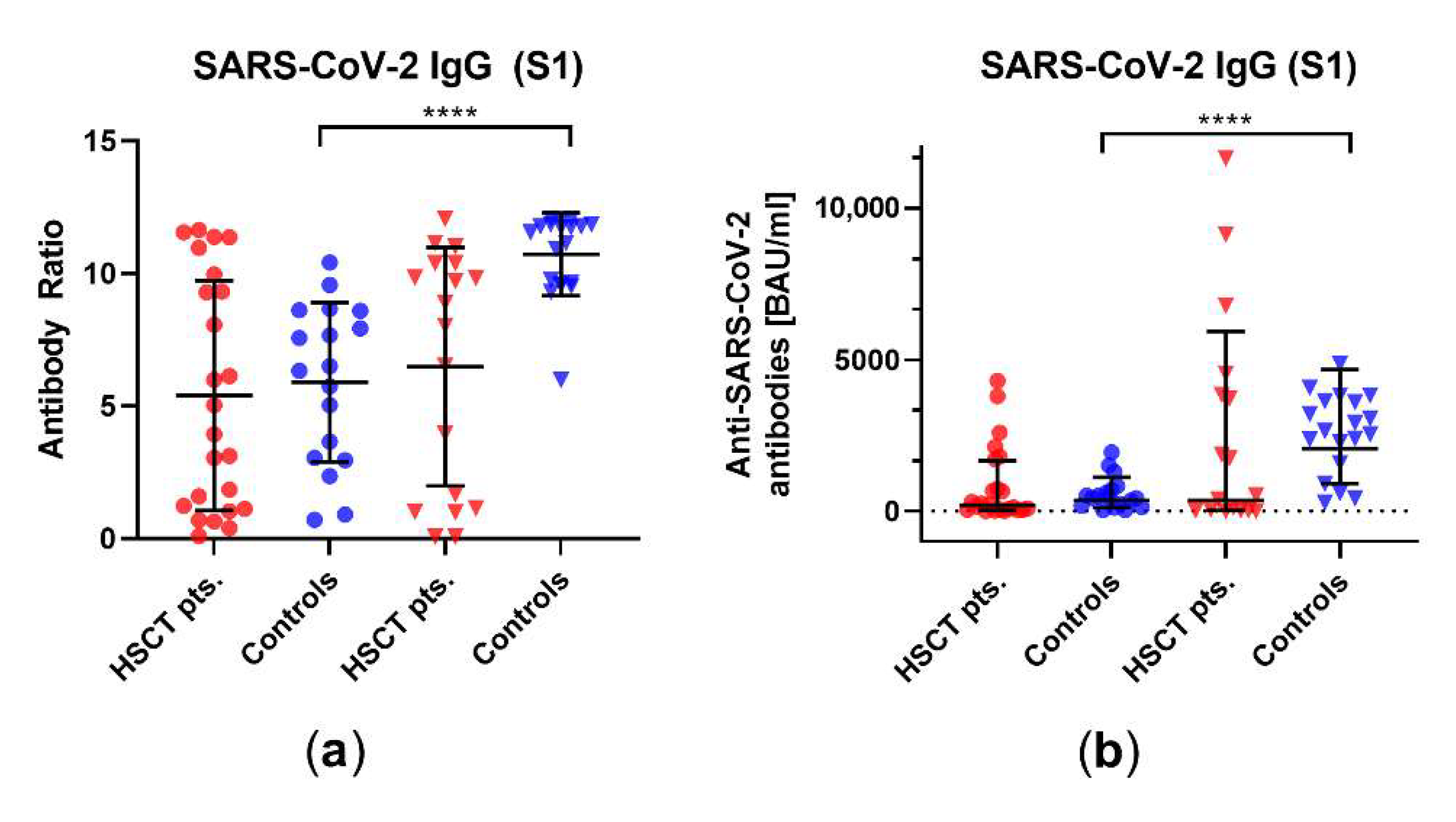
3.3. Comparison of Humoral Vaccination Responses to Wildtype Virus in Patients after Hematopoietic Stem-Cell Transplantation and Healthy Controls
3.4. Correlation of SARS-CoV-2-Specific Immune Responses with Age and Interval between HSCT and Blood Collection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eberhardt, C.S.; Balletto, E.; Cornberg, M.; Mikulska, M. Coronavirus disease 2019 vaccination in transplant recipients. Curr. Opin. Infect. Dis. 2021, 34, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Grupper, A.; Rabinowich, L.; Schwartz, D.; Schwartz, I.F.; Ben-Yehoyada, M.; Shashar, M.; Katchman, E.; Halperin, T.; Turner, D.; Goykhman, Y.; et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transplant. 2021, 21, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Caillard, S.; Chavarot, N.; Francois, H.; Matignon, M.; Greze, C.; Kamar, N.; Gatault, P.; Thaunat, O.; Legris, T.; Frimat, L.; et al. Is COVID-19 infection more severe in kidney transplant recipients? Am. J. Transplant. 2021, 21, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Coronavirus (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int/ (accessed on 15 June 2022).
