B Cell Responses upon Human Papillomavirus (HPV) Infection and Vaccination
Abstract
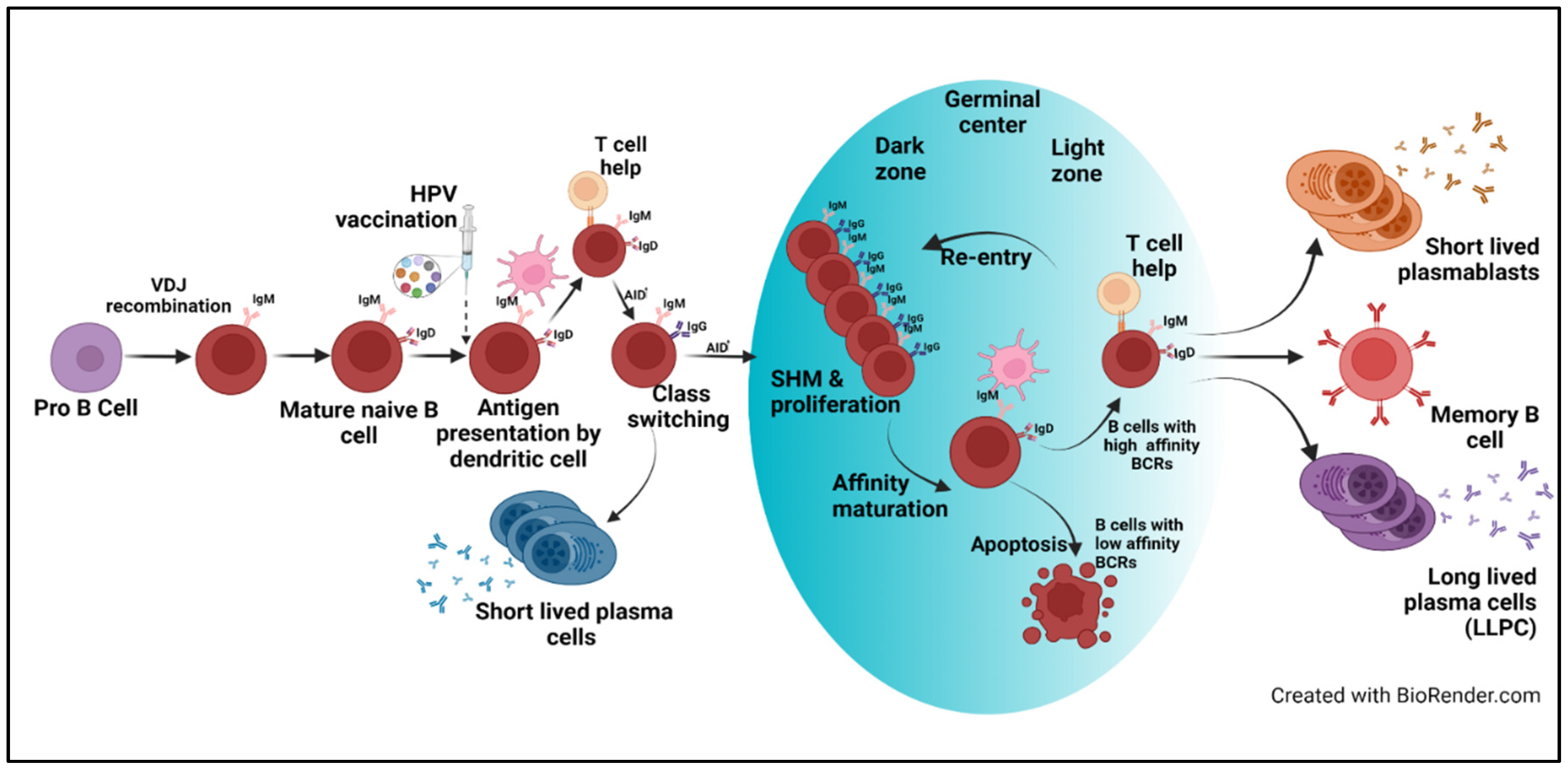
1. Introduction
2. Vaccines—Mode of Response
3. Techniques to Study B-Cell Responses
4. Evidence, Importance, and Comparison of B-Cell Responses across HPV Vaccines
5. Effect of Number of Doses, Recipient Age, and Type of Adjuvant in Vaccine-Induced B-Cell Response
6. Molecular Signatures/Patterns/Properties of HPV-Specific B-Cell Responses
7. B-Cell Response to Natural Infection with HPV
8. Lessons from Other Vaccines/Infection
9. Summary and Open Questions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- zur Hausen, H. Papillomaviruses in the causation of human cancers—A brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Paavonen, J.; Jenkins, D.; Bosch, F.X.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.N.; Apter, D.L.; Kitchener, H.C.; Castellsague, X.; et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: An interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007, 369, 2161–2170. [Google Scholar] [CrossRef]
- Herrero, R.; Hildesheim, A.; Rodríguez, A.C.; Wacholder, S.; Bratti, C.; Solomon, D.; González, P.; Porras, C.; Jiménez, S.; Guillen, D.; et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine 2008, 26, 4795–4808. [Google Scholar] [CrossRef] [PubMed]
- The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 2007, 356, 1915–1927. [Google Scholar] [CrossRef]
- Joura, E.A.; Giuliano, A.R.; Iversen, O.E.; Bouchard, C.; Mao, C.; Mehlsen, J.; Moreira, E.D., Jr.; Ngan, Y.; Petersen, L.K.; Lazcano-Ponce, E.; et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015, 372, 711–723. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Du, J. Human Papillomavirus Vaccines: An Updated Review. Vaccines 2020, 8, 391. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Artemchuk, H.; Eriksson, T.; Poljak, M.; Surcel, H.M.; Dillner, J.; Lehtinen, M.; Faust, H. Long-term Antibody Response to Human Papillomavirus Vaccines: Up to 12 Years of Follow-up in the Finnish Maternity Cohort. J. Infect. Dis. 2019, 219, 582–589. [Google Scholar] [CrossRef]
- Harper, D.M.; Franco, E.L.; Wheeler, C.M.; Moscicki, A.B.; Romanowski, B.; Roteli-Martins, C.M.; Jenkins, D.; Schuind, A.; Costa Clemens, S.A.; Dubin, G. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: Follow-up from a randomised control trial. Lancet 2006, 367, 1247–1255. [Google Scholar] [CrossRef]
- Joura, E.A.; Kjaer, S.K.; Wheeler, C.M.; Sigurdsson, K.; Iversen, O.E.; Hernandez-Avila, M.; Perez, G.; Brown, D.R.; Koutsky, L.A.; Tay, E.H.; et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine 2008, 26, 6844–6851. [Google Scholar] [CrossRef]
