Characterization of the Protective Immune Responses Conferred by Recombinant BCG Overexpressing Components of Mycobacterium tuberculosis Sec Protein Export System
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Generation of BCGSecDFG Strains
2.3. Western Blot
2.4. Macrophage Infection
2.5. Vaccination of Mice and M. tuberculosis Challenge
2.6. Organ Collection and Processing
2.7. IFNγ ELISPOT
2.8. Flow Cytometry
2.9. Statistical Analysis
3. Results
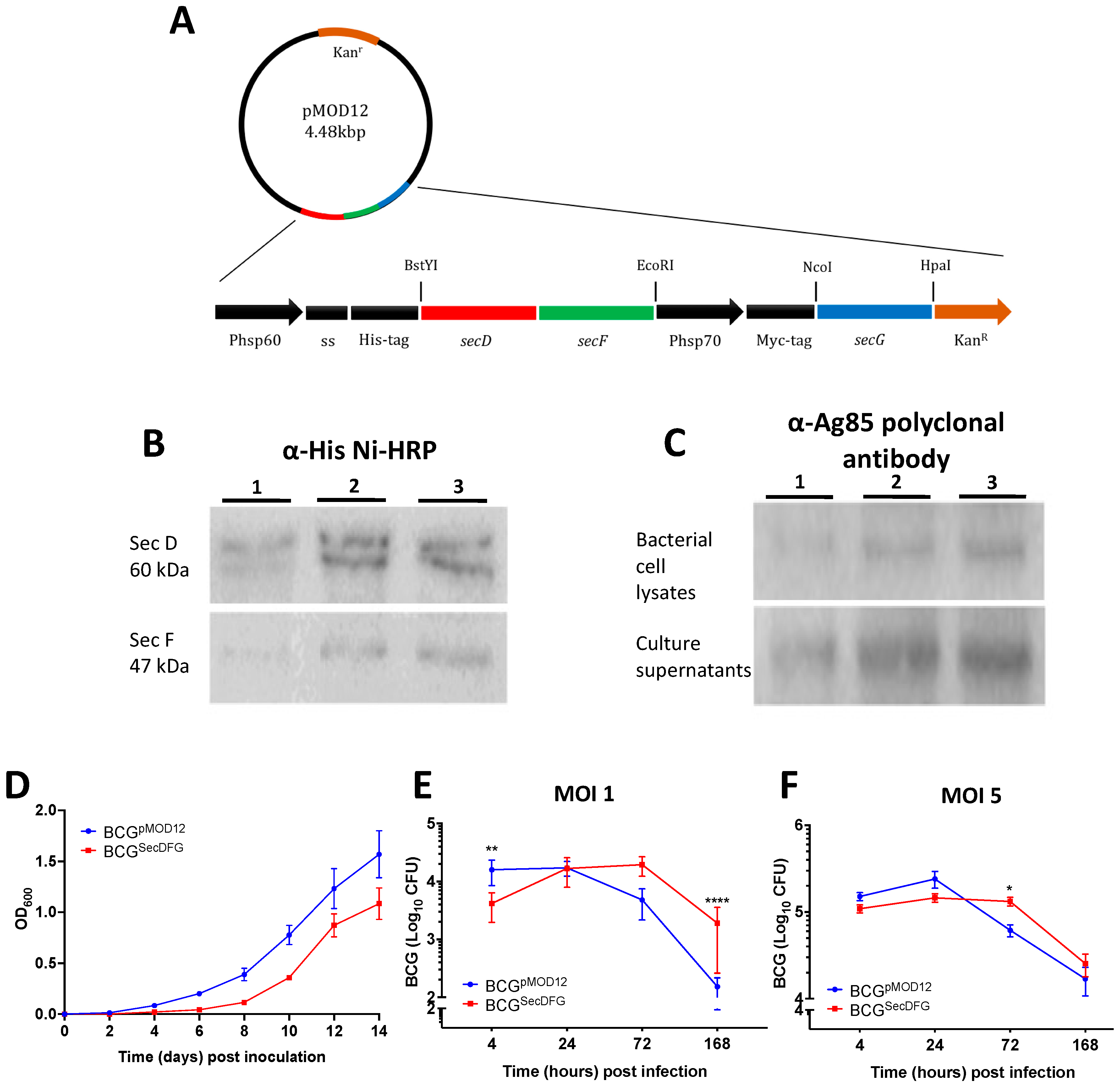
3.1. Construction and Characterization of rBCG Strains Overexpressing the SecDFG Components from the M. tuberculosis Sec Export System
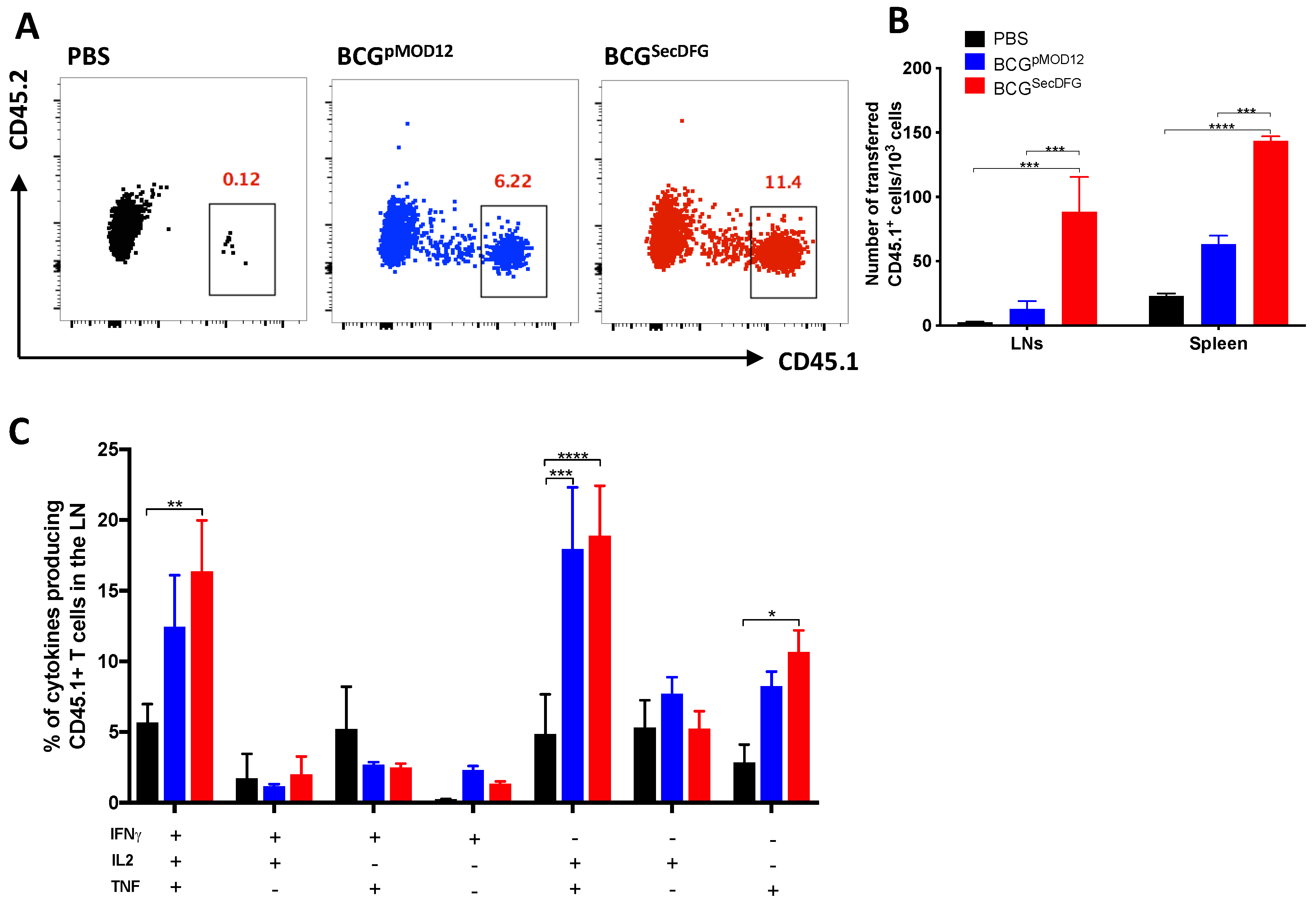
3.2. Increased In Vitro Intracellular Persistence and In Vivo Immunity by BCGSecDFG
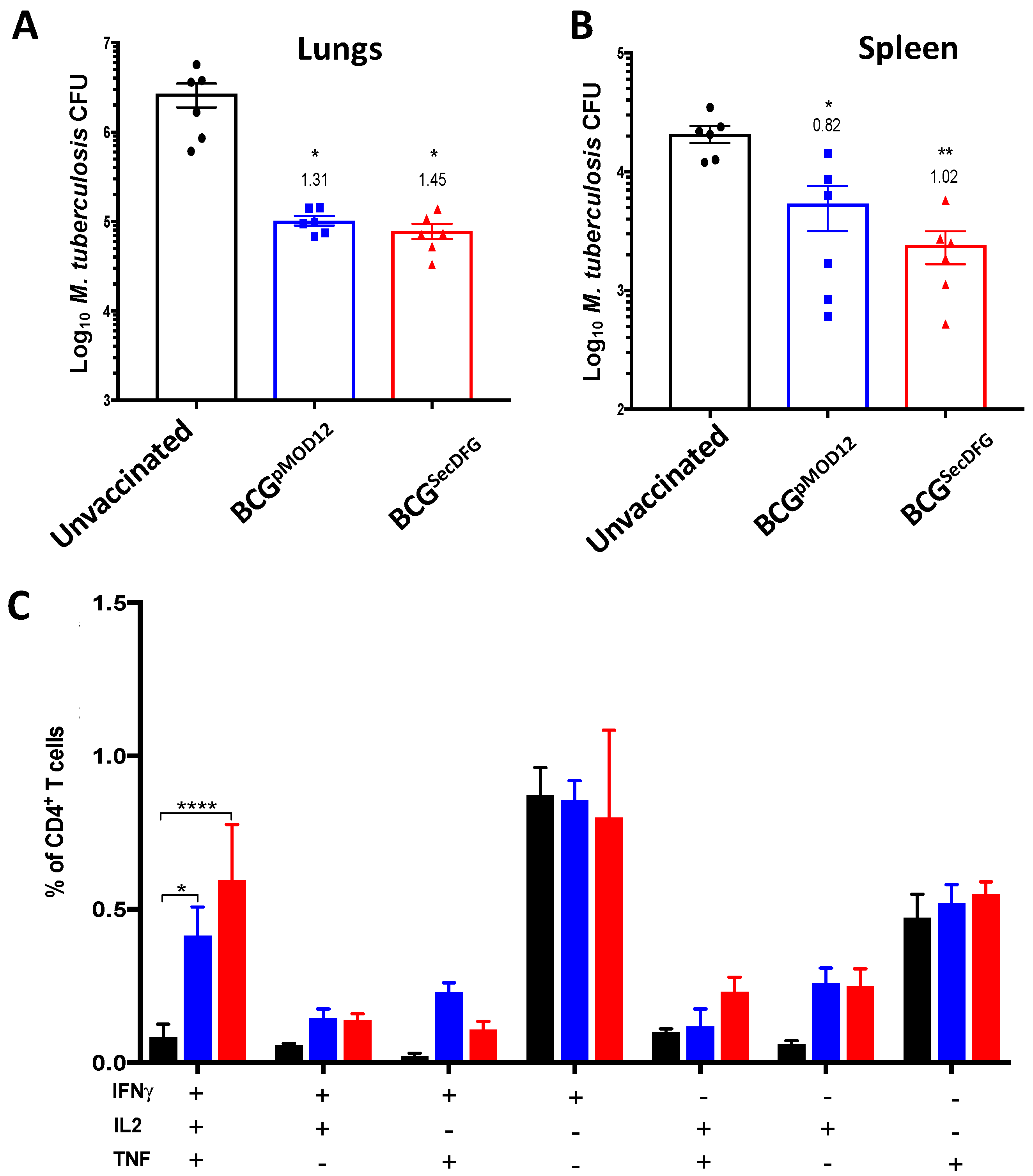
3.3. Protective Efficacy of BCGSecDFG against Aerosol M. tuberculosis Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pai, M.; Kasaeva, T.; Swaminathan, S. COVID-19’s Devastating Effect on Tuberculosis Care—A Path to Recovery. N. Engl. J. Med. 2022, 386, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Ottenhoff, T.H.; Kaufmann, S.H. Vaccines against tuberculosis: Where are we and where do we need to go? PLoS Pathog. 2012, 8, e1002607. [Google Scholar] [CrossRef] [PubMed]
- Aguilo, N.; Gonzalo-Asensio, J.; Alvarez-Arguedas, S.; Marinova, D.; Gomez, A.B.; Uranga, S.; Spallek, R.; Singh, M.; Audran, R.; Spertini, F.; et al. Reactogenicity to major tuberculosis antigens absent in BCG is linked to improved protection against Mycobacterium tuberculosis. Nat. Commun. 2017, 8, 16085. [Google Scholar] [CrossRef] [PubMed]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.M.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F.; et al. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin. Infect. Dis. 2013, 58, 470–480. [Google Scholar] [CrossRef]
