Positive Attribute Framing Increases COVID-19 Booster Vaccine Intention for Unfamiliar Vaccines
Abstract
:1. Introduction
2. Methods and Materials
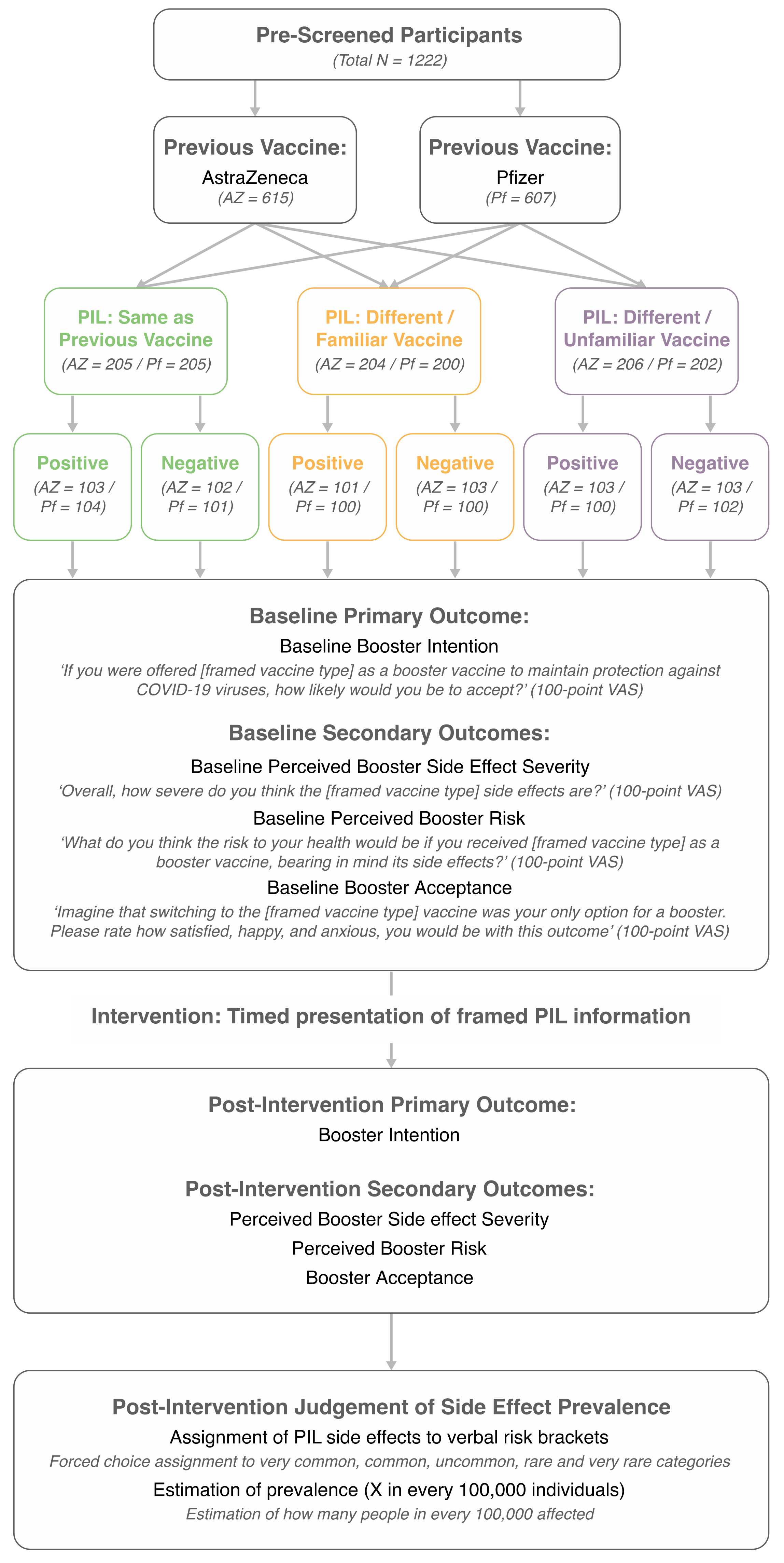
2.1. Participants
2.2. Design
2.3. Data Collection: Primary and Secondary Outcomes
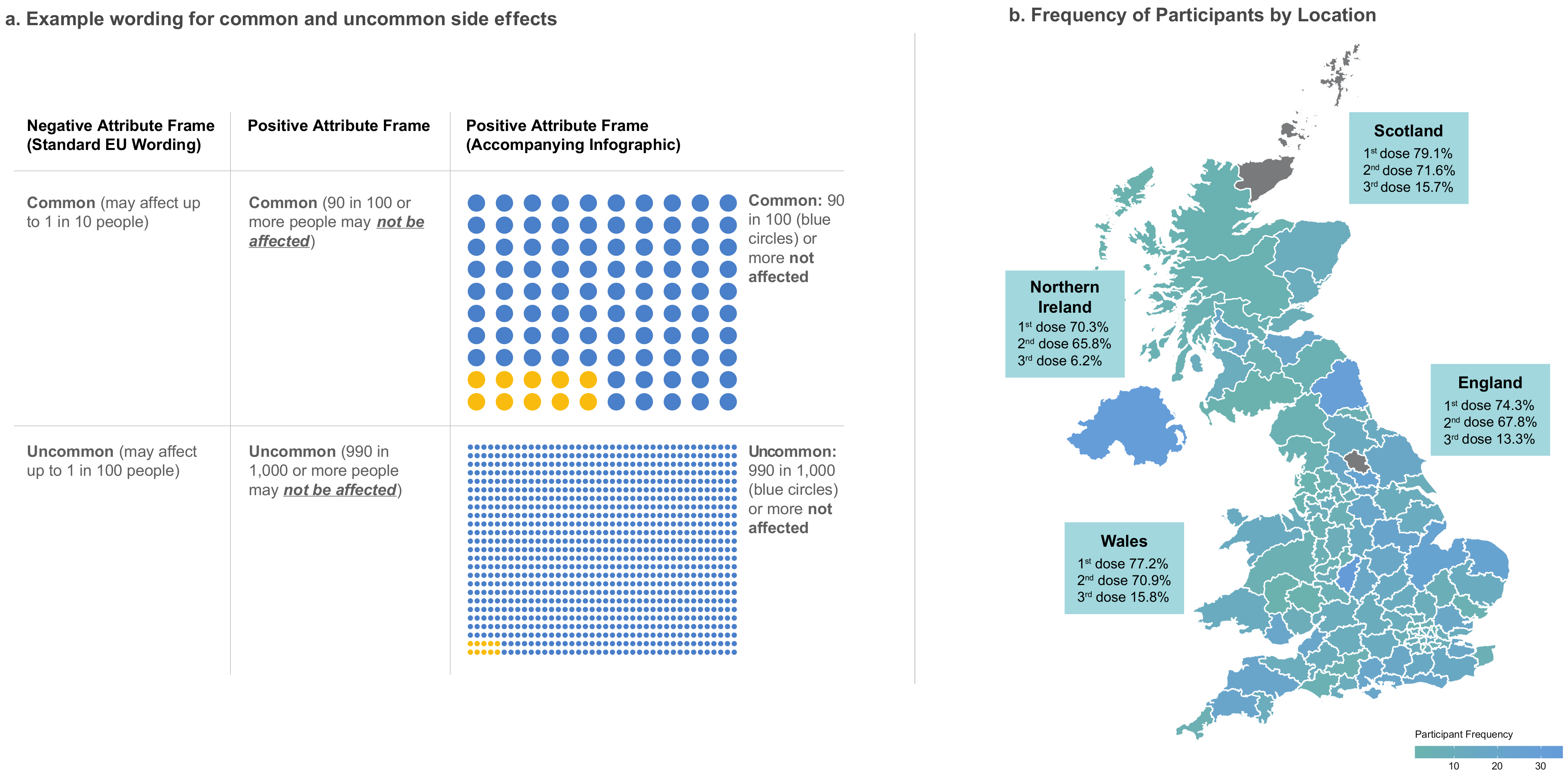
2.4. Data Collection and Quotas
2.5. Procedure
2.6. Survey Materials: Descriptive Variables
2.6.1. Demographic Information
2.6.2. Previous Exposure to COVID-19
2.6.3. Previous COVID-19 Vaccination History
2.6.4. Familiarity with COVID-19 Vaccine Side Effects
2.7. Survey Materials: Inferential Variables
2.7.1. Primary and Secondary Outcomes