- Nygård, M.; Saah, A.; Munk, C.; Tryggvadottir, L.; Enerly, E.; Hortlund, M.; Sigurdardottir, L.G.; Vuocolo, S.; Kjaer, S.K.; Dillner, J. Evaluation of the Long-Term Anti-Human Papillomavirus 6 (HPV6), 11, 16, and 18 Immune Responses Generated by the Quadrivalent HPV Vaccine. Clin. Vaccine Immunol. 2015, 22, 943–948. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Prabhu, P.R.; Pawlita, M.; Gheit, T.; Bhatla, N.; Muwonge, R.; Nene, B.M.; Esmy, P.O.; Joshi, S.; Poli, U.R.; et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multicentre prospective cohort study. Lancet Oncol. 2016, 17, 67–77. [Google Scholar] [CrossRef]
- Guevara, A.; Cabello, R.; Woelber, L.; Moreira, E.D.; Joura, E.; Reich, O.; Shields, C.; Ellison, M.C.; Joshi, A.; Luxembourg, A. Antibody persistence and evidence of immune memory at 5years following administration of the 9-valent HPV vaccine. Vaccine 2017, 35, 5050–5057. [Google Scholar] [CrossRef]
- Kjaer, S.K.; Nygård, M.; Sundström, K.; Munk, C.; Berger, S.; Dzabic, M.; Fridrich, K.E.; Waldstrøm, M.; Sørbye, S.W.; Bautista, O.; et al. Long-term effectiveness of the nine-valent human papillomavirus vaccine in Scandinavian women: Interim analysis after 8 years of follow-up. Hum. Vaccines Immunother. 2021, 17, 943–949. [Google Scholar] [CrossRef]
- Harper, D.M.; DeMars, L.R. HPV vaccines–A review of the first decade. Gynecol. Oncol. 2017, 146, 196–204. [Google Scholar] [CrossRef]
- Muramatsu, M.; Kinoshita, K.; Fagarasan, S.; Yamada, S.; Shinkai, Y.; Honjo, T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000, 102, 553–563. [Google Scholar] [CrossRef]
- Nutt, S.L.; Hodgkin, P.D.; Tarlinton, D.M.; Corcoran, L.M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 2015, 15, 160–171. [Google Scholar] [CrossRef]
- Amanna, I.J.; Slifka, M.K. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol. Rev. 2010, 236, 125–138. [Google Scholar] [CrossRef]
- Mayr, L.M.; Su, B.; Moog, C. Non-Neutralizing Antibodies Directed against HIV and Their Functions. Front. Immunol. 2017, 8, 1590. [Google Scholar] [CrossRef]
- Tyler, D.S.; Lyerly, H.K.; Weinhold, K.J. Minireview Anti-HIV-1 ADCC. AIDS Res. Hum. Retrovir. 1989, 5, 557–563. [Google Scholar] [CrossRef]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef]
- Wang, J.W.; Wu, W.H.; Huang, T.C.; Wong, M.; Kwak, K.; Ozato, K.; Hung, C.F.; Roden, R.B.S. Roles of Fc Domain and Exudation in L2 Antibody-Mediated Protection against Human Papillomavirus. J. Virol. 2018, 92, e00572-18. [Google Scholar] [CrossRef]
- Bernasconi, N.L.; Traggiai, E.; Lanzavecchia, A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 2002, 298, 2199–2202. [Google Scholar] [CrossRef]
- Corti, D.; Lanzavecchia, A. Efficient Methods To Isolate Human Monoclonal Antibodies from Memory B Cells and Plasma Cells. Microbiol. Spectr. 2014, 2, 2–5. [Google Scholar] [CrossRef]
- Walsh, P.N.; Friedrich, D.P.; Williams, J.A.; Smith, R.J.; Stewart, T.L.; Carter, D.K.; Liao, H.-X.; McElrath, M.J.; Frahm, N.; Network, N.H.V.T. Optimization and qualification of a memory B-cell ELISpot for the detection of vaccine-induced memory responses in HIV vaccine trials. J. Immunol. Methods 2013, 394, 84–93. [Google Scholar] [CrossRef]