- Triccas, J.A. Recombinant BCG as a vaccine vehicle to protect against tuberculosis. Bioeng. Bugs 2010, 1, 110–115. [Google Scholar] [CrossRef]
- Pieters, J. Mycobacterium tuberculosis and the macrophage: Maintaining a balance. Cell Host Microbe 2008, 3, 399–407. [Google Scholar] [CrossRef]
- Gupta, S.; Rodriguez, G.M. Mycobacterial extracellular vesicles and host pathogen interactions. Pathog. Dis. 2018, 76, fty031. [Google Scholar] [CrossRef]
- Feltcher, M.E.; Sullivan, J.T.; Braunstein, M. Protein export systems of Mycobacterium tuberculosis: Novel targets for drug development? Future Microbiol. 2010, 5, 1581–1597. [Google Scholar] [CrossRef]
- Sullivan, J.T.; Young, E.F.; McCann, J.R.; Braunstein, M. The Mycobacterium tuberculosis SecA2 system subverts phagosome maturation to promote growth in macrophages. Infect. Immun. 2012, 80, 996–1006. [Google Scholar] [CrossRef]
- Bottai, D.; Serafini, A.; Cascioferro, A.; Brosch, R.; Manganelli, R. Targeting type VII/ESX secretion systems for development of novel antimycobacterial drugs. Curr. Pharm. Des. 2014, 20, 4346–4356. [Google Scholar] [CrossRef]
- Feltcher, M.E.; Gibbons, H.S.; Ligon, L.S.; Braunstein, M. Protein export by the mycobacterial SecA2 system is determined by the preprotein mature domain. J. Bacteriol. 2013, 195, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Ligon, L.S.; Hayden, J.D.; Braunstein, M. The ins and outs of Mycobacterium tuberculosis protein export. Tuberculosis 2012, 92, 121–132. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.R.; Kurtz, S.; Braunstein, M. Secreted and Exported Proteins Important to Mycobacterium. In Bacterial Secreted Proteins: Secretory Mechanisms and Role in Pathogenesis; Caister Academic Press: Norfolk, UK, 2009; p. 265. [Google Scholar]
