Manifestation of Subclinical Extrapulmonary Tuberculosis after COVID-19 Vaccination as Supraclavicular Lymphadenopathy
Abstract
:1. Introduction
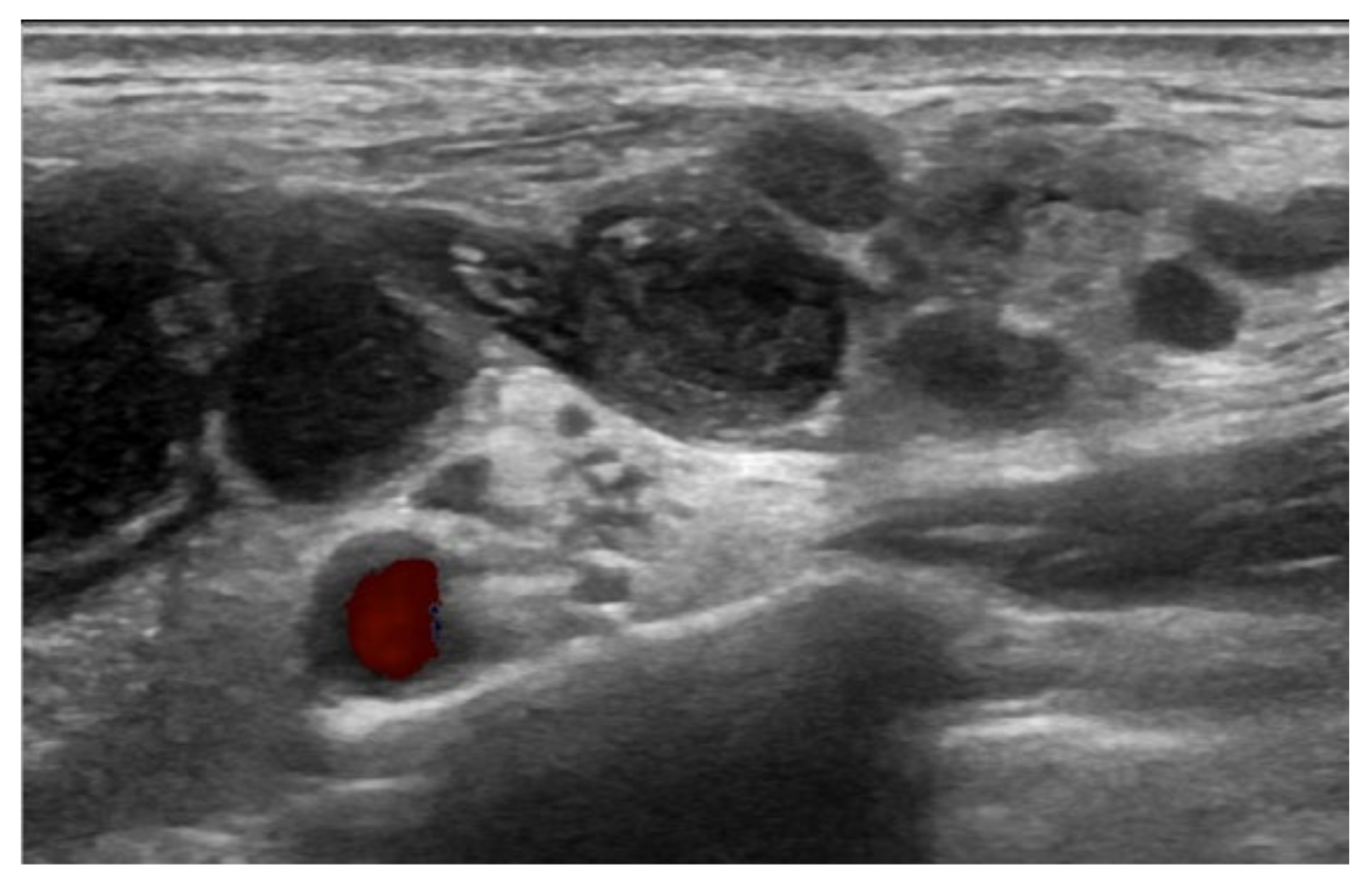
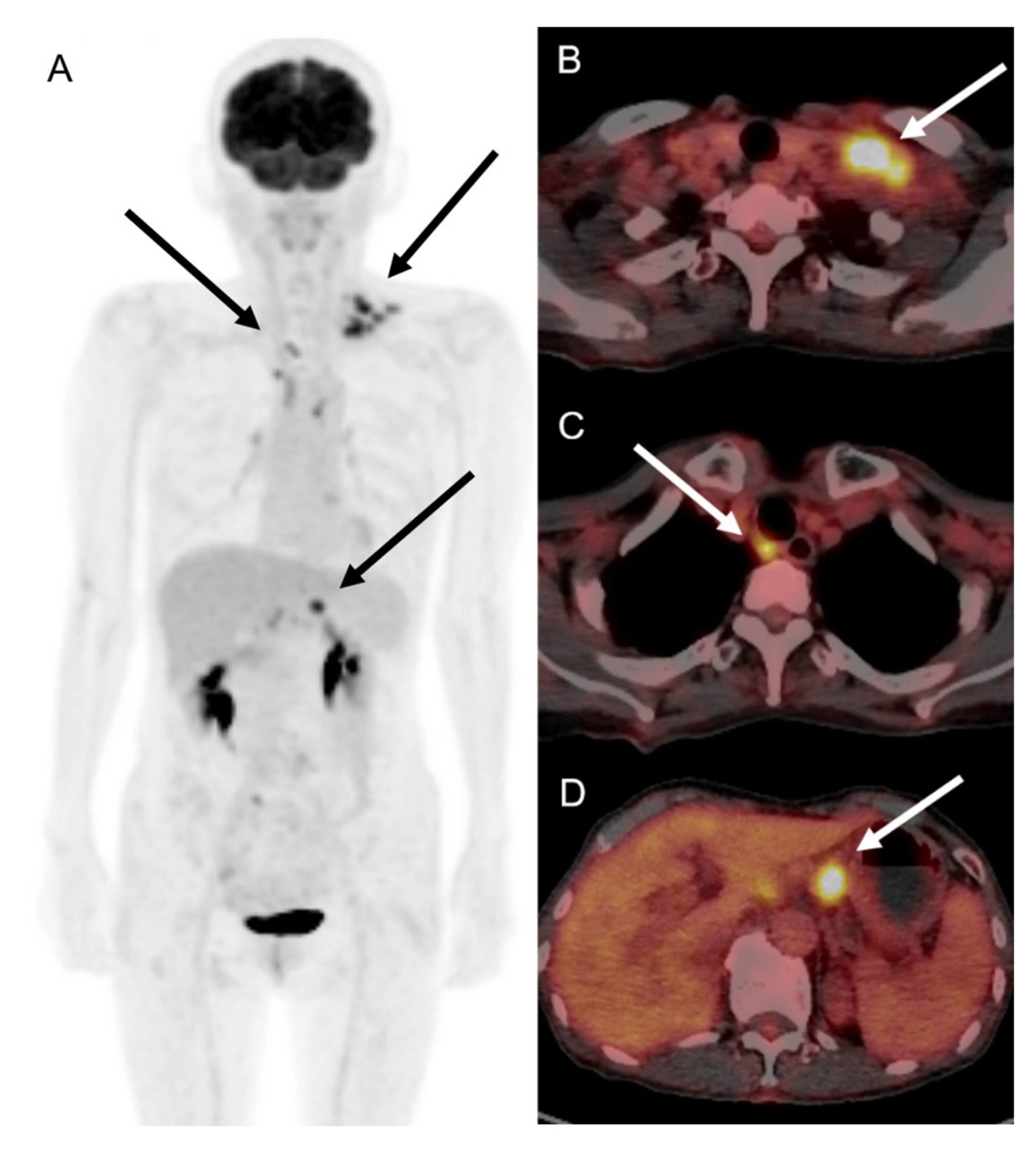
2. Case Description
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garreffa, E.; Hamad, A.; O’Sullivan, C.C.; Hazim, A.Z.; York, J.; Puri, S.; Turnbull, A.; Robertson, J.F.; Goetz, M.P. Regional lymphadenopathy following COVID-19 vaccination: Literature review and considerations for patient management in breast cancer care. Eur. J. Cancer 2021, 159, 38–51. [Google Scholar] [CrossRef]
- Keshavarz, P.; Yazdanpanah, F.; Rafiee, F.; Mizandari, M. Lymphadenopathy Following COVID-19 Vaccination: Imaging Findings Review. Acad. Radiol. 2021, 28, 1058–1071. [Google Scholar] [CrossRef]
- Cohen, D.; Krauthammer, S.H.; Wolf, I.; Even-Sapir, E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: Incidence assessed by [(18)F]FDG PET-CT and relevance to study interpretation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1854–1863. [Google Scholar] [CrossRef]