- Giesen, N.; Sprute, R.; Rüthrich, M.; Khodamoradi, Y.; Mellinghoff, S.C.; Beutel, G.; Lueck, C.; Koldehoff, M.; Hentrich, M.; Sandherr, M.; et al. Evidence-based management of COVID-19 in cancer patients: Guideline by the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). Eur. J. Cancer 2020, 140, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Giesen, N.; Sprute, R.; Rüthrich, M.; Khodamoradi, Y.; Mellinghoff, S.C.; Beutel, G.; Lueck, C.; Koldehoff, M.; Hentrich, M.; Sandherr, M.; et al. 2021 update of the AGIHO guideline on evidence-based management of COVID-19 in patients with cancer regarding diagnostics, viral shedding, vaccination and therapy. Eur. J. Cancer 2021, 147, 154–160. [Google Scholar] [CrossRef]
- Gagelmann, N.; Passamonti, F.; Wolschke, C.; Massoud, R.; Niederwieser, C.; Adjallé, R.; Mora, B.; Ayuk, F.; Kröger, N. Antibody response after vaccination against SARS-CoV-2 in adults with haematological malignancies: A systematic review and meta-analysis. Haematologica 2021. ahead of print. [Google Scholar] [CrossRef]
- Washington State Department of Health. SARS-CoV-2 Vaccine Breakthrough Surveillance and Case Information Resource. 2022. Available online: https://www.doh.wa.gov/Portals/1/Documents/1600/coronavirus/data-tables/420-339-VaccineBreakthroughReport.pdf (accessed on 12 June 2022).
- Statista. 2021. Available online: https://de.statista.com/statistik/daten/studie/1273795/umfrage/anteil-impfdurchbrueche-unter-symptomatischen-covid-19-faellen-in-deutschland/#professional (accessed on 20 May 2022).
- Werbel, W.A.; Boyarsky, B.J.; Ou, M.T.; Massie, A.B.; Tobian, A.A.R.; Garonzik-Wang, J.M.; Segev, D.L. Safety and Immunogenicity of a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Ann. Intern. Med. 2021, 174, 1330–1332. [Google Scholar] [CrossRef]
- Hall, V.G.; Ferreira, V.H.; Ku, T.; Ierullo, M.; Majchrzak-Kita, B.; Chaparro, C.; Selzner, N.; Schiff, J.; McDonald, M.; Tomlinson, G.; et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N. Engl. J. Med. 2021, 385, 1244–1246. [Google Scholar] [CrossRef]
- Peled, Y.; Ram, E.; Lavee, J.; Segev, A.; Matezki, S.; Wieder-Finesod, A.; Halperin, R.; Mandelboim, M.; Indenbaum, V.; Levy, I.; et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: Immunogenicity and clinical experience. J. Heart Lung Transplant. 2022, 41, 148–157. [Google Scholar] [CrossRef]
- Redjoul, R.; Le Bouter, A.; Parinet, V.; Fourati, S.; Maury, S. Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT. Lancet Haematol. 2021, 8, e681–e683. [Google Scholar] [CrossRef]
- Pettini, E.; Ciabattini, A.; Pastore, G.; Polvere, J.; Lucchesi, S.; Fiorino, F.; Montagnani, F.; Bucalossi, A.; Tozzi, M.; Marotta, G.; et al. A third dose of mRNA-1273 vaccine improves SARS-CoV-2 immunity in HCT recipients with low antibody response after 2 doses. Blood Adv. 2022, 6, 2247–2249. [Google Scholar] [CrossRef]
- Schwarzkopf, S.; Krawczyk, A.; Knop, D.; Klump, H.; Heinold, A.; Heinemann, F.M.; Thümmler, L.; Temme, C.; Breyer, M.; Witzke, O.; et al. Cellular Immunity in COVID-19 Convalescents with PCR-Confirmed Infection but with Undetectable SARS-CoV-2-Specific IgG. Emerg. Infect. Dis. 2021, 27, 122. [Google Scholar] [CrossRef]
