mRNA COVID-19 Vaccine Reactogenicity among Healthcare Workers: Results from an Active Survey in a Pediatric Hospital from Bucharest, January–February 2021
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine Emergency Use Authorization Review Memorandum; Food and Drug Administration: Silver Spring, MD, USA, 2020. Available online: https://www.fda.gov/media/144416/download (accessed on 1 April 2022).
- European Medicines Agency. EMA Receives Application for Conditional Marketing Authorisation of COVID-19 mRNA Vaccine BNT162b2. Available online: https://www.ema.europa.eu/en/news/ema-receives-application-conditional-marketing-authorisation-covid-19-mrna-vaccine-bnt162b2 (accessed on 9 April 2022).
- COVID-19 Vaccines. European Medicines Agency. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines (accessed on 9 April 2022).
- Dooling, K.; Marin, M.; Wallace, M.; McClung, N.; Chamberland, M.; Lee, G.M.; Talbot, H.K.; Romero, J.R.; Bell, B.P.; Oliver, S.E. The Advisory Committee on Immunization Practices’ Updated Interim Recommendation for Allocation of COVID-19 vaccin-United States, December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 69, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- European Commission. EU Vaccines Strategy. Available online: https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/public-health/eu-vaccines-strategy_en (accessed on 9 April 2022).
- Strategia de Vaccinare împotriva COVID-19 în România. Available online: Strategia-vaccinare-02-12-2020-CL-FINAL-cu-COVID-19-tabel-2_CUPRINS-UPDATE-1.pdf (accessed on 9 April 2022).
- Dascalu, S.; Geambasu, O.; Covaciu, O.; Chereches, R.M.; Diaconu, G.; Dumitra, G.G.; Gheorghita, V.; Popovici, E.D. Prospects of COVID-19 Vaccination in Romania: Challenges and Potential Solutions. Front. Public Health 2021, 9, 644538. [Google Scholar] [CrossRef] [PubMed]
- Ordin Comun Pentru Stabilirea Normelor Privind Autorizarea, Organizarea și Funcționarea Centrelor de Vaccinare împotriva COVID-19. Available online: https://www.cnscbt.ro/index.php/legislatie_cov/2175-ordin-ms-2171-181-4380-m223-autorizare-organizare-functionare-centre-vaccinare/file (accessed on 9 April 2022).
- Papagiannis, D.; Malli, F. Registry Systems for COVID-19 Vaccines and Rate of Acceptability for Vaccination Before and After Availability of Vaccines in 12 Countries: A Narrative Review. Infect. Dis. Rep. 2022, 14, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.; Rocha, J.V.; Moniz, M.; Gama, A.; Laires, P.A.; Pedro, A.R.; Dias, S.; Leite, A.; Nunes, C. Factors Associated with COVID-19 Vaccine Hesitancy. Vaccines 2021, 9, 300. [Google Scholar] [CrossRef]
- Pitigoi, D. Epidemiologie-Curs și Lucrări Practice; Editura Universitară “Carol Davila”: Bucharest, Romania, 2019; pp. 42–43. [Google Scholar]
- Centrul Național de Supraveghere și Control Boli Transmisibile. Metodologia de Supraveghere a Reacțiilor Postvaccinale indezirabile-RAPI. Available online: http://www.cnscbt.ro/index.php/metodologii/rapi/662-metodologie-rapi/file (accessed on 9 April 2022).
- Agentia Nationala a Medicamentului si a Dispozitivelor Medicale din Romania. Available online: https://www.anm.ro/medicamente-de-uz-uman/farmacovigilenta/raportarea-reactiilor-adverse-suspectate-vaccin-covid-19 (accessed on 9 April 2022).
- Comirnaty Product Information. Available online: https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_ro.pdf (accessed on 15 January 2021).
- Dean, A.G.; Arner, T.G.; Sunki, G.G. Epi Info™, a Database and Statistics Program for Public Health Professionals; Centers for Diseases Control: Atlanta, GA, USA, 2011.
- MedCalc Software Ltd. Comparison of Proportions Calculator. Version 20.106. Available online: https://www.medcalc.org/calc/comparison_of_proportions.php (accessed on 1 April 2022).
- Centers for Diseases Control. Pfizer-BioNTech COVID-19 Vaccine (also known as COMIRNATY) Overview and Safety. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html (accessed on 25 March 2021).