2.7.2. Post-Intervention Judgement of Side Effect Prevalence
2.8. Patient Information Leaflets (PILs)
2.9. Statistical Analysis and Sample Size
3. Results
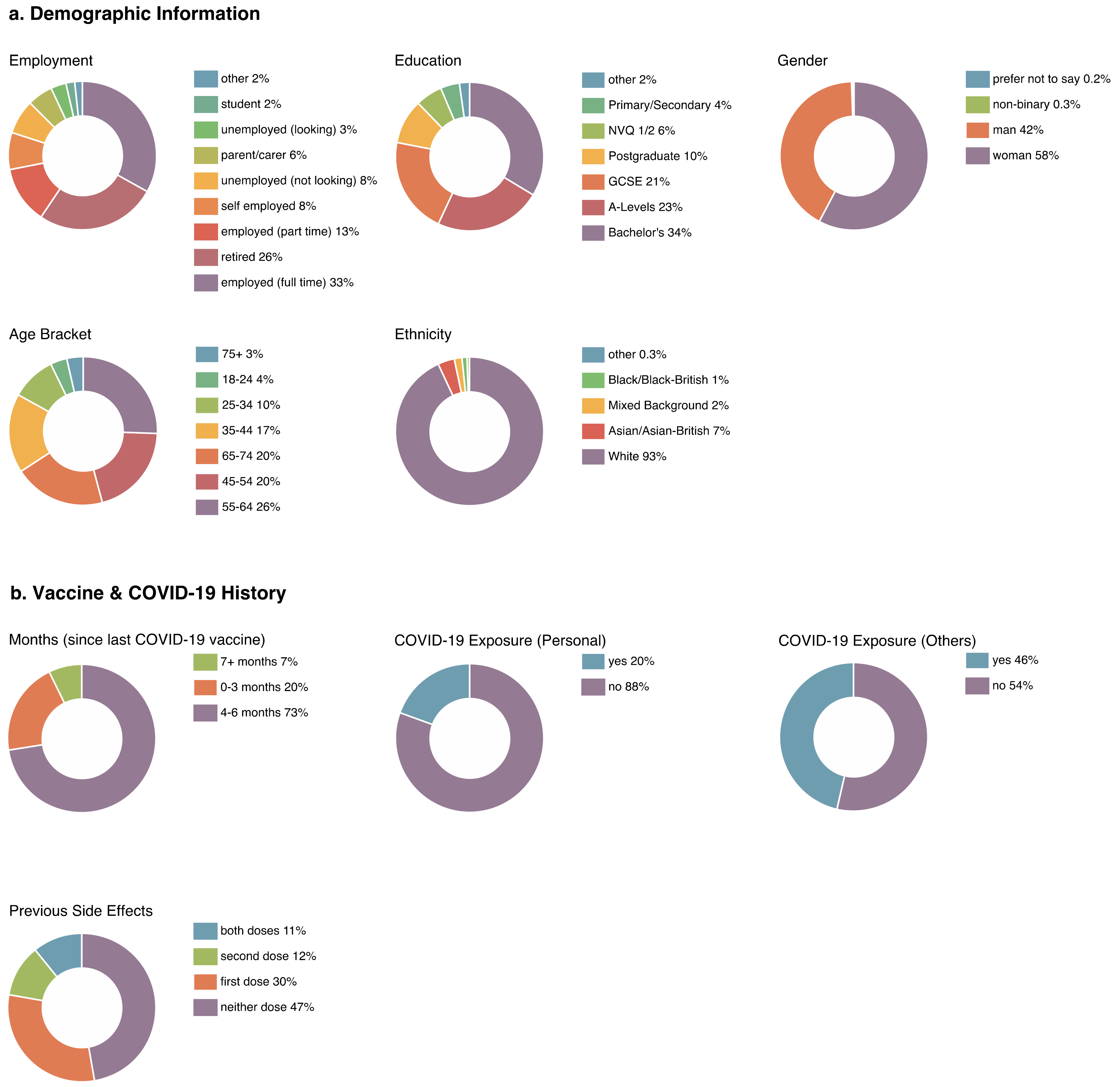
3.1. Sample
3.2. Descriptive Statistics
3.3. Primary Analysis
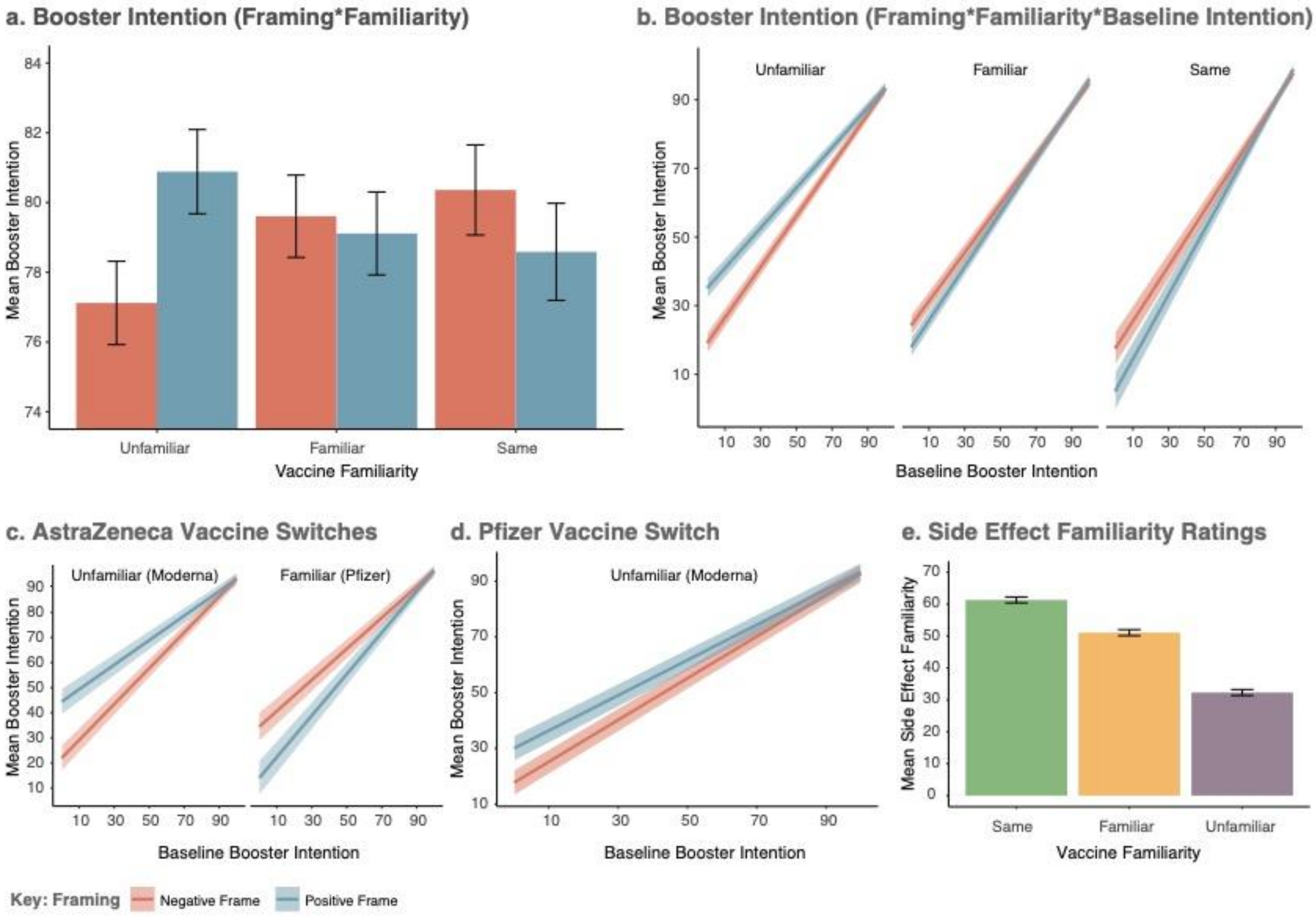
3.3.1. Knowledge of Vaccine Side Effects Mirrors Categorical Levels of the Familiarity Factor
3.3.2. Baseline Vaccine Intention
3.3.3. Effect of Framing and Familiarity on the Intention to Be Vaccinated
3.4. The Effect of Framing on Vaccine Switches
3.4.1. Interactions between Previous Vaccine Type and Experimental Factors
3.4.2. Previous Vaccine: AstraZeneca
3.4.3. Previous Vaccine: Pfizer
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Chand, M.; Brown, K.; Ladhani, S.N.; et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK. medRxiv 2021. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning immunity of the BNT162b2 vaccine: A nationwide study from Israel. medRxiv 2021. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khan, K.; Khoury, D.S.; Moyo-Gwete, T.; Tegally, H.; Scheepers, C.; Amoako, D.; Karim, F.; Bernstein, M.; et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv 2021. [Google Scholar] [CrossRef]
- Basile, K.; Rockett, R.J.; McPhie, K.; Fennell, M.; Johnson-Mackinnon, J.; Agius, J.E.; Fong, W.; Rahman, H.; Ko, D.; Donavan, L.; et al. Improved neutralization of the SARS-CoV-2 Omicron variant after Pfizer-BioNTech BNT162b2 COVID-19 vaccine boosting. bioRxiv 2021. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19 booster vaccines: What we know and who’s doing what. BMJ 2021, 374, n2082. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, N.E.; Eskola, J.; Liang, X.; Chaudhuri, M.; Dube, E.; Gellin, B.; Goldstein, S.; Larson, H.; Manzo, M.L.; Reingold, A.; et al. Vaccine Hesitancy: Definition, Scope and Determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Ten Threats to Global Health in 2019. 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 12 December 2021).