- Thümmler, L.; Schwarzkopf, S.; Knop, D.; Ross, J.A.; Berg, V.; Horn, P.A.; Lindemann, M. Comparison of SARS-CoV-2- and HCoV-Specific T Cell Response Using IFN-γ ELISpot. Diagnostics 2021, 11, 1439. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, M.; Lenz, V.; Knop, D.; Klump, H.; Alt, M.; Aufderhorst, U.W.; Schipper, L.; Schwarzkopf, S.; Meller, L.; Steckel, N.; et al. Convalescent plasma treatment of critically ill intensive care COVID-19 patients. Transfusion 2021, 61, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.; Abedin, S.; Fenske, T.; Chhabra, S.; Ledeboer, N.; Hari, P.; Hamadani, M. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR T-cell therapy. Blood 2021, 138, 1278–1281. [Google Scholar] [CrossRef]
- Bird, S.; Panopoulou, A.; Shea, R.L.; Tsui, M.; Saso, R.; Sud, A.; West, S.; Smith, K.; Barwood, J.; Kaczmarek, E.; et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021, 8, e389–e392. [Google Scholar] [CrossRef]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv, T.; Shorer Arbel, Y.; Scarfò, L.; et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021, 137, 3165–3173. [Google Scholar] [CrossRef]
- Ljungman, P.; Engelhard, D.; de la Cámara, R.; Einsele, H.; Locasciulli, A.; Martino, R.; Ribaud, P.; Ward, K.; Cordonnier, C.; for the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation; et al. Vaccination of stem cell transplant recipients: Recommendations of the Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant. 2005, 35, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Chiarucci, M.; Paolasini, S.; Isidori, A.; Guiducci, B.; Loscocco, F.; Capalbo, M.; Visani, G. Immunological Response Against SARS-COV-2 After BNT162b2 Vaccine Administration Is Impaired in Allogeneic but Not in Autologous Stem Cell Transplant Recipients. Front. Oncol. 2021, 11, 737300. [Google Scholar] [CrossRef]
- Einarsdottir, S.; Martner, A.; Waldenström, J.; Nicklasson, M.; Ringlander, J.; Arabpour, M.; Törnell, A.; Wiktorin, H.G.; Nilsson, S.; Bittar, R.; et al. Deficiency of SARS-CoV-2 T-cell responses after vaccination in long-term allo-HSCT survivors translates into abated humoral immunity. Blood Adv. 2022, 6, 2723–2730. [Google Scholar] [CrossRef]
- Abid, M.B.; Rubin, M.; Ledeboer, N.; Szabo, A.; Longo, W.; Mohan, M.; Shah, N.N.; Fenske, T.S.; Abedin, S.; Runaas, L.; et al. Efficacy of a third SARS-CoV-2 mRNA vaccine dose among hematopoietic cell transplantation, CAR T cell, and BiTE recipients. Cancer Cell 2022, 40, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, P.; Jullien, M.; Peterlin, P.; Garnier, A.; Le Bourgeois, A.; Coste-Burel, M.; Béné, M.C.; Guillaume, T. Effectiveness of a third dose of BNT162b2 anti-SARS-CoV-2 mRNA vaccine over a 6-month follow-up period in allogenic hematopoietic stem cells recipients. Hematol. Oncol. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Jarisch, A.; Wiercinska, E.; Daqiq-Mirdad, S.; Hellstern, H.; Ajib, S.; Cremer, A.; Nguyen, N.T.T.; Dukat, A.; Ullrich, E.; Ciesek, S.; et al. SARS-CoV-2-specific T cells are generated in less than half of allogeneic HSCT recipients failing to seroconvert after COVID-19 vaccination. Eur. J. Immunol. 2022. ahead of print. [Google Scholar] [CrossRef]