- Gee, J.; Marquez, P.; Su, J.; Calvert, G.M.; Liu, R.; Myers, T.; Nair, N.; Martin, S.; Clark, T.; Markowitz, L.; et al. First Month of COVID-19 Vaccine Safety Monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 283–288. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- David, S.S.B.; Baruch Gez, S.; Rahamim-Cohen, D.; Shamir-Stein, N.; Lerner, U.; Zohar, A.E. Immediate side effects of Comirnaty COVID-19 vaccine: A nationwide survey of vaccinated people in Israel, December 2020 to March 2021. Eurosurveillance 2022, 27, 2100540. [Google Scholar]
- European Centre for Disease Prevention and Control. COVID-19 Vaccine Tracker. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/covid-19/vaccine-tracker.html#uptake-tab (accessed on 8 April 2022).
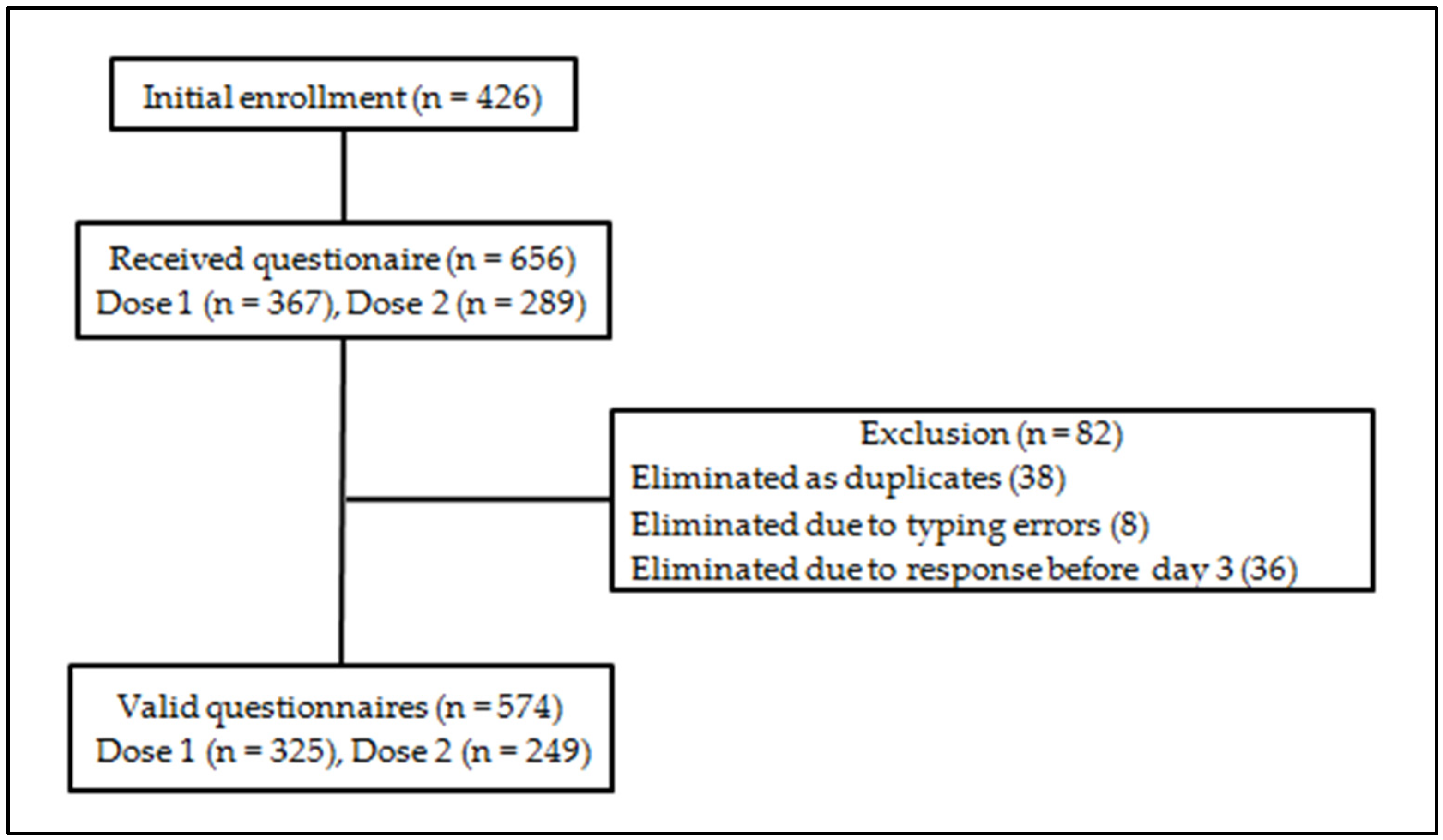
| Characteristics | Dose 1 (n = 325) | Dose 2 (n = 249) | Total (n = 574) | |
|---|---|---|---|---|
| Median age (years), IQR | 42, IQR 31–49 | 43, IQR 33–50 | 42, IQR 32–50 | |
| Age group | 18–55 years of age | 290 (89.2%) | 225 (90.4%) | 515 (89.7%) |
| 56 years of age and older | 35 (10.8%) | 24 (9.6%) | 59 (10.3%) | |
| Gender distribution | Female | 263 (80.9%) | 207 (83.1%) | 470 (81.9%) |
| Male | 62 (19.1%) | 42 (16.9%) | 104 (18.1%) | |
| Adverse Event | Hospital Survey (n = 290) Number (%) | Reference Study- Pfizer-BioNTec [1] (n = 2291) Number (%) | Statistical Analysis * |
|---|---|---|---|
| Local reactions | |||
| Redness (any) | 16 (5.5) | 104 (4.5) | p = 0.44, χ2(1) = 0.58 |
| Swelling (any) | 25 (8.6) | 132 (5.8) | p = 0.06, χ2(1) = 3.51 |
