Pneumococcal Carriage in Infants Post-PCV10 Introduction in Pakistan: Results from Serial Cross-Sectional Surveys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Laboratory Methods
2.3. Sample Size
2.4. Data Management and Quality Assurance
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collaborators GBDLRI. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [Green Version]
- Oligbu, G.; Fry, N.K.; Ladhani, S.N. The Epidemiology and Biostatistics of Pneumococcus. Methods Mol. Biol. 2019, 1968, 215–224. [Google Scholar]
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T.; et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 2009, 374, 893–902. [Google Scholar] [CrossRef]
- McAllister, D.A.; Liu, L.; Shi, T.; Chu, Y.; Reed, C.; Burrows, J.; Adeloye, D.; Rudan, I.; Black, R.E.; Campbell, H. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: A systematic analysis. Lancet Glob. Health 2019, 7, e47–e57. [Google Scholar] [CrossRef] [Green Version]
- Johnson, H.L.; Deloria-Knoll, M.; Levine, O.S.; Stoszek, S.K.; Freimanis, H.L.; Reithinger, R.; Muenz, L.R.; O’Brien, K.L. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: The pneumococcal global serotype project. PLoS Med. 2010, 7, e1000348. [Google Scholar] [CrossRef] [Green Version]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef]
- Publication, W. Pneumococcal vaccines WHO position paper–2012–Recommendations. Vaccine 2012, 30, 4717–4718. [Google Scholar] [CrossRef]
- Neves, F.P.G.; Cardoso, N.T.; Snyder, R.E.; Marlow, M.A.; Cardoso, C.A.A.; Teixeira, L.M.; Riley, L.W. Pneumococcal carriage among children after four years of routine 10-valent pneumococcal conjugate vaccine use in Brazil: The emergence of multidrug resistant serotype 6C. Vaccine 2017, 35, 2794–2800. [Google Scholar] [CrossRef]
- Bruce, M.G.; Singleton, R.; Bulkow, L.; Rudolph, K.; Zulz, T.; Gounder, P.; Hurlburt, D.; Bruden, D.; Hennessy, T. Impact of the 13-valent pneumococcal conjugate vaccine (pcv13) on invasive pneumococcal disease and carriage in Alaska. Vaccine 2015, 33, 4813–4819. [Google Scholar] [CrossRef] [Green Version]
- Izurieta, P.; Bahety, P.; Adegbola, R.; Clarke, C.; Hoet, B. Public health impact of pneumococcal conjugate vaccine infant immunization programs: Assessment of invasive pneumococcal disease burden and serotype distribution. Expert Rev. Vaccines 2018, 17, 479–493. [Google Scholar] [CrossRef] [Green Version]
- Gavi TVA. Countries Approved for Support; GAVI: Geneva, Switzerland, 2020. [Google Scholar]
- PATH. Phase 3 Study of 10-Valent Pneumococcal Conjugate Vaccine (PNEUMOSIL) in Healthy Infants. 2020. Available online: https://ClinicalTrials.gov/show/NCT03197376 (accessed on 25 January 2021).
- Pfizer. Phase 3 Study of 10-Valent Pneumococcal Conjugate Vaccine (PNEUMOSIL) in Healthy Infants. 2021. Available online: https://ClinicalTrials.gov/show/NCT03197376 (accessed on 25 January 2021).
- Greenberg, D.; Hoover, P.A.; Vesikari, T.; Peltier, C.; Hurley, D.C.; McFetridge, R.D.; Coller, B.G.; Stek, J.E.; Abeygunawardana, C.; Winters, M.A.; et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Vaccine 2018, 36, 6883–6891. [Google Scholar] [CrossRef]
- Pfizer. Pfizer Announces Presentation of Data from a Phase 2 Study of Its 20-Valent Pneumococcal Conjugate Vaccine Candidate Being Investigated for the Prevention of Invasive Disease and Pneumonia in Adults Aged 18 Years and Older 2019. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer_announces_presentation_of_data_from_a_phase_2_study_of_its_20valent_pneumococcal_conjugate_vaccine_candidate_being_investigated_for_the_prevention_of_invasive_disease_and_pneumonia_in_adults_aged_18_years (accessed on 10 October 2021).
- Ali, A.; Husain, S.; Riaz, A.; Khawar, H. Status of introduction of pneumococcal conjugate vaccine in Pakistan. Pediatr. Infect. Dis. 2016, 8, 64–66. [Google Scholar] [CrossRef]
- Satzke, C.; Turner, P.; Virolainen-Julkunen, A.; Adrian, P.V.; Antonio, M.; Hare, K.M.; Henao-Restrepo, A.M.; Leach, A.J.; Klugman, K.P.; Porter, B.D.; et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: Updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine 2013, 32, 165–179. [Google Scholar] [CrossRef]
- EPI. Newsletter 2020. Available online: http://www.epi.gov.pk/wp-content/uploads/2020/12/EPI-Newsletter-November-2020-Edition.pdf (accessed on 10 October 2021).