- Dauner, J.G.; Pan, Y.; Hildesheim, A.; Harro, C.; Pinto, L.A. Characterization of the HPV-specific memory B cell and systemic antibody responses in women receiving an unadjuvanted HPV16 L1 VLP vaccine. Vaccine 2010, 28, 5407–5413. [Google Scholar] [CrossRef]
- Dessy, F.J.; Giannini, S.L.; Bougelet, C.A.; Kemp, T.J.; David, M.P.; Poncelet, S.M.; Pinto, L.A.; Wettendorff, M.A. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum. Vaccines 2008, 4, 425–434. [Google Scholar] [CrossRef]
- Scherer, E.M.; Smith, R.A.; Simonich, C.A.; Niyonzima, N.; Carter, J.J.; Galloway, D.A. Characteristics of Memory B Cells Elicited by a Highly Efficacious HPV Vaccine in Subjects with No Pre-existing Immunity. PLoS Pathog. 2014, 10, e1004461. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scherer, E.M.; Smith, R.A.; Carter, J.J.; Wipf, G.C.; Gallego, D.F.; Stern, M.; Wald, A.; Galloway, D.A. Analysis of Memory B-Cell Responses Reveals Suboptimal Dosing Schedule of a Licensed Vaccine. J. Infect. Dis. 2018, 217, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Muecksch, F.; Schaefer-Babajew, D.; Wang, Z.; Finkin, S.; Gaebler, C.; Ramos, V.; Cipolla, M.; Mendoza, P.; Agudelo, M.; et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature 2021, 600, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L.; Schiepers, A.; Ersching, J.; Barbulescu, A.; Cavazzoni, C.B.; Angelini, A.; Okada, T.; Kurosaki, T.; Victora, G.D. Restricted Clonality and Limited Germinal Center Reentry Characterize Memory B Cell Reactivation by Boosting. Cell 2020, 180, 92–106.e11. [Google Scholar] [CrossRef]
- Haderxhanaj, L.T.; Leichliter, J.S.; Aral, S.O.; Chesson, H.W. Sex in a lifetime: Sexual behaviors in the United States by lifetime number of sex partners, 2006–2010. Sex. Transm. Dis. 2014, 41, 345–352. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Einstein, M.H.; Baron, M.; Levin, M.J.; Chatterjee, A.; Edwards, R.P.; Zepp, F.; Carletti, I.; Dessy, F.J.; Trofa, A.F.; Schuind, A.; et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum. Vaccines 2009, 5, 705–719. [Google Scholar] [CrossRef]
- Einstein, M.H.; Levin, M.J.; Chatterjee, A.; Chakhtoura, N.; Takacs, P.; Catteau, G.; Dessy, F.J.; Moris, P.; Lin, L.; Struyf, F.; et al. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: Follow-up through Month 48 in a Phase III randomized study. Hum. Vaccines Immunother. 2014, 10, 3455–3465. [Google Scholar] [CrossRef]
- Moscicki, A.B.; Wheeler, C.M.; Romanowski, B.; Hedrick, J.; Gall, S.; Ferris, D.; Poncelet, S.; Zahaf, T.; Moris, P.; Geeraerts, B.; et al. Immune responses elicited by a fourth dose of the HPV-16/18 AS04-adjuvanted vaccine in previously vaccinated adult women. Vaccine 2012, 31, 234–241. [Google Scholar] [CrossRef]
- Nicoli, F.; Mantelli, B.; Gallerani, E.; Telatin, V.; Bonazzi, I.; Marconi, P.; Gavioli, R.; Gabrielli, L.; Lazzarotto, T.; Barzon, L.; et al. HPV-Specific Systemic Antibody Responses and Memory B Cells are Independently Maintained up to 6 Years and in a Vaccine-Specific Manner Following Immunization with Cervarix and Gardasil in Adolescent and Young Adult Women in Vaccination Programs in Italy. Vaccines 2020, 8, 26. [Google Scholar] [CrossRef]