- Henao-Tamayo, M.; Junqueira-Kipnis, A.P.; Ordway, D.; Gonzales-Juarrero, M.; Stewart, G.R.; Young, D.B.; Wilkinson, R.J.; Basaraba, R.J.; Orme, I.M. A mutant of Mycobacterium tuberculosis lacking the 19-kDa lipoprotein Rv3763 is highly attenuated in vivo but retains potent vaccinogenic properties. Vaccine 2007, 25, 7153–7159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sulzenbacher, G.; Canaan, S.; Bordat, Y.; Neyrolles, O.; Stadthagen, G.; Roig-Zamboni, V.; Rauzier, J.; Maurin, D.; Laval, F.; Daffé, M.; et al. LppX is a lipoprotein required for the translocation of phthiocerol dimycocerosates to the surface of Mycobacterium tuberculosis. EMBO J. 2006, 25, 1436–1444. [Google Scholar] [CrossRef]
- Fabiana, B.; Andrea, G.; Laura, K.; María, S.; Alicia, A.; Karina, C.; Virginia, M.; Martin, Z.; Oscar, T.; María, R. The knockout of the lprG-Rv1410 operon produces strong attenuation of Mycobacterium tuberculosis. Microbes Infect. 2004, 6, 182–187. [Google Scholar]
- Målen, H.; Berven, F.S.; Fladmark, K.E.; Wiker, H.G. Comprehensive analysis of exported proteins from Mycobacterium tuberculosis H37Rv. Proteomics 2007, 7, 1702–1718. [Google Scholar] [CrossRef]
- Palma, C.; Iona, E.; Giannoni, F.; Pardini, M.; Brunori, L.; Orefici, G.; Fattorini, L.; Cassone, A. The Ag85B protein of Mycobacterium tuberculosis may turn a protective immune response induced by Ag85B-DNA vaccine into a potent but non-protective Th1 immune response in mice. Cell. Microbiol. 2007, 9, 1455–1465. [Google Scholar] [CrossRef]
- Karbalaei, M.Z.B.; Soleimanpour, S.; Rezaee, S. Antigen 85 complex as a powerful Mycobacterium tuberculosis immunogene: Biology, immune-pathogenicity, applications in diagnosis, and vaccine design. Microb. Pathog. 2017, 112, 20–29. [Google Scholar] [CrossRef]
- Wiker, H.G.; Harboe, M. The antigen 85 complex: A major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 1992, 56, 648–661. [Google Scholar] [CrossRef]