- Simell, B.; Auranen, K.; Käyhty, H.; Goldblatt, D.; Dagan, R.; O’Brien, K.L. The fundamental link between pneumococcal carriage and disease. Expert Rev. Vaccines 2012, 11, 841–855. [Google Scholar] [CrossRef] [Green Version]
- Nisar, M.I.; Nayani, K.; Akhund, T.; Riaz, A.; Irfan, O.; Shakoor, S.; Muneer, S.; Muslim, S.; Hotwani, A.; Kabir, F.; et al. Nasopharyngeal carriage of Streptococcus pneumoniae in children under 5 years of age before introduction of pneumococcal vaccine (PCV10) in urban and rural districts in Pakistan. BMC Infect Dis. 2018, 18, 672. [Google Scholar] [CrossRef]
- da Gloria Carvalho, M.; Pimenta, F.C.; Jackson, D.; Roundtree, A.; Ahmad, Y.; Millar, E.V.; O’Brien, K.L.; Whitney, C.G.; Cohen, A.L.; Beall, B.W. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J. Clin. Microbiol. 2010, 48, 1611–1618. [Google Scholar] [CrossRef] [Green Version]
- Pai, R.; Gertz, R.E.; Beall, B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J. Clin. Microbiol. 2006, 44, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Jin, P.; Xiao, M.; Kong, F.; Oftadeh, S.; Zhou, F.; Liu, C.; Gilbert, G.L. Simple, accurate, serotype-specific PCR assay to differentiate Streptococcus pneumoniae serotypes 6A, 6B, and 6C. J. Clin. Microbiol. 2009, 47, 2470–2474. [Google Scholar] [CrossRef] [Green Version]
- Carvalho Mda, G.; Tondella, M.L.; McCaustland, K.; Weidlich, L.; McGee, L.; Mayer, L.W.; Steigerwalt, A.; Whaley, M.; Facklam, R.R.; Fields, B.; et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J. Clin. Microbiol. 2007, 45, 2460–2466. [Google Scholar] [CrossRef] [Green Version]
- Nisar, M.I.; Ahmed, S.; Jehan, F.; Shahid, S.; Shakoor, S.; Kabir, F.; Hotwani, A.; Munir, S.; Muhammad, S.; Khalid, F.; et al. Direct and indirect effect of 10 valent pneumococcal vaccine on nasopharyngeal carriage in children under 2 years of age in Matiari, Pakistan. Vaccine 2021, 39, 1319–1327. [Google Scholar] [CrossRef]