- Olsson, S.E.; Villa, L.L.; Costa, R.L.; Petta, C.A.; Andrade, R.P.; Malm, C.; Iversen, O.E.; Høye, J.; Steinwall, M.; Riis-Johannessen, G.; et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine 2007, 25, 4931–4939. [Google Scholar] [CrossRef]
- Cao, Y.; Gordic, M.; Kobold, S.; Lajmi, N.; Meyer, S.; Bartels, K.; Hildebrandt, Y.; Luetkens, T.; Ihloff, A.S.; Kröger, N.; et al. An optimized assay for the enumeration of antigen-specific memory B cells in different compartments of the human body. J. Immunol. Methods 2010, 358, 56–65. [Google Scholar] [CrossRef]
- Farber, D.L. Tissues, not blood, are where immune cells function. Nature 2021, 593, 506–509. [Google Scholar] [CrossRef]
- Smolen, K.K.; Gelinas, L.; Franzen, L.; Dobson, S.; Dawar, M.; Ogilvie, G.; Krajden, M.; Fortuno, E.S.; Kollmann, T.R. Age of recipient and number of doses differentially impact human B and T cell immune memory responses to HPV vaccination. Vaccine 2012, 30, 3572–3579. [Google Scholar] [CrossRef]
- Siegrist, C.-A. 2-Vaccine Immunology. In Plotkin’s Vaccines, 7th ed.; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Edwards, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 16–34.e7. [Google Scholar] [CrossRef]
- WHO. Human papillomavirus vaccines. Wkly. Epidemiol. Rec. 2017; 19, 241–268. [Google Scholar]
- Giannini, S.L.; Hanon, E.; Moris, P.; Van Mechelen, M.; Morel, S.; Dessy, F.; Fourneau, M.A.; Colau, B.; Suzich, J.; Losonksy, G.; et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 2006, 24, 5937–5949. [Google Scholar] [CrossRef]
- Schiller, J.T.; Lowy, D.R. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat. Rev. Microbiol. 2012, 10, 681–692. [Google Scholar] [CrossRef]
- Mankarious, S.; Lee, M.; Fischer, S.; Pyun, K.H.; Ochs, H.D.; Oxelius, V.A.; Wedgwood, R.J. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J. Lab. Clin. Med. 1988, 112, 634–640. [Google Scholar]
- Manz, R.A.; Löhning, M.; Cassese, G.; Thiel, A.; Radbruch, A. Survival of long-lived plasma cells is independent of antigen. Int. Immunol. 1998, 10, 1703–1711. [Google Scholar] [CrossRef]
- Bovay, A.; Nassiri, S.; Maby–El Hajjami, H.; Marcos Mondéjar, P.; Akondy, R.S.; Ahmed, R.; Lawson, B.; Speiser, D.E.; Fuertes Marraco, S.A. Minimal immune response to booster vaccination against Yellow Fever associated with pre-existing antibodies. Vaccine 2020, 38, 2172–2182. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.F.; Kaur, K.; Pauli, N.T.; Huang, M.; Huang, Y.; Wilson, P.C. High preexisting serological antibody levels correlate with diversification of the influenza vaccine response. J. Virol. 2015, 89, 3308–3317. [Google Scholar] [CrossRef]