| Pain at the injection site (any) | 244 (84.1) | 1904 (83.1) | p = 0.67, χ2(1) = 0.18 |
| Systemic reactions | |||
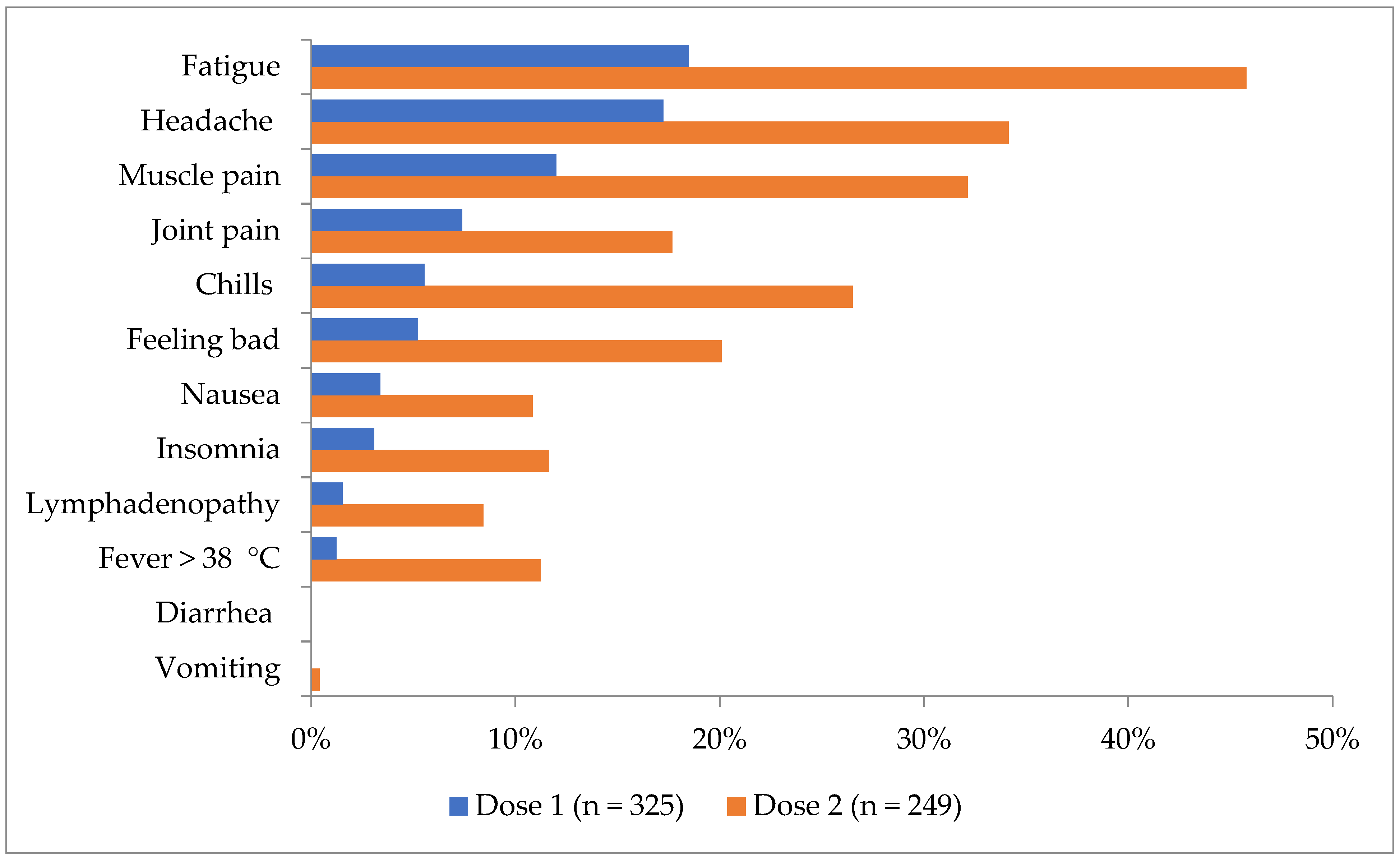
| Fever > 38 °C | 3 (1.0) | 85 (3.7) | p = 0.02, χ2(1) = 5.71 |
| Fatigue (any) | 55 (19.0) | 1085 (47.4) | p < 0.0001, χ2(1) = 84.14 |
| Headache (any) | 52 (17.9) | 959 (41.9) | p < 0.0001, χ2(1) = 62.18 |
| Chills (any) | 14 (4.8) | 321 (14.0) | p < 0.0001, χ2(1) = 19.29 |
| Vomiting (any) | 0 | 28 (1.2) | p = 0.06, χ2(1) = 3.51 |
| Muscle pain (any) | 35 (12.1) | 487 (21.3) | p < 0.0001, χ2(1) = 13.47 |
| Joint pain (any) | 22 (7.6) | 251 (11.0) | p = 0.08, χ2(1) = 3.14 |
| Adverse Event | Hospital Survey (n = 225) Number (%) | Reference Study- Pfizer-BioNTech [1] (n = 2098) Number (%) | Statistical Analysis * |
|---|---|---|---|
| Local reactions | |||
| Redness (any) | 11 (4.9) | 123 (5.9) | p = 0.54, χ2(1) = 0.37 |
| Swelling (any) | 13 (5.8) | 132 (6.3) | p = 0.76, χ2(1) = 0.09 |
| Pain at the injection site (any) | 176 (78.2) | 1632 (77.8) | p = 0.89, χ2(1) = 0.01 |
| Systemic reactions | |||
| Fever > 38 °C | 26 (11.6) | 331 (15.8) | p = 0.10, χ2(1) = 2.75 |
| Fatigue (any) | 107 (47.6) | 1247 (59.4) | p = 0.0006, χ2(1) = 11.63 |
| Headache (any) | 81 (36.0) | 1085 (51.7) | p < 0.0001, χ2(1) = 20.02 |
| Chills (any) | 60 (26.7) | 737 (35.1) | p = 0.011, χ2(1) = 6.36 |
| Vomiting (any) | 1 (0.4) | 40 (1.9) | p = 0.10, χ2(1) = 2.65 |
| Muscle pain (any) | 75 (33.3) | 783 (37.3) | p = 0.24, χ2(1) = 1.39 |