- Dunne, E.M.; Satzke, C.; Ratu, F.T.; Neal, E.F.G.; Boelsen, L.K.; Matanitobua, S.; Pell, C.L.; Nation, M.L.; Ortika, B.D.; Reyburn, R.; et al. Effect of ten-valent pneumococcal conjugate vaccine introduction on pneumococcal carriage in Fiji: Results from four annual cross-sectional carriage surveys. Lancet Glob. Health 2018, 6, e1375–e1385. [Google Scholar] [CrossRef] [Green Version]
- Hammitt, L.L.; Etyang, A.O.; Morpeth, S.C.; Ojal, J.; Mutuku, A.; Mturi, N.; Moisi, J.C.; Adetifa, I.M.; Akech, D.O.; Otiende, M.; et al. Effect of ten-valent pneumococcal conjugate vaccine on invasive pneumococcal disease and nasopharyngeal carriage in Kenya: A longitudinal surveillance study. Lancet 2019, 393, 2146–2154. [Google Scholar] [CrossRef] [Green Version]
- Brandileone, M.C.; Zanella, R.C.; Almeida, S.C.G.; Brandao, A.P.; Ribeiro, A.F.; Carvalhanas, T.M.P.; Sato, H.; Andrade, A.L.; Verani, J.R.; Pneumococcal Carriage Study Group. Effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae among children in São Paulo, Brazil. Vaccine 2016, 34, 5604–5611. [Google Scholar] [CrossRef] [Green Version]
- Veenhoven, R.; Bogaert, D.; Uiterwaal, C.; Brouwer, C.; Kiezebrink, H.; Bruin, J.; IJzerman, E.; Hermans, P.; de Groot, R.; Zegers, B.; et al. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: A randomised study. Lancet 2003, 361, 2189–2195. [Google Scholar] [CrossRef]
- Sigaúque, B.; Moiane, B.; Massora, S.; Pimenta, F.; Verani, J.R.; Mucavele, H.; Chaúque, A.; Quintó, L.; Dos Santos, R.T.; Carvalho, M.D.G.; et al. Early Declines in Vaccine Type Pneumococcal Carriage in Children Less Than 5 Years Old after Introduction of 10-valent Pneumococcal Conjugate Vaccine in Mozambique. Pediatr. Infect. Dis. J. 2018, 37, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, D.; de Groot, R.; Hermans, P. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect. Dis. 2004, 4, 144–154. [Google Scholar] [CrossRef]