- Kruh-Garcia, N.A.; Murray, M.; Prucha, J.G.; Dobos, K.M. Antigen 85 variation across lineages of Mycobacterium tuberculosis—Implications for vaccine and biomarker success. J. Proteom. 2014, 97, 141–150. [Google Scholar] [CrossRef][Green Version]
- Launois, P.; Drowart, A.; Bourreau, E.; Couppie, P.; Farber, C.M.; Van Vooren, J.P.; Huygen, K. T cell reactivity against mycolyl transferase antigen 85 of M. tuberculosis in HIV-TB coinfected subjects and in AIDS patients suffering from tuberculosis and nontuberculous mycobacterial infections. Clin. Dev. Immunol. 2010, 2011, 640309. [Google Scholar] [PubMed]
- Fletcher, A.H.; Schrager, L. TB vaccine development and the End TB Strategy: Importance and current status. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Sable, S.B.; Posey, J.E.; Scriba, T.J. Tuberculosis vaccine development: Progress in clinical evaluation. Clin. Microbiol. Rev. 2019, 33, e00100-19. [Google Scholar] [CrossRef] [PubMed]
- Feltcher, M.E.; Braunstein, M. Emerging themes in SecA2-mediated protein export. Nat. Rev. Microbiol. 2012, 10, 779–789. [Google Scholar] [CrossRef]
- Nijeholt, J.A.L.A.; Driessen, A.J. The bacterial Sec-translocase: Structure and mechanism. Phil. Trans. R. Soc. B 2012, 367, 1016–1028. [Google Scholar] [CrossRef]
- Chung, M.A.; Luo, Y.; O’Donnell, M.; Rodriguez, C.; Heber, W.; Sharma, S.; Chang, H.R. Development and preclinical evaluation of a Bacillus Calmette-Guerin-MUC1-based novel breast cancer vaccine. Cancer Res. 2003, 63, 1280–1287. [Google Scholar]
- Counoupas, C.; Pinto, R.; Nagalingam, G.; Britton, W.J.; Triccas, J.A. Protective efficacy of recombinant BCG over-expressing protective, stage-specific antigens of Mycobacterium tuberculosis. Vaccine 2018, 36, 2619–2629. [Google Scholar] [CrossRef]
- Counoupas, C.; Pinto, R.; Nagalingam, G.; Hill-Cawthorne, G.; Feng, C.; Britton, W.J.; Triccas, J.A. Mycobacterium tuberculosis components expressed during chronic infection of the lung contribute to long-term control of pulmonary tuberculosis in mice. NPJ Vaccines 2016, 1, 16012. [Google Scholar] [CrossRef]