- Mohseni, S.; Shojaiefard, A.; Khorgami, Z.; Alinejad, S.; Ghorbani, A.; Ghafouri, A. Peripheral lymphadenopathy: Approach and diagnostic tools. Iran J. Med. Sci. 2014, 39 (Suppl. S2), 158–170. [Google Scholar]
- Turan, A.; Kaplanoglu, H.; Kaplanoglu, V. Frequency of Ipsilateral Axillary Lymphadenopathy After the Inactivated COVID-19 Vaccine. Curr. Med. Imaging 2022. ahead of print. [Google Scholar] [CrossRef]
- Co, M.; Wong, P.C.P.; Kwong, A. COVID-19 vaccine associated axillary lymphadenopathy—A systematic review. Cancer Treat Res. Commun. 2022, 31, 100546. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; Galdiero, R.; Picone, C.; Izzo, F.; D’Aniello, R.; Miele, V.; Grassi, R.; Grassi, R.; et al. Lymphadenopathy after BNT162b2 COVID-19 Vaccine: Preliminary Ultrasound Findings. Biology 2021, 10, 214. [Google Scholar] [CrossRef]
- Hiller, N.; Goldberg, S.N.; Cohen-Cymberknoh, M.; Vainstein, V.; Simanovsky, N. Lymphadenopathy Associated With the COVID-19 Vaccine. Cureus 2021, 13, e13524. [Google Scholar] [CrossRef]