- Ghaffar, F.; Friedland, I.R.; McCracken, G.H., Jr. Dynamics of nasopharyngeal colonization by Streptococcus pneumoniae. Pediatr. Infect Dis. J. 1999, 18, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, D.; Koppen, S.; Boelens, H. (Eds.) Epidemiology and Determinants of Nasopharyngeal Carriage of Bacterial Pathogens in Healthy Dutch Children. In Program and Abstracts of the 21st Annual Meeting of the European Society for Paediatric Infectious Diseases; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Hadjipanayis, A.; Efstathiou, E.; Alexandrou, M.; Panayiotou, L.; Zachariadou, C.; Petrou, P.; Papaevangelou, V. Nasopharyngeal Pneumococcal Carriage among Healthy Children in Cyprus Post Widespread Simultaneous Implementation of PCV10 and PCV13 Vaccines. PLoS ONE 2016, 11, e0163269. [Google Scholar] [CrossRef]
- Ghaffar, F.; Muniz, L.S.; Katz, K.; Smith, J.L.; Shouse, T.; Davis, P.; McCracken, G.H., Jr. Effects of large dosages of amoxicillin/clavulanate or azithromycin on nasopharyngeal carriage of Streptococcus pneumoniae, Haemophilus influenzae, nonpneumococcal alpha-hemolytic streptococci, and Staphylococcus aureus in children with acute otitis media. Clin. Infect Dis. 2002, 34, 1301–1309. [Google Scholar] [CrossRef] [Green Version]
- Faden, H.; Duffy, L.; Wasielewski, R.; Wolf, J.; Krystofik, D.; Tung, Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. J. Infect. Dis. 1997, 175, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Halloran, M.E.; Struchiner, C.J.; Longini, I.M., Jr. Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am. J. Epidemiol. 1997, 146, 789–803. [Google Scholar] [CrossRef]
- Neal, E.F.G.; Flasche, S.; Nguyen, C.D.; Ratu, F.T.; Dunne, E.M.; Koyamaibole, L.; Reyburn, R.; Rafai, E.; Kama, M.; Ortika, B.D.; et al. Associations between ethnicity, social contact, and pneumococcal carriage three years post-PCV10 in Fiji. Vaccine 2020, 38, 202–211. [Google Scholar] [CrossRef]
| Characteristics | 2014 | 2015 | 2016 |
|---|---|---|---|
| n = 224 | n = 221 | n = 220 | |
| Age, months (Mean ± SD) | 7.1 ± 2.60 | 7.5 ± 2.7 | 7.8 ± 2.94 |
| Gender | |||
| Male | 108 (48.2%) | 113 (51.1%) | 113 (51.6%) |
| Female | 116 (51.8%) | 108 (48.9%) | 106 (48.4%) |
| Education of Caretaker | |||
| Illiterate | 150 (81.1%) | 119 (84.4%) | 184 (84.8%) |
| 1–5 Years | 25 (13.5%) | 17 (12.1%) | 25 (11.5%) |
| 6–16 Years | 10 (5.4%) | 5 (3.5%) | 8 (3.7%) |