| Joint pain (any) | 40 (17.8) | 459 (21.9) | p = 0.15, χ2(1) = 2.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crăciun, M.-D.; Nițescu, G.V.; Golumbeanu, M.; Tănase, A.-A.; Pițigoi, D.; Săndulescu, O.; Crăciun, P.; Enciu, B.G.; Bălănescu, R.N.; Ulici, A. mRNA COVID-19 Vaccine Reactogenicity among Healthcare Workers: Results from an Active Survey in a Pediatric Hospital from Bucharest, January–February 2021. Vaccines 2022, 10, 836. https://doi.org/10.3390/vaccines10060836
Crăciun M-D, Nițescu GV, Golumbeanu M, Tănase A-A, Pițigoi D, Săndulescu O, Crăciun P, Enciu BG, Bălănescu RN, Ulici A. mRNA COVID-19 Vaccine Reactogenicity among Healthcare Workers: Results from an Active Survey in a Pediatric Hospital from Bucharest, January–February 2021. Vaccines. 2022; 10(6):836. https://doi.org/10.3390/vaccines10060836
Chicago/Turabian StyleCrăciun, Maria-Dorina, Gabriela Viorela Nițescu, Mihaela Golumbeanu, Alina-Andreea Tănase, Daniela Pițigoi, Oana Săndulescu, Petru Crăciun, Bianca Georgiana Enciu, Radu Ninel Bălănescu, and Alexandru Ulici. 2022. "mRNA COVID-19 Vaccine Reactogenicity among Healthcare Workers: Results from an Active Survey in a Pediatric Hospital from Bucharest, January–February 2021" Vaccines 10, no. 6: 836. https://doi.org/10.3390/vaccines10060836
APA StyleCrăciun, M.-D., Nițescu, G. V., Golumbeanu, M., Tănase, A.-A., Pițigoi, D., Săndulescu, O., Crăciun, P., Enciu, B. G., Bălănescu, R. N., & Ulici, A. (2022). mRNA COVID-19 Vaccine Reactogenicity among Healthcare Workers: Results from an Active Survey in a Pediatric Hospital from Bucharest, January–February 2021. Vaccines, 10(6), 836. https://doi.org/10.3390/vaccines10060836