- Fernandez-Prada, M.; Rivero-Calle, I.; Calvache-Gonzalez, A.; Martinon-Torres, F. Acute onset supraclavicular lymphadenopathy coinciding with intramuscular mRNA vaccination against COVID-19 may be related to vaccine injection technique, Spain, January and February 2021. Euro. Surveill. 2021, 26, 2100193. [Google Scholar] [CrossRef]
- Eshet, Y.; Tau, N.; Alhoubani, Y.; Kanana, N.; Domachevsky, L.; Eifer, M. Prevalence of Increased FDG PET/CT Axillary Lymph Node Uptake Beyond 6 Weeks after mRNA COVID-19 Vaccination. Radiology 2021, 300, E345–E347. [Google Scholar] [CrossRef]
- Tan, N.J.H.; Tay, K.X.J.; Wong, S.B.J.; Nga, M.E. COVID-19 post-vaccination lymphadenopathy: Report of cytological findings from fine needle aspiration biopsy. Diagn. Cytopathol. 2021, 49, E467–E470. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). Recommendations of the NCCN Advisory Committee on COVID-19 Vaccination and Pre-Exposure Prophylaxis. Available online: https://www.nccn.org/docs/default-source/covid-19/2021_covid-19_vaccination_guidance_v5-0.pdf (accessed on 27 April 2022).