| Education of Primary wage earner | |||
| No Education | 122 (54.5%) | 76 (42.0%) | 128 (59.0%) |
| 1–5 years | 34 (15.2%) | 61 (33.7%) | 46 (21.2%) |
| 6–16 years | 68 (30.4%) | 44 (24.3%) | 43 (19.8%) |
| People in the household, Median (IQR) | 8 (6–11.5) | 8 (6–11) | 8 (6–11) |
| No. of rooms in house, Median (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–2) |
| Crowding index, * Median (IQR) | 6 (4–8) | 5 (4–7) | 5.5 (4–8) |
| Hospital Admissions in the last year | |||
| None | 206 (92.0%) | 207 (93.7%) | 217 (99.1%) |
| One | 16 (7.1%) | 12 (5.4%) | 1 (0.5%) |
| Two or more | 2 (0.9%) | 2 (0.9%) | 1 (0.5%) |
| Outpatient visits in the last month | |||
| None | 118 (52.7%) | 109 (49.5%) | 166 (75.8%) |
| One | 66 (29.5%) | 58 (26.4%) | 18 (8.2%) |
| Two or more | 40 (17.9%) | 53 (24.1%) | 35 (16.0%) |
| Smoker in household | |||
| Yes | 136 (60.7%) | 101 (45.7%) | 101 (46.1%) |
| No | 88 (39.3%) | 120 (54.3%) | 118 (53.9%) |
| Exposed to smoke during cooking | |||
| Yes | 179 (79.9%) | 47 (21.3%) | 66 (30.1%) |
| No | 44 (19.6%) | 174 (78.7%) | 153 (69.9%) |
| Don′t Know | 1 (0.4%) | 0 (0.0%) | 0 (0.0%) |
| Ever Vaccinated | |||
| Yes | 184 (82.1%) | 165 (74.7%) | 184 (83.6%) |
| No | 40 (17.9%) | 56 (25.3%) | 36 (16.4%) |
| Year | 2014 | 2015 | 2016 |
|---|---|---|---|
| (n= 224) | (n = 221) | (n = 220) | |
| Positive for pneumococcus (n) | 180 | 187 | 180 |
| Prevalence of pneumococcus % (95% CI) * | 80.3 (74.5–85.3) | 84.6 (79.1–89.1) | 81.8 (76.0–86.6) |
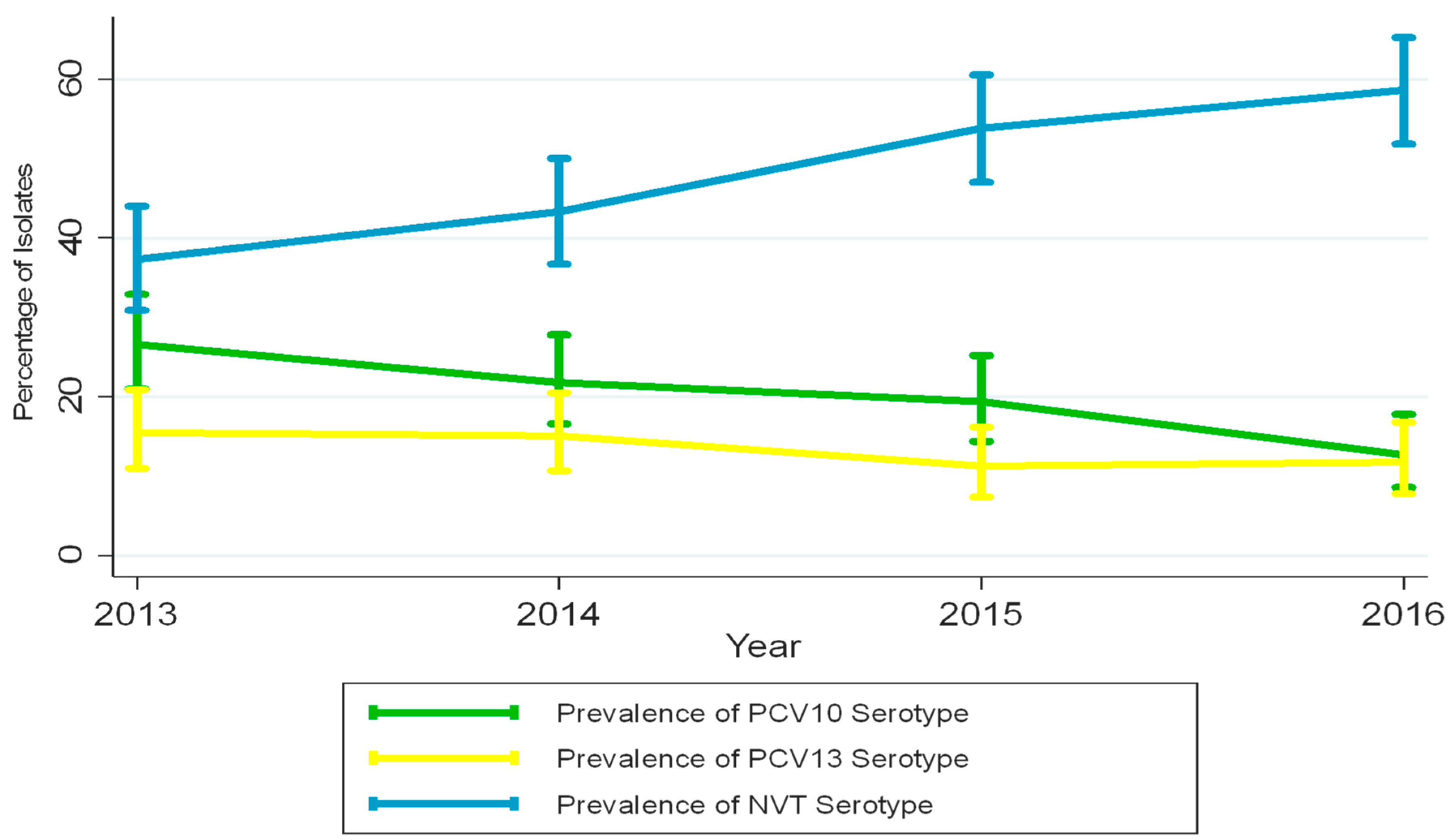
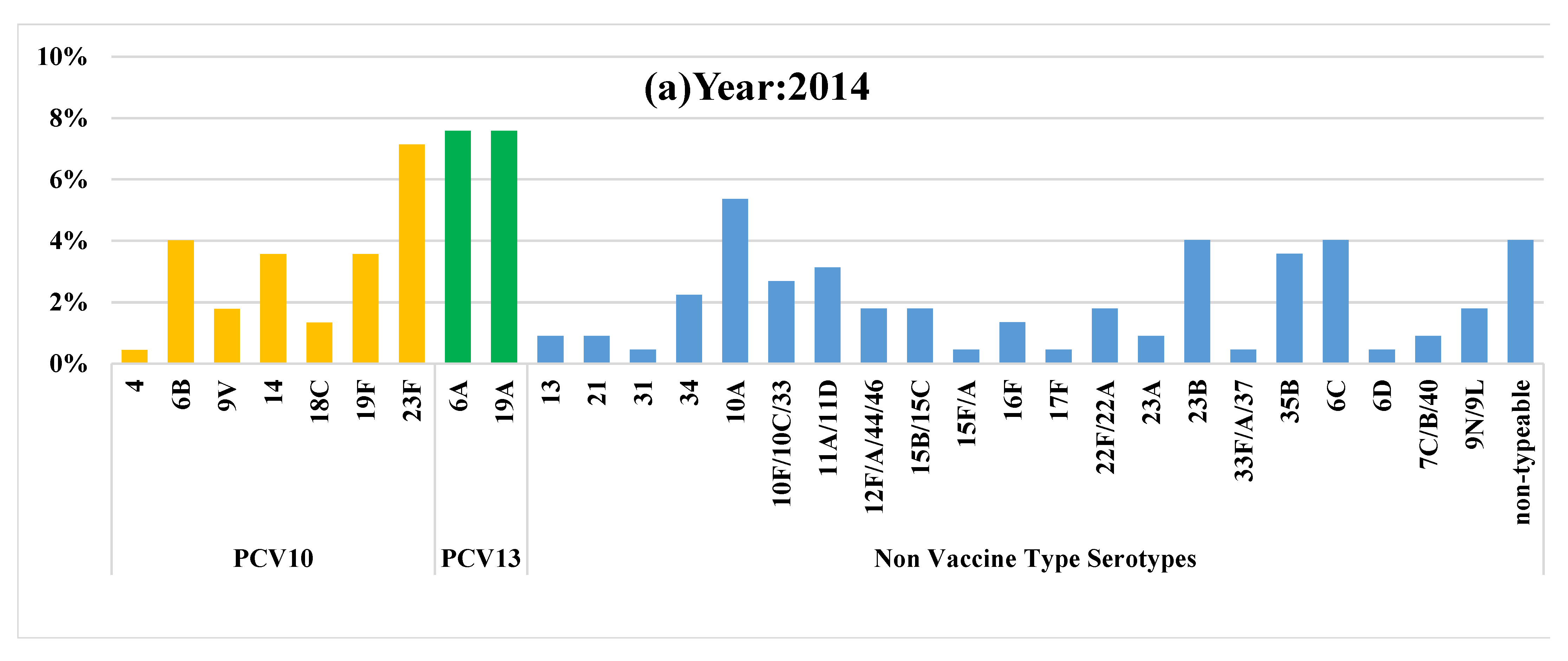
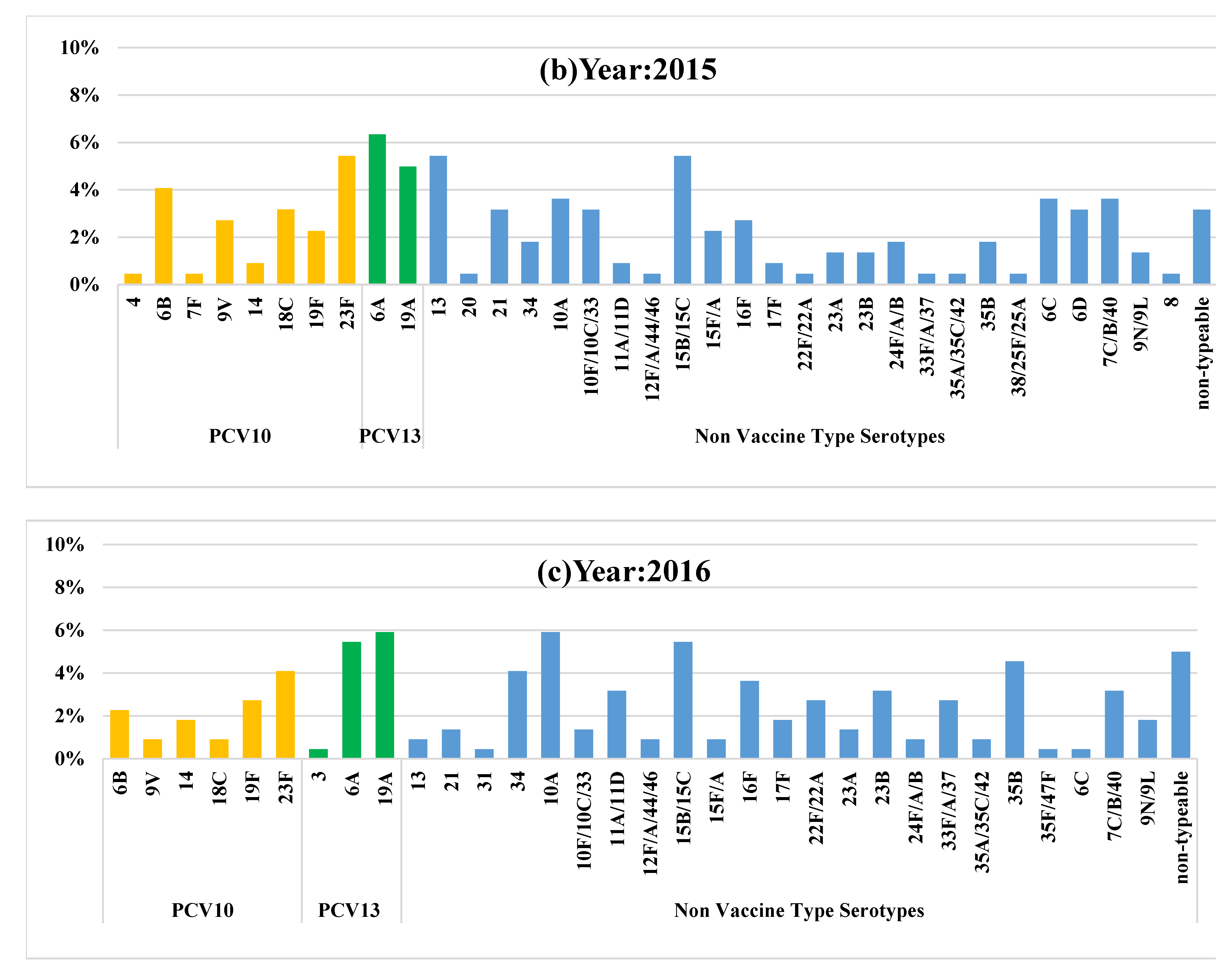
| Vaccine type serotypes (n) | 49 | 43 | 28 |
| Prevalence of vaccine type serotypes % (95% CI) ᵝ | 21.8 (16.6–27.8) | 19.4 (14.4–25.2) | 12.7 (8.6–17.8) |
| NVT serotypes (n) | 131 | 144 | 152 |
| Prevalence of NVT serotypes % (95% CI) ᵞ | 58.4 (51.7–65.0) | 65.1 (58.4–71.4) | 69.0 (62.5–75.1) |
| PCV10 Vaccination Status | 2014 | 2015 | 2016 |
|---|---|---|---|
| Unvaccinated, % (95% CI) * | 67.4 (60.8–73.5) | 37.5 (31.1–44.3) | 23.1 (17.7–29.3) |