- Tomisawa, S.; Abe, C.; Kamiya, M.; Kikukawa, T.; Demura, M.; Kawano, K.; Aizawa, T. A new approach to detect small peptides clearly and sensitively by Western blotting using a vacuum-assisted detection method. Biophysics 2013, 9, 79–83. [Google Scholar] [CrossRef]
- Prendergast, K.A.; Counoupas, C.; Leotta, L.; Eto, C.; Bitter, W.; Winter, N.; Triccas, J.A. The Ag85B protein of the BCG vaccine facilitates macrophage uptake but is dispensable for protection against aerosol Mycobacterium tuberculosis infection. Vaccine 2016, 34, 2608–2615. [Google Scholar] [CrossRef]
- Gröschel, M.I.; Sayes, F.; Shin, S.J.; Frigui, W.; Pawlik, A.; Orgeur, M.; Canetti, R.; Honoré, N.; Simeone, R.; van der Werf, T.; et al. Recombinant BCG Expressing ESX-1 of Mycobacterium marinum Combines Low Virulence with Cytosolic Immune Signaling and Improved TB Protection. Cell Rep. 2017, 18, 2752–2765. [Google Scholar] [CrossRef] [PubMed]
- Bottai, D.; Frigui, W.; Clark, S.; Rayner, E.; Zelmer, A.; Andreu, N.; de Jonge, M.I.; Bancroft, G.J.; Williams, A.; Brodin, P.; et al. Increased protective efficacy of recombinant BCG strains expressing virulence-neutral proteins of the ESX-1 secretion system. Vaccine 2015, 33, 2710–2718. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.; Chen, C.; Portnoy, D.A. Strategies used by bacteria to grow in macrophages. Microbiol. Spectr. 2016, 4, 701–725. [Google Scholar] [CrossRef] [PubMed]
- Forrellad, M.A.; Klepp, L.I.; Gioffré, A.; Sabio y García, J.; Morbidoni, H.R.; de la Paz Santangelo, M.; Cataldi, A.A.; Bigi, F. Virulence factors of the Mycobacterium tuberculosis complex. Virulence 2013, 4, 3–66. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.W.; Brandt, L.; Agger, E.M.; van Pinxteren, L.A.H.; Andersen, P. The influence of remaining live BCG organisms in vaccinated mice on the maintenance of immunity to tuberculosis. Scand. J. Immunol. 2004, 60, 273–277. [Google Scholar] [CrossRef]