- Arce, J.S.S.; Warren, S.S.; Meriggi, N.F.; Scacco, A.; McMurry, N.; Voors, M.; Syunyaev, G.; Malik, A.A.; Aboutajdine, S.; Adeojo, O.; et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 2021, 27, 1385–1394. [Google Scholar] [CrossRef]
- Why Won’t Americans Get Vaccinated? [Internet] [Economist/YouGov poll]. 16 July 2021. Available online: https://today.yougov.com/topics/politics/articles-reports/2021/07/15/why-wont-americans-get-vaccinated-poll-data (accessed on 10 December 2021).
- Rzymski, P.; Poniedziałek, B.; Fal, A. Willingness to Receive the Booster COVID-19 Vaccine Dose in Poland. Vaccines 2021, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Data for Action: Achieving High Uptake of COVID-19 vaccines: Gathering and Using Data on the Behavioural and Social Drivers of Vaccination: A Guidebook for Immunization Programmes and Implementing Partners: Interim guidance, 1 April 2021. Available online: https://apps.who.int/iris/handle/10665/340645 (accessed on 11 December 2021).
- Levin, I.P.; Schneider, S.; Gaeth, G.J. All Frames Are Not Created Equal: A Typology and Critical Analysis of Framing Effects. Organ. Behav. Hum. Decis. Process. 1998, 76, 149–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, K.; Faasse, K.; Geers, A.L.; Helfer, S.G.; Sharpe, L.; Colloca, L.; Colagiuri, B. Can Positive Framing Reduce Nocebo Side Effects? Current Evidence and Recommendation for Future Research. Front. Pharmacol. 2019, 10, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, A.M.; Pennie, R.A.; Dales, R.E. Framing effects on expectations, decisions, and side effects experienced: The case of influenza immunization. J. Clin. Epidemiol. 1996, 49, 1271–1276. [Google Scholar] [CrossRef]
- Bigman, C.A.; Cappella, J.N.; Hornik, R.C. Effective or ineffective: Attribute framing and the human papillomavirus (HPV) vaccine. Patient Educ. Couns. 2010, 81, S70–S76. [Google Scholar] [CrossRef] [Green Version]
- Sudharsanan, N.; Favaretti, C.; Hachaturyan, V.; Bärnighausen, T.; Vandormael, A. Effects of Side-Effect Risk Framing Strategies on COVID-19 Vaccine Intentions in the United States and the United Kingdom: A Randomized Controlled Trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Chen, T.; Dai, M.; Xia, S.; Zhou, Y. Do Messages Matter? Investigating the Combined Effects of Framing, Outcome Uncertainty, and Number Format on COVID-19 Vaccination Attitudes and Intention. Health Commun. 2021, 37, 944–951. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, W. Promoting COVID-19 Vaccination: The Interplay of Message Framing, Psychological Uncertainty, and Public Agency as a Message Source. Sci. Commun. 2021, 44, 3–29. [Google Scholar] [CrossRef]
- Betta, S.; Castellini, G.; Acampora, M.; Barello, S. The Effect of Message Framing on COVID-19 Vaccination Intentions among the Younger Age Population Groups: Results from an Experimental Study in the Italian Context. Vaccines 2022, 10, 559. [Google Scholar] [CrossRef] [PubMed]
- Lentzen, M.-P.; Huebenthal, V.; Kaiser, R.; Kreppel, M.; Zoeller, J.E.; Zirk, M. A retrospective analysis of social media posts pertaining to COVID-19 vaccination side effects. Vaccine 2022, 40, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Jamison, A.M.; Broniatowski, D.A.; Dredze, M.; Wood-Doughty, Z.; Khan, D.; Quinn, S.C. Vaccine-related advertising in the Facebook Ad Archive. Vaccine 2020, 38, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Ndwandwe, D.; Wiysonge, C.S. COVID-19 vaccines. Curr. Opin. Immunol. 2021, 71, 111–116. [Google Scholar] [CrossRef]
- Donovan, R.J.; Jalleh, G. Positive versus Negative Framing of a Hypothetical Infant Immunization: The Influence of Involvement. Health Educ. Behav. 2000, 27, 82–95. [Google Scholar] [CrossRef]
- Benjamin, D.; Por, H.-H.; Budescu, D. Climate Change Versus Global Warming: Who Is Susceptible to the Framing of Climate Change? Environ. Behav. 2016, 49, 745–770. [Google Scholar] [CrossRef]
- Haydarov, R.; Gordon, J.C. Effect of combining attribute and goal framing within messages to change vaccination behavior. J. Commun. Healthc. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Interim Statement on COVID-19 Vaccines in the Context of the Circulation of the Omicron SARS-CoV-2 Variant from the WHO Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC) [Internet] [World Health Organization]. 11 January 2022. Available online: https://www.who.int/news/item/11-01-2022-interim-statement-on-covid-19-vaccines-in-the-context-of-the-circulation-of-the-omicron-sars-cov-2-variant-from-the-who-technical-advisory-group-on-covid-19-vaccine-composition (accessed on 13 January 2022).