| Partially vaccinated, % (95% CI) * | 18.3 (13.4–24.0) | 32.5 (26.4–39.1) | 28.6 (22.7–35.0) |
| Fully vaccinated, % (95% CI) * | 14.2 (9.9–19.5) | 29.8 (23.9–36.3) | 48.1 (41.4–54.9) |
| PCV10 Vaccination Status | 2014 | 2015 | 2016 |
|---|---|---|---|
| Unvaccinated, % (95% CI) * | 25.1 (18.4–32.8) | 16.8 (9.5–26.6) | 15.6 (7.0–28.5) |
| Partially vaccinated, % (95% CI) * | 19.5 (8.8–34.8) | 31.9 (21.4–43.9) | 19.0 (10.2–30.9) |
| Fully vaccinated, % (95% CI) * | 9.3 (1.9–25.0) | 9.0 (3.4–18.7) | 7.5 (3.3–14.3) |
| Predictors | Culture Negative | Culture Positive | Unadjusted OR(CI) | Adjusted OR(CI) |
|---|---|---|---|---|
| n = 118 | n = 547 | |||
| Age, months (Mean ± SD) | 6.9 ± 2.7 | 7.6 ± 2.7 | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) |
| Gender | ||||
| Male | 66 (56.4%) | 268 (49.0%) | Reference | |
| Female | 51 (43.6%) | 279 (51.0%) | 1.3 (0.9–2.0) | |
| Education of Caretaker | ||||
| Illiterate | 76 (77.6%) | 377 (84.7%) | Reference | |
| 1–5 Years | 16 (16.3%) | 51 (11.5%) | 0.6 (0.3–1.1) | |
| 6–16 Years | 6 (6.1%) | 17 (3.8%) | 0.6 (0.2–1.5) | |
| Education of Primary wage earner | ||||
| No Education | 59 (53.6%) | 267 (52.1%) | Reference | |
| 1–5 years | 20 (18.2%) | 121 (23.6%) | 1.3 (0.7–2.3) | |
| 6–16 years | 31 (28.2%) | 124 (24.2%) | 0.8 (0.5–1.4) | |
| Total No. people in child′s house, Median (IQR) | 8 (6–11) | 8 (6–11) | 1.0 (0.9–1.0) | |
| No. of rooms in child′s house, Median (IQR) | 1 (1–2) | 1 (1–2) | 1.0 (0.9–1.0) | |
| Crowding index, Median (IQR) | 5 (4–8) | 5.5 (4–7.6) | 1.0 (0.9–1.1) | |
| Hospital Admissions in the last year | ||||
| None | 107 (91.5%) | 523 (95.6%) | Reference | |
| One | 8 (6.8%) | 21 (3.8%) | 0.5 (0.2–1.2) | |
| Two or more | 2 (1.7%) | 3 (0.5%) | 0.3 (0.05–1.9) | |
| Outpatient visits in the last month | ||||
| None | 56 (47.9%) | 337 (61.7%) | Reference | |
| One | 34 (29.1%) | 108 (19.8%) | 0.5 (0.3–0.9) | |
| Two or more | 27 (23.1%) | 101 (18.5%) | 0.6 (0.4–1.0) | 0.5 (0.4–0.9) |
| Smoker in household | ||||
| Yes | 59 (50.4%) | 267 (48.8%) | 1.0 (0.7–1.6) | |