- Lightman, S.M.; Utley, A.; Lee, K.P. Survival of Long-Lived Plasma Cells (LLPC): Piecing Together the Puzzle. Front. Immunol. 2019, 10, 965. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.A.; Castle, P.E.; Roden, R.B.; Harro, C.D.; Lowy, D.R.; Schiller, J.T.; Wallace, D.; Williams, M.; Kopp, W.; Frazer, I.H.; et al. HPV-16 L1 VLP vaccine elicits a broad-spectrum of cytokine responses in whole blood. Vaccine 2005, 23, 3555–3564. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Murillo, F.M.; Delannoy, M.J.; Blosser, R.L.; Yutzy, W.H.; Uematsu, S.; Takeda, K.; Akira, S.; Viscidi, R.P.; Roden, R.B.S. B Lymphocyte Activation by Human Papillomavirus-Like Particles Directly Induces Ig Class Switch Recombination via TLR4-MyD88. J. Immunol. 2005, 174, 7912–7919. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.O.; West, A.P.; Huey-Tubman, K.E.; Hoffmann, M.A.G.; Sharaf, N.G.; Hoffman, P.R.; Koranda, N.; Gristick, H.B.; Gaebler, C.; Muecksch, F.; et al. Structures of human antibodies bound to SARS-CoV-2 spike reveal common epitopes and recurrent features of antibodies. bioRxiv 2020. [Google Scholar] [CrossRef]
- Corti, D.; Bianchi, S.; Vanzetta, F.; Minola, A.; Perez, L.; Agatic, G.; Guarino, B.; Silacci, C.; Marcandalli, J.; Marsland, B.J.; et al. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature 2013, 501, 439–443. [Google Scholar] [CrossRef]
- Ehrhardt, S.A.; Zehner, M.; Krähling, V.; Cohen-Dvashi, H.; Kreer, C.; Elad, N.; Gruell, H.; Ercanoglu, M.S.; Schommers, P.; Gieselmann, L.; et al. Polyclonal and convergent antibody response to Ebola virus vaccine rVSV-ZEBOV. Nat. Med. 2019, 25, 1589–1600. [Google Scholar] [CrossRef]
- Galson, J.D.; Pollard, A.J.; Trück, J.; Kelly, D.F. Studying the antibody repertoire after vaccination: Practical applications. Trends Immunol. 2014, 35, 319–331. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Bozzacco, L.; Keeffe, J.R.; Khouri, R.; Olsen, P.C.; Gazumyan, A.; Schaefer-Babajew, D.; Avila-Rios, S.; Nogueira, L.; Patel, R.; et al. Recurrent Potent Human Neutralizing Antibodies to Zika Virus in Brazil and Mexico. Cell 2017, 169, 597–609.e511. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent Antibody Responses to SARS-CoV-2 Infection in Convalescent Individuals. bioRxiv 2020. [Google Scholar] [CrossRef]
- Scheid, J.F.; Mouquet, H.; Ueberheide, B.; Diskin, R.; Klein, F.; Oliveira, T.Y.; Pietzsch, J.; Fenyo, D.; Abadir, A.; Velinzon, K.; et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 2011, 333, 1633–1637. [Google Scholar] [CrossRef]
- Wu, N.C.; Yamayoshi, S.; Ito, M.; Uraki, R.; Kawaoka, Y.; Wilson, I.A. Recurring and Adaptable Binding Motifs in Broadly Neutralizing Antibodies to Influenza Virus Are Encoded on the D3-9 Segment of the Ig Gene. Cell Host Microbe 2018, 24, 569–578.e564. [Google Scholar] [CrossRef]
- Yu, L.; Guan, Y. Immunologic Basis for Long HCDR3s in Broadly Neutralizing Antibodies against HIV-1. Front. Immunol. 2014, 5, 250. [Google Scholar] [CrossRef]