- Einarsdottir, S.; Martner, A.; Nicklasson, M.; Wiktorin, H.G.; Arabpour, M.; Törnell, A.; Vaht, K.; Waldenström, J.; Ringlander, J.; Bergström, T.; et al. Reduced immunogenicity of a third COVID-19 vaccination among recipients of allogeneic haematopoietic stem cell transplantation. Haematologica 2022, 107, 6. [Google Scholar] [CrossRef]
- Lindemann, M.; Klisanin, V.; Thümmler, L.; Fisenkci, N.; Tsachakis-Mück, N.; Ditschkowski, M.; Schwarzkopf, S.; Klump, H.; Reinhardt, H.C.; Horn, P.A.; et al. Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients. Vaccines 2021, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Geurtsvan Kessel, C.H.; Geers, D.; Schmitz, K.S.; Mykytyn, A.Z.; Lamers, M.M.; Bogers, S.; Scherbeijn, S.; Gommers, L.; Sablerolles, R.S.G.; Nieuwkoop, N.N.; et al. Divergent SARS-CoV-2 Omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci. Immunol. 2022, 7, eabo2202. [Google Scholar] [CrossRef] [PubMed]
| Mutation | HV69-70 del | Y144 del | K417N | K417T | L452R | E484K | E484Q | N501Y | A570D | D614G | P681H | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variant | ||||||||||||
| Wildtype | ||||||||||||
| B 1.1.7 (alpha) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| B 1.351 (beta) | ✓ | ✓ | ✓ | ✓ | ||||||||
| P.1 (gamma) | ✓ | ✓ | ✓ | ✓ | ||||||||
| B 1.617.2/AY.1/.2 (delta/delta plus) | ✓ | ✓ | ||||||||||
| B 1.427/B 1.429 (epsilon) | ✓ | ✓ | ||||||||||
| B 1.525 (eta) | ✓ | ✓ | ✓ | |||||||||
| B 1.526 (iota) | ✓ | ✓ | ||||||||||
| B 1.617.1/B 1.17.3 (kappa) | ✓ | ✓ | ||||||||||
| C.37 (lambda) | ✓ | |||||||||||
| B 1.621 (mu) | ✓ | ✓ | ✓ | ✓ | ||||||||
| B 1.1.529 (omicron) | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thümmler, L.; Koldehoff, M.; Fisenkci, N.; Brochhagen, L.; Horn, P.A.; Krawczyk, A.; Lindemann, M. Cellular and Humoral Immunity after the Third Vaccination against SARS-CoV-2 in Hematopoietic Stem-Cell Transplant Recipients. Vaccines 2022, 10, 972. https://doi.org/10.3390/vaccines10060972
Thümmler L, Koldehoff M, Fisenkci N, Brochhagen L, Horn PA, Krawczyk A, Lindemann M. Cellular and Humoral Immunity after the Third Vaccination against SARS-CoV-2 in Hematopoietic Stem-Cell Transplant Recipients. Vaccines. 2022; 10(6):972. https://doi.org/10.3390/vaccines10060972
Chicago/Turabian StyleThümmler, Laura, Michael Koldehoff, Neslinur Fisenkci, Leonie Brochhagen, Peter A. Horn, Adalbert Krawczyk, and Monika Lindemann. 2022. "Cellular and Humoral Immunity after the Third Vaccination against SARS-CoV-2 in Hematopoietic Stem-Cell Transplant Recipients" Vaccines 10, no. 6: 972. https://doi.org/10.3390/vaccines10060972
APA StyleThümmler, L., Koldehoff, M., Fisenkci, N., Brochhagen, L., Horn, P. A., Krawczyk, A., & Lindemann, M. (2022). Cellular and Humoral Immunity after the Third Vaccination against SARS-CoV-2 in Hematopoietic Stem-Cell Transplant Recipients. Vaccines, 10(6), 972. https://doi.org/10.3390/vaccines10060972