| No | 58 (49.6%) | 280 (51.2%) | Reference | |
| Exposed to smoke during cooking | ||||
| Yes | 46 (39.3%) | 246 (45.0%) | 1.3 (0.8–1.9) | |
| No | 71 (60.7%) | 300 (54.8%) | Reference | |
| Don′t Know | 0 (0.0%) | 1 (0.2%) | - | |
| PCV10 Vaccination Status | ||||
| Non-Vaccinated | 59 (50.0%) | 226 (41.3%) | 0.6 (0.4–1.0) | |
| Partially Vaccinated | 30 (25.4%) | 146 (26.7%) | 0.8 (0.5–1.4) | |
| Fully Vaccinated | 29 (24.6%) | 175 (32.0%) | Reference | |
| Year of Enrollment | ||||
| 2014 | 44 (37.3%) | 180 (32.9%) | Reference | |
| 2015 | 34 (28.8%) | 187 (34.2%) | 1.3 (0.8–2.2) | |
| 2016 | 40 (33.9%) | 180 (32.9%) | 1.1 (0.7–1.8) |
| Predictors | PCV10 Negative | PCV10 Positive | Unadjusted OR(CI) | Adjusted OR(CI) |
|---|---|---|---|---|
| n = 710 | n = 180 | |||
| Age, months (Mean ± SD) | 7.6 (2.8) | 7.0 (2.6) | 0.9 (0.8–1.0) | |
| Gender | ||||
| Male | 271 (49.8%) | 63 (52.5%) | Reference | |
| Female | 273 (50.2%) | 57 (47.5%) | 0.9 (0.6–1.3) | |
| Education of Caretaker | ||||
| Illiterate | 365 (81.7%) | 88 (91.7%) | Reference | |
| 1–5 Years | 60 (13.4%) | 7 (7.3%) | 0.5 (0.2–1.1) | |
| 6–16 Years | 22 (4.9%) | 1 (1.0%) | 0.2 (0.2–1.4) | |
| Education of Primary wage earner | ||||
| No Education | 266 (52.3%) | 60 (53.1%) | Reference | |
| 1–5 years | 113 (22.2%) | 28 (24.8%) | 1.1 (0.6–1.8) | |
| 6–16 years | 130 (25.5%) | 25 (22.1%) | 0.9 (0.5–1.4) | |
| Total No. people in child′s house, Median (IQR) | 8 (6–11) | 8 (6–11) | 1.0 (0.9–1.0) | |
| No. of rooms in child′s house, Median (IQR) | 1 (1–2) | 1 (1–2) | 1.0 (0.9–1.0) | |
| Crowding index Median (IQR) | 5.3 (4–7.6) | 6 (4.1–8) | 1.0 (0.9–1.1) | |
| Hospital Admissions in the last year | ||||
| None | 518 (95.2%) | 112 (93.3%) | Reference | |
| One | 22 (4.0%) | 7 (5.8%) | 1.5 (0.6–3.5) | |
| Two or more | 4 (0.7%) | 1 (0.8%) | 1.2 (0.1–10.4) | |
| Outpatient visits in the last month | ||||
| None | 323 (59.5%) | 70 (58.3%) | Reference | |
| One | 121 (22.3%) | 21 (17.5%) | 0.8 (0.4–1.3) | |
| Two or more | 99 (18.2%) | 29 (24.2%) | 1.3 (0.8–2.2) | |
| Smoker in household | ||||