- Kaveh, D.A.; Bachy, V.S.; Hewinson, R.G.; Hogarth, P.J. Systemic BCG immunization induces persistent lung mucosal multifunctional CD4 TEM cells which expand following virulent mycobacterial challenge. PLoS ONE 2011, 6, e21566. [Google Scholar] [CrossRef]
- Nandakumar, S.; Kannanganat, S.; Posey, J.E.; Amara, R.R.; Sable, S.B. Attrition of T-cell functions and simultaneous upregulation of inhibitory markers correspond with the waning of BCG-induced protection against tuberculosis in mice. PLoS ONE 2014, 9, e113951. [Google Scholar] [CrossRef]
- Giri, P.K.; Kruh, N.A.; Dobos, K.M.; Schorey, J.S. Proteomic analysis identifies highly antigenic proteins in exosomes from M. tuberculosis-infected and culture filtrate protein-treated macrophages. Proteomics 2010, 10, 3190–3202. [Google Scholar]
- Kong, C.U.; Ng, L.G.; Nambiar, J.K.; Spratt, J.M.; Weninger, W.; Triccas, J.A. Targeted induction of antigen expression within dendritic cells modulates antigen-specific immunity afforded by recombinant BCG. Vaccine 2011, 29, 1374–1381. [Google Scholar] [CrossRef]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P. The immune response in tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef]
- Seder, R.A.; Darrah, P.A.; Roederer, M. T-cell quality in memory and protection: Implications for vaccine design. Nat. Rev. Immunol. 2008, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Lindenstrøm, T.; Knudsen, N.P.; Agger, E.M.; Andersen, P. Control of chronic Mycobacterium tuberculosis infection by CD4 KLRG1− IL-2–secreting central memory cells. J. Immunol. 2013, 190, 1300248. [Google Scholar] [CrossRef] [PubMed]