- Pfizer plans to manufacture up to 100 million doses of omicron-specific vaccine by spring. The Washington Post, 11 January 2022. Available online: https://www.washingtonpost.com/business/2022/01/11/pfizer-omicron-specific-vaccine/(accessed on 13 January 2022).
- Webster, R.K.; Rubin, G.J. The Effect of Positively Framing Side-Effect Risk in Two Different Formats on Side-Effect Expectations, Informed Consent and Credibility: A Randomised Trial of 16- to 75-Year-Olds in England. Drug Saf. 2020, 43, 1011–1022. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Carter, P.; Blair, E. Attribute Framing and Goal Framing Effects in Health Decisions. Organ. Behav. Hum. Decis. Process. 2001, 85, 382–399. [Google Scholar] [CrossRef] [Green Version]
- Levin, I.P.; Schnittjer, S.K.; Thee, S.L. Information framing effects in social and personal decisions. J. Exp. Soc. Psychol. 1988, 24, 520–529. [Google Scholar] [CrossRef]
- Zimmermann, C.; Baldo, C.; Molino, A. Framing of outcome and probability of recurrence: Breast cancer patients’ choice of adjuvant chemotherapy (ACT) in hypothetical patient scenarios. Breast Cancer Res. Treat. 2000, 60, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Marteau, T.M. Framing of information: Its influence upon decisions of doctors and patients. Br. J. Soc. Psychol. 1989, 28, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.K.; Rubin, G.J. Predicting Expectations of Side-Effects for Those Which Are Warned Versus Not Warned About in Patient Information Leaflets. Ann. Behav. Med. 2021, 55, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Herber, O.R.; Gies, V.; Schwappach, D.; Thürmann, P.; Wilm, S. Patient information leaflets: Informing or frightening? A focus group study exploring patients’ emotional reactions and subsequent behavior towards package leaflets of commonly prescribed medications in family practices. BMC Fam. Pract. 2014, 15, 163. [Google Scholar] [CrossRef] [Green Version]
- Berry, D.C.; Raynor, D.K.; Knapp, P. Communicating risk of medication side effects: An empirical evaluation of EU recommended terminology. Psychol. Health Med. 2003, 8, 251–263. [Google Scholar] [CrossRef]
- Research and Analysis: Coronavirus Vaccine—Weekly Summary of Yellow Card Reporting [Internet] [gov.uk]. 22 September 2021. Available online: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting (accessed on 15 December 2021).