| No | 265 (48.7%) | 61 (50.8%) | Reference | |
| Yes | 279 (51.3%) | 59 (49.2%) | 0.9 (0.6–1.3) | |
| Exposed to smoke during cooking | ||||
| No | 308 (56.6%) | 63 (52.5%) | Reference | |
| Yes | 235 (43.2%) | 57 (47.5%) | 1.2 (0.7–1.7) | |
| PCV10 Vaccination Status | ||||
| Non-Vaccinated | 225 (41.3%) | 60 (50.0%) | 2.9 (1.6, 5.1) | 2.4 (1.2–4.8) |
| Partially Vaccinated | 133 (24.4%) | 43 (35.8%) | 3.5 (1.9, 6.5) | 2.8 (1.3–5.9) |
| Fully Vaccinated | 187 (34.3%) | 17 (14.2%) | Reference | Ref |
| Year of Enrollment | ||||
| 2014 | 175 (32.1%) | 49 (40.8%) | Reference | |
| 2015 | 178 (32.7%) | 43 (35.8%) | 0.8 (0.5–1.3) | |
| 2016 | 192 (35.2%) | 28 (23.3%) | 0.5 (0.3–0.8) | 0.5 (0.3–1.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahid, S.; Khan, A.; Nisar, M.I.; Khalid, F.; Qazi, M.F.; Ahmed, S.; Kabir, F.; Hotwani, A.; Muneer, S.; Ali, S.A.; et al. Pneumococcal Carriage in Infants Post-PCV10 Introduction in Pakistan: Results from Serial Cross-Sectional Surveys. Vaccines 2022, 10, 971. https://doi.org/10.3390/vaccines10060971
Shahid S, Khan A, Nisar MI, Khalid F, Qazi MF, Ahmed S, Kabir F, Hotwani A, Muneer S, Ali SA, et al. Pneumococcal Carriage in Infants Post-PCV10 Introduction in Pakistan: Results from Serial Cross-Sectional Surveys. Vaccines. 2022; 10(6):971. https://doi.org/10.3390/vaccines10060971
Chicago/Turabian StyleShahid, Shahira, Amala Khan, Muhammad Imran Nisar, Farah Khalid, Muhammad Farrukh Qazi, Sheraz Ahmed, Furqan Kabir, Aneeta Hotwani, Sahrish Muneer, Syed Asad Ali, and et al. 2022. "Pneumococcal Carriage in Infants Post-PCV10 Introduction in Pakistan: Results from Serial Cross-Sectional Surveys" Vaccines 10, no. 6: 971. https://doi.org/10.3390/vaccines10060971
APA StyleShahid, S., Khan, A., Nisar, M. I., Khalid, F., Qazi, M. F., Ahmed, S., Kabir, F., Hotwani, A., Muneer, S., Ali, S. A., Whitney, C. G., Zaidi, A. K. M., & Jehan, F. (2022). Pneumococcal Carriage in Infants Post-PCV10 Introduction in Pakistan: Results from Serial Cross-Sectional Surveys. Vaccines, 10(6), 971. https://doi.org/10.3390/vaccines10060971