- Mittrücker, H.-W.; Steinhoff, U.; Köhler, A.; Krause, M.; Lazar, D.; Mex, P.; Miekley, D.; Kaufmann, S.H.E. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc. Natl. Acad. Sci. USA 2007, 104, 12434–12439. [Google Scholar] [CrossRef] [PubMed]
- Palendira, U.; Spratt, J.M.; Britton, W.J.; Triccas, J.A. Expanding the antigenic repertoire of BCG improves protective efficacy against aerosol Mycobacterium tuberculosis infection. Vaccine 2005, 23, 1680–1685. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, M.A.; Harth, G.; Dillon, B.J.; Masleša-Galić, S. Recombinant bacillus Calmette–Guérin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 2000, 97, 13853–13858. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisa, A.; Counoupas, C.; Pinto, R.; Britton, W.J.; Triccas, J.A. Characterization of the Protective Immune Responses Conferred by Recombinant BCG Overexpressing Components of Mycobacterium tuberculosis Sec Protein Export System. Vaccines 2022, 10, 945. https://doi.org/10.3390/vaccines10060945
Nisa A, Counoupas C, Pinto R, Britton WJ, Triccas JA. Characterization of the Protective Immune Responses Conferred by Recombinant BCG Overexpressing Components of Mycobacterium tuberculosis Sec Protein Export System. Vaccines. 2022; 10(6):945. https://doi.org/10.3390/vaccines10060945
Chicago/Turabian StyleNisa, Annuurun, Claudio Counoupas, Rachel Pinto, Warwick J. Britton, and James A. Triccas. 2022. "Characterization of the Protective Immune Responses Conferred by Recombinant BCG Overexpressing Components of Mycobacterium tuberculosis Sec Protein Export System" Vaccines 10, no. 6: 945. https://doi.org/10.3390/vaccines10060945
APA StyleNisa, A., Counoupas, C., Pinto, R., Britton, W. J., & Triccas, J. A. (2022). Characterization of the Protective Immune Responses Conferred by Recombinant BCG Overexpressing Components of Mycobacterium tuberculosis Sec Protein Export System. Vaccines, 10(6), 945. https://doi.org/10.3390/vaccines10060945