- Chen, F.; Tzarum, N.; Wilson, I.A.; Law, M. V(H)1-69 antiviral broadly neutralizing antibodies: Genetics, structures, and relevance to rational vaccine design. Curr. Opin. Virol. 2019, 34, 149–159. [Google Scholar] [CrossRef]
- Stanley, M.; Lowy, D.R.; Frazer, I. Chapter 12: Prophylactic HPV vaccines: Underlying mechanisms. Vaccine 2006, 24, S106–S113. [Google Scholar] [CrossRef]
- Muñoz-Alía, M.; Nace, R.A.; Zhang, L.; Russell, S.J. Serotypic evolution of measles virus is constrained by multiple co-dominant B cell epitopes on its surface glycoproteins. Cell Rep. Med. 2021, 2, 100225. [Google Scholar] [CrossRef]
- Schiller, J.T.; Day, P.M.; Kines, R.C. Current understanding of the mechanism of HPV infection. Gynecol. Oncol. 2010, 118, S12–S17. [Google Scholar] [CrossRef]
- Carter, J.J.; Koutsky, L.A.; Hughes, J.P.; Lee, S.K.; Kuypers, J.; Kiviat, N.; Galloway, D.A. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 2000, 181, 1911–1919. [Google Scholar] [CrossRef]
- Dillner, J. The serological response to papillomaviruses. Semin. Cancer Biol. 1999, 9, 423–430. [Google Scholar] [CrossRef]
- Harro, C.D.; Pang, Y.Y.; Roden, R.B.; Hildesheim, A.; Wang, Z.; Reynolds, M.J.; Mast, T.C.; Robinson, R.; Murphy, B.R.; Karron, R.A.; et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 2001, 93, 284–292. [Google Scholar] [CrossRef]
- Tong, Y.; Ermel, A.; Tu, W.; Shew, M.; Brown, D.R. Association of HPV types 6, 11, 16, and 18 DNA detection and serological response in unvaccinated adolescent women. J. Med. Virol. 2013, 85, 1786–1793. [Google Scholar] [CrossRef]
- Safaeian, M.; Porras, C.; Schiffman, M.; Rodriguez, A.C.; Wacholder, S.; Gonzalez, P.; Quint, W.; van Doorn, L.-J.; Sherman, M.E.; Xhenseval, V.; et al. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J. Natl. Cancer Inst. 2010, 102, 1653–1662. [Google Scholar] [CrossRef]
- Scherer, E.M.; Smith, R.A.; Gallego, D.F.; Carter, J.J.; Wipf, G.C.; Hoyos, M.; Stern, M.; Thurston, T.; Trinklein, N.D.; Wald, A.; et al. A Single Human Papillomavirus Vaccine Dose Improves B Cell Memory in Previously Infected Subjects. EBioMedicine 2016, 10, 55–64. [Google Scholar] [CrossRef]
- Villa, L.L.; Ault, K.A.; Giuliano, A.R.; Costa, R.L.R.; Petta, C.A.; Andrade, R.P.; Brown, D.R.; Ferenczy, A.; Harper, D.M.; Koutsky, L.A.; et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine 2006, 24, 5571–5583. [Google Scholar] [CrossRef]
- Taub, D.D.; Ershler, W.B.; Janowski, M.; Artz, A.; Key, M.L.; McKelvey, J.; Muller, D.; Moss, B.; Ferrucci, L.; Duffey, P.L.; et al. Immunity from smallpox vaccine persists for decades: A longitudinal study. Am. J. Med. 2008, 121, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S.; Felgner, P.; Davies, H.; Glidewell, J.; Villarreal, L.; Ahmed, R. Cutting Edge: Long-Term B Cell Memory in Humans after Smallpox Vaccination. J. Immunol. 2003, 171, 4969–4973. [Google Scholar] [CrossRef] [PubMed]