- Ha, S.M.; Chu, A.J.; Lee, J.; Kim, S.Y.; Lee, S.H.; Yoen, H.; Cho, N.; Moon, W.K.; Chang, J.M. US Evaluation of Axillary Lymphadenopathy Following COVID-19 Vaccination: A Prospective Longitudinal Study. Radiology 2022, 26, 220543. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.W.; Hwang, S.; Kim, J.; Lee, J.M.; Cho, H.J.; Moon, J.H.; Hwang, N.; Jeong, J.Y.; Lee, S.-W.; Sohn, S.K.; et al. Patients presenting high fever with lymphadenopathy after COVID-19 vaccination were diagnosed with hemophagocytic lymphohistiocytosis. Infect. Dis. 2021, 54, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, J.G.; Tampe, B.; Korsten, P. First Report of Two Cases of Lofgren’s Syndrome after SARS-CoV-2 Vaccination-Coincidence or Causality? Vaccines 2021, 9, 1313. [Google Scholar] [CrossRef]
- Soub, H.A.; Ibrahim, W.; Maslamani, M.A.; Gawahir, A.A.; Ummer, W.; Abu-Dayeh, A. Kikuchi-Fujimoto disease following SARS CoV2 vaccination: Case report. IDCases 2021, 25, e01253. [Google Scholar] [CrossRef]
- Guimaraes, L.E.; Baker, B.; Perricone, C.; Shoenfeld, Y. Vaccines, adjuvants and autoimmunity. Pharmacol. Res. 2015, 100, 190–209. [Google Scholar] [CrossRef]
- Ozutemiz, C.; Krystosek, L.A.; Church, A.L.; Chauhan, A.; Ellermann, J.M.; Domingo-Musibay, E.; Steinberger, D. Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncologic Patients. Radiology 2021, 300, E296–E300. [Google Scholar] [CrossRef]
- Xu, G.; Lu, Y. COVID-19 mRNA Vaccination-Induced Lymphadenopathy Mimics Lymphoma Progression on FDG PET/CT. Clin. Nucl. Med. 2021, 46, 353–354. [Google Scholar] [CrossRef]
- Bazemore, A.W.; Smucker, D.R. Lymphadenopathy and malignancy. Am. Fam. Physician 2002, 66, 2103–2110. [Google Scholar]
- Solovic, I.; Jonsson, J.; Korzeniewska-Kosela, M.; Chiotan, D.I.; Pace-Asciak, A.; Slump, E.; Rumetshofer, R.; Abubakar, I.; Kos, S.; Svetina-Sorli, P.; et al. Challenges in diagnosing extrapulmonary tuberculosis in the European Union, 2011. Euro. Surveill. 2013, 18, 20432. [Google Scholar] [CrossRef]
- Sama, J.N.; Chida, N.; Polan, R.M.; Nuzzo, J.; Page, K.; Shah, M. High proportion of extrapulmonary tuberculosis in a low prevalence setting: A retrospective cohort study. Public Health 2016, 138, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selwyn, P.A.; Hartel, D.; Lewis, V.A.; Schoenbaum, E.E.; Vermund, S.H.; Klein, R.S.; Walker, A.T.; Friedland, G.H. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N. Engl. J. Med. 1989, 320, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Uplekar, M.; Weil, D.; Lonnroth, K.; Jaramillo, E.; Lienhardt, C.; Dias, H.M.; Falzon, D.; Floyd, K.; Gargioni, G.; Getahun, H.; et al. WHO’s new end TB strategy. Lancet 2015, 385, 1799–1801. [Google Scholar] [CrossRef]