- Kreiner, H.; Gamliel, E. Are highly numerate individuals invulnerable to attribute framing bias? Comparing numerically and graphically represented attribute framing. Eur. J. Soc. Psychol. 2017, 47, 775–782. [Google Scholar] [CrossRef]
- Myers, J.L.; Well, A.D. Research Design and Statistical Analysis, 2nd ed.; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2003. [Google Scholar]
- Cardinal, R.N.; Aitken, M.R.F. ANOVA for the Behavioural Sciences Researcher; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2006. [Google Scholar]
- Llewellyn-Thomas, H.A.; McGreal, M.J.; Thiel, E.C. Cancer Patients’ Decision Making and Trial-entry Preferences: The Effects of “Framing” Information about Short-term Toxicity and Long-term Survival. Med. Decis. Mak. 1995, 15, 4–12. [Google Scholar] [CrossRef]
- Ferguson, E.; Gallagher, L. Message framing with respect to decisions about vaccination: The roles of frame valence, frame method and perceived risk. Br. J. Psychol. 2007, 98, 667–680. [Google Scholar] [CrossRef]
- Jasper, J.D.; Goel, R.; Einarson, A.; Gallo, M.; Koren, G. Effects of framing on teratogenic risk perception in pregnant women. Lancet 2001, 358, 1237–1238. [Google Scholar] [CrossRef]
- Webster, R.K.; Weinman, J.; Rubin, G.J. Explaining all without causing unnecessary harm: Is there scope for positively framing medical risk information? Patient Educ. Couns. 2019, 102, 602–603. [Google Scholar] [CrossRef] [Green Version]
- Freling, T.H.; Vincent, L.H.; Henard, D.H. When not to accentuate the positive: Re-examining valence effects in attribute framing. Organ. Behav. Hum. Decis. Process. 2014, 124, 95–109. [Google Scholar] [CrossRef]
- Dan, V.; Dixon, G.N. Fighting the Infodemic on Two Fronts: Reducing False Beliefs Without Increasing Polarization. Sci. Commun. 2021, 43, 674–682. [Google Scholar] [CrossRef]
- Nyhan, B.; Reifler, J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 2015, 33, 459–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, R.K.; Weinman, J.; Rubin, G.J. People’s Understanding of Verbal Risk Descriptors in Patient Information Leaflets: A Cross-Sectional National Survey of 18- to 65-Year-Olds in England. Drug Saf. 2017, 40, 743–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, D.C.; Knapp, P.R.; Raynor, T. Is 15 per cent very common? Informing people about the risks of medication side effects. Int. J. Pharm. Pract. 2002, 10, 145–151. [Google Scholar] [CrossRef]
- Berry, D.C.; Raynor, D.; Knapp, P.; Bersellini, E. Patients’ Understanding of Risk Associated with Medication Use. Drug Saf. 2003, 26, 1–11. [Google Scholar] [CrossRef]
- Knapp, P.; Raynor, D.K.; Woolf, E.; Gardner, P.; Carrigan, N.; McMillan, B.; Knapp, P. Communicating the Risk of Side Effects to Patients. Drug Saf. 2009, 32, 837–849. [Google Scholar] [CrossRef]
- Gerend, M.A.; Shepherd, J.E. Predicting human papillomavirus vaccine uptake in young adult women: Comparing the health belief model and theory of planned behavior. Ann. Behav. Med. 2012, 44, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, B.A.; Ruiter, R.A.C.; Chapman, G.; Kok, G. The intention to get vaccinated against influenza and actual vaccination uptake of Dutch healthcare personnel. Vaccine 2014, 32, 6986–6991. [Google Scholar] [CrossRef] [Green Version]
- Juraskova, I.; Bari, R.A.; O’Brien, M.T.; McCaffery, K.J. HPV Vaccine Promotion: Does Referring to Both Cervical Cancer and Genital Warts Affect Intended and Actual Vaccination Behavior? Women’s Health Issues 2011, 21, 71–79. [Google Scholar] [CrossRef]
- Jensen, U.; Ayers, S.; Koskan, A. Video-based messages to reduce COVID-19 vaccine hesitancy and nudge uptake. PsyArXiv 2021. Available online: https://psyarxiv.com/df9qw/ (accessed on 21 January 2022).