- Buisman, A.M.; de Rond, C.G.H.; Öztürk, K.; ten Hulscher, H.I.; van Binnendijk, R.S. Long-term presence of memory B-cells specific for different vaccine components. Vaccine 2009, 28, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Koff, R.S. Immunogenicity of hepatitis B vaccines: Implications of immune memory. Vaccine 2002, 20, 3695–3701. [Google Scholar] [CrossRef]
- Banatvala, J.; Van Damme, P.; Oehen, S. Lifelong protection against hepatitis B: The role of vaccine immunogenicity in immune memory. Vaccine 2000, 19, 877–885. [Google Scholar] [CrossRef]
- Dentico, P.; Crovari, P.; Lai, P.L.; Ponzio, F.; Safary, A.; Pellegrino, A.; Meurice, F.; Di Pasquale, A.; Tornieporth, N.; Volpe, A.; et al. Anamnestic response to administration of purified non-adsorbed hepatitis B surface antigen in healthy responders to hepatitis B vaccine with long-term non-protective antibody titres. Vaccine 2002, 20, 3725–3730. [Google Scholar] [CrossRef]
- Ward, S.M.; Phalora, P.; Bradshaw, D.; Leyendeckers, H.; Klenerman, P. Direct Ex Vivo Evaluation of Long-Lived Protective Antiviral Memory B Cell Responses against Hepatitis B Virus. J. Infect. Dis. 2008, 198, 813–817. [Google Scholar] [CrossRef]
- Leyendeckers, H.; Odendahl, M.; Löhndorf, A.; Irsch, J.; Spangfort, M.; Miltenyi, S.; Hunzelmann, N.; Assenmacher, M.; Radbruch, A.; Schmitz, J. Correlation analysis between frequencies of circulating antigen-specific IgG-bearing memory B cells and serum titers of antigen-specific IgG. Eur. J. Immunol. 1999, 29, 1406–1417. [Google Scholar] [CrossRef]
- Amanna, I.J.; Carlson, N.E.; Slifka, M.K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 2007, 357, 1903–1915. [Google Scholar] [CrossRef]
- Hammarlund, E.; Thomas, A.; Amanna, I.J.; Holden, L.A.; Slayden, O.D.; Park, B.; Gao, L.; Slifka, M.K. Plasma cell survival in the absence of B cell memory. Nat. Commun. 2017, 8, 1781. [Google Scholar] [CrossRef]
- Davis, C.W.; Jackson, K.J.L.; McCausland, M.M.; Darce, J.; Chang, C.; Linderman, S.L.; Chennareddy, C.; Gerkin, R.; Brown, S.J.; Wrammert, J.; et al. Influenza vaccine–induced human bone marrow plasma cells decline within a year after vaccination. Science 2020, 370, 237–241. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabhu, P.R.; Carter, J.J.; Galloway, D.A. B Cell Responses upon Human Papillomavirus (HPV) Infection and Vaccination. Vaccines 2022, 10, 837. https://doi.org/10.3390/vaccines10060837
Prabhu PR, Carter JJ, Galloway DA. B Cell Responses upon Human Papillomavirus (HPV) Infection and Vaccination. Vaccines. 2022; 10(6):837. https://doi.org/10.3390/vaccines10060837
Chicago/Turabian StylePrabhu, Priya R., Joseph J. Carter, and Denise A. Galloway. 2022. "B Cell Responses upon Human Papillomavirus (HPV) Infection and Vaccination" Vaccines 10, no. 6: 837. https://doi.org/10.3390/vaccines10060837
APA StylePrabhu, P. R., Carter, J. J., & Galloway, D. A. (2022). B Cell Responses upon Human Papillomavirus (HPV) Infection and Vaccination. Vaccines, 10(6), 837. https://doi.org/10.3390/vaccines10060837