- Asai, S.; Miyachi, H.; Suzuki, K.; Shimamura, K.; Ando, Y. Ultrasonographic differentiation between tuberculous lymphadenitis and malignant lymph nodes. J. Ultrasound Med. 2001, 20, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dutta, R.; Kannan, U.; Kumar, R.; Khilnani, G.C.; Gupta, S.D. Evaluation of mediastinal lymph nodes using F-FDG PET/CT scan and its histopathologic correlation. Ann. Thorac. Med. 2011, 6, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, B.S.; Lee, D.S.; Chung, J.; Lee, M.C.; Kim, S.; Kang, W.J. 18F-FDG PET/CT in mediastinal lymph node staging of non-small-cell lung cancer in a tuberculosis-endemic country: Consideration of lymph node calcification and distribution pattern to improve specificity. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1794–1802. [Google Scholar] [CrossRef]
- Napoli, C.; Ferretti, F.; Di Ninno, F.; Orioli, R.; Marani, A.; Sarlo, M.G.; Prestigiacomo, C.; de Luca, A.; Orsi, G.B. Screening for Tuberculosis in Health Care Workers: Experience in an Italian Teaching Hospital. Biomed. Res. Int. 2017, 2017, 7538037. [Google Scholar] [CrossRef]
- Sheerin, D.; Abhimanyu Wang, X.; Johnson, W.E.; Coussens, A. Systematic evaluation of transcriptomic disease risk and diagnostic biomarker overlap between COVID-19 and tuberculosis: A patient-level meta-analysis. MedRxiv 2020. [Google Scholar] [CrossRef]
- Flynn, J.L.; Chan, J. Tuberculosis: Latency and reactivation. Infect. Immun. 2001, 69, 4195–4201. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.F.; Yen, C.L.; Fu, Y.C.; Cheng, C.M.; Shen, T.C.; Chang, P.D.; Cheng, K.; Liu, C.; Chang, Y.; Chen, P.; et al. Innate Immune Responses of Vaccinees Determine Early Neutralizing Antibody Production After ChAdOx1nCoV-19 Vaccination. Front. Immunol. 2022, 13, 807454. [Google Scholar] [CrossRef]
- Flynn, J.L.; Chan, J. Immunology of tuberculosis. Annu. Rev. Immunol. 2001, 19, 93–129. [Google Scholar] [CrossRef] [PubMed]
- Adin, M.E.; Isufi, E.; Kulon, M.; Pucar, D. Association of COVID-19 mRNA Vaccine With Ipsilateral Axillary Lymph Node Reactivity on Imaging. JAMA Oncol. 2021, 7, 1241–1242. [Google Scholar] [CrossRef] [PubMed]

| 1st Dose | 2nd Dose | |
|---|---|---|
| Symptoms | Fatigue | Lymphadenopathy |
| Injection site tenderness |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, H.G.; Kim, D.G.; Choi, J.H. Manifestation of Subclinical Extrapulmonary Tuberculosis after COVID-19 Vaccination as Supraclavicular Lymphadenopathy. Vaccines 2022, 10, 964. https://doi.org/10.3390/vaccines10060964
Cha HG, Kim DG, Choi JH. Manifestation of Subclinical Extrapulmonary Tuberculosis after COVID-19 Vaccination as Supraclavicular Lymphadenopathy. Vaccines. 2022; 10(6):964. https://doi.org/10.3390/vaccines10060964
Chicago/Turabian StyleCha, Han Gyu, Dong Gyu Kim, and Joon Ho Choi. 2022. "Manifestation of Subclinical Extrapulmonary Tuberculosis after COVID-19 Vaccination as Supraclavicular Lymphadenopathy" Vaccines 10, no. 6: 964. https://doi.org/10.3390/vaccines10060964
APA StyleCha, H. G., Kim, D. G., & Choi, J. H. (2022). Manifestation of Subclinical Extrapulmonary Tuberculosis after COVID-19 Vaccination as Supraclavicular Lymphadenopathy. Vaccines, 10(6), 964. https://doi.org/10.3390/vaccines10060964