- Sheeran, P. Intention—Behavior Relations: A Conceptual and Empirical Review. Eur. Rev. Soc. Psychol. 2002, 12, 1–36. [Google Scholar] [CrossRef]
| Same Positive N = 207 | Same Negative N = 203 | Familiar Positive N = 201 | Familiar Negative N = 203 | Unfamiliar Positive N = 203 | Unfamiliar Negative N = 205 | |
|---|---|---|---|---|---|---|
| Employment | ||||||
| Employed full-time | 68 | 63 | 70 | 61 | 72 | 73 |
| Employed part-time | 16 | 28 | 21 | 36 | 23 | 29 |
| Self employed | 16 | 19 | 14 | 17 | 11 | 21 |
| Unemployed (looking) | 8 | 6 | 5 | 9 | 6 | 4 |
| Unemployed (not looking)/long-term sick or disabled | 17 | 16 | 14 | 16 | 14 | 15 |
| Parent/Carer | 10 | 13 | 10 | 10 | 17 | 10 |
| Student | 3 | 4 | 2 | 6 | 4 | 5 |
| Retired | 66 | 52 | 58 | 47 | 52 | 46 |
| Other | 3 | 2 | 7 | 1 | 4 | 2 |
| Education | ||||||
| Primary/Secondary (no qualifications) | 11 | 15 | 10 | 7 | 7 | 3 |
| GCSE (or equivalent) | 36 | 44 | 39 | 48 | 44 | 44 |
| NVQ 1/2 | 16 | 10 | 10 | 13 | 10 | 11 |
| A/AS-Levels (or equivalent) | 53 | 50 | 44 | 46 | 45 | 47 |
| Bachelor’s degree ;(or equivalent) | 65 | 66 | 76 | 61 | 74 | 71 |
| Post-Graduate | 21 | 14 | 17 | 21 | 21 | 27 |
| Other | 5 | 4 | 5 | 7 | 2 | 2 |
| Gender | ||||||
| Woman | 117 | 122 | 106 | 123 | 122 | 116 |
| Man | 90 | 81 | 93 | 79 | 81 | 87 |
| Non-binary | 0 | 0 | 1 | 1 | 0 | 1 |
| Prefer not to say (other) | 0 | 0 | 1 | 0 | 0 | 1 |
| Age bracket (years) | ||||||
| 18–24 | 8 | 8 | 3 | 10 | 7 | 11 |
| 25–34 | 17 | 23 | 20 | 18 | 18 | 20 |
| 35–44 | 42 | 27 | 30 | 41 | 32 | 39 |
| 45–54 | 34 | 49 | 38 | 38 | 46 | 41 |
| 55–64 | 52 | 49 | 59 | 52 | 53 | 50 |
| 65–74 | 48 | 38 | 41 | 39 | 40 | 39 |
| 75+ | 6 | 9 | 10 | 5 | 7 | 5 |
| Ethnicity | ||||||
| Asian or Asian British a | 5 | 10 | 6 | 3 | 9 | 11 |
| Black or Black British b | 5 | 4 | 3 | 1 | 1 | 2 |
| Mixed background c | 4 | 2 | 5 | 4 | 1 | 4 |
| Other d | 3 | 0 | 0 | 0 | 1 | 0 |
| White e | 190 | 187 | 187 | 195 | 191 | 188 |
| Same Positive N = 207 | Same Negative N = 203 | Familiar Positive N = 201 | Familiar Negative N = 203 | Unfamiliar Positive N = 203 | Unfamiliar Negative N = 205 | |
|---|---|---|---|---|---|---|
| Months (since last COVID-19 vaccination) | ||||||
| 0–3 | 41 | 44 | 38 | 48 | 34 | 42 |
| 4–6 | 156 | 148 | 147 | 143 | 153 | 141 |
| 7+ | 10 | 11 | 16 | 12 | 16 | 22 |
| COVID-19 Exposure: Personal infection | ||||||
| Yes | 24 | 23 | 27 | 26 | 22 | 26 |
| No | 183 | 180 | 174 | 177 | 181 | 179 |
| COVID-19 Exposure: Significant others | ||||||
| Yes | 108 | 86 | 87 | 98 | 92 | 94 |
| No | 99 | 117 | 114 | 105 | 111 | 111 |
| Previous Side Effects | ||||||
| Yes (First Dose) | 65 | 61 | 57 | 61 | 66 | 62 |
| Yes (Second Dose) | 23 | 26 | 20 | 28 | 20 | 24 |
| Yes (Both Doses) | 23 | 17 | 22 | 21 | 21 | 26 |
| No | 96 | 99 | 102 | 93 | 96 | 93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnes, K.; Colagiuri, B. Positive Attribute Framing Increases COVID-19 Booster Vaccine Intention for Unfamiliar Vaccines. Vaccines 2022, 10, 962. https://doi.org/10.3390/vaccines10060962
Barnes K, Colagiuri B. Positive Attribute Framing Increases COVID-19 Booster Vaccine Intention for Unfamiliar Vaccines. Vaccines. 2022; 10(6):962. https://doi.org/10.3390/vaccines10060962
Chicago/Turabian StyleBarnes, Kirsten, and Ben Colagiuri. 2022. "Positive Attribute Framing Increases COVID-19 Booster Vaccine Intention for Unfamiliar Vaccines" Vaccines 10, no. 6: 962. https://doi.org/10.3390/vaccines10060962
APA StyleBarnes, K., & Colagiuri, B. (2022). Positive Attribute Framing Increases COVID-19 Booster Vaccine Intention for Unfamiliar Vaccines. Vaccines, 10(6), 962. https://doi.org/10.3390/vaccines10060962






