Impact of Moderna mRNA-1273 Booster Vaccine on Fully Vaccinated High-Risk Chronic Dialysis Patients after Loss of Humoral Response
Abstract
:1. Introduction
2. Materials and Methods
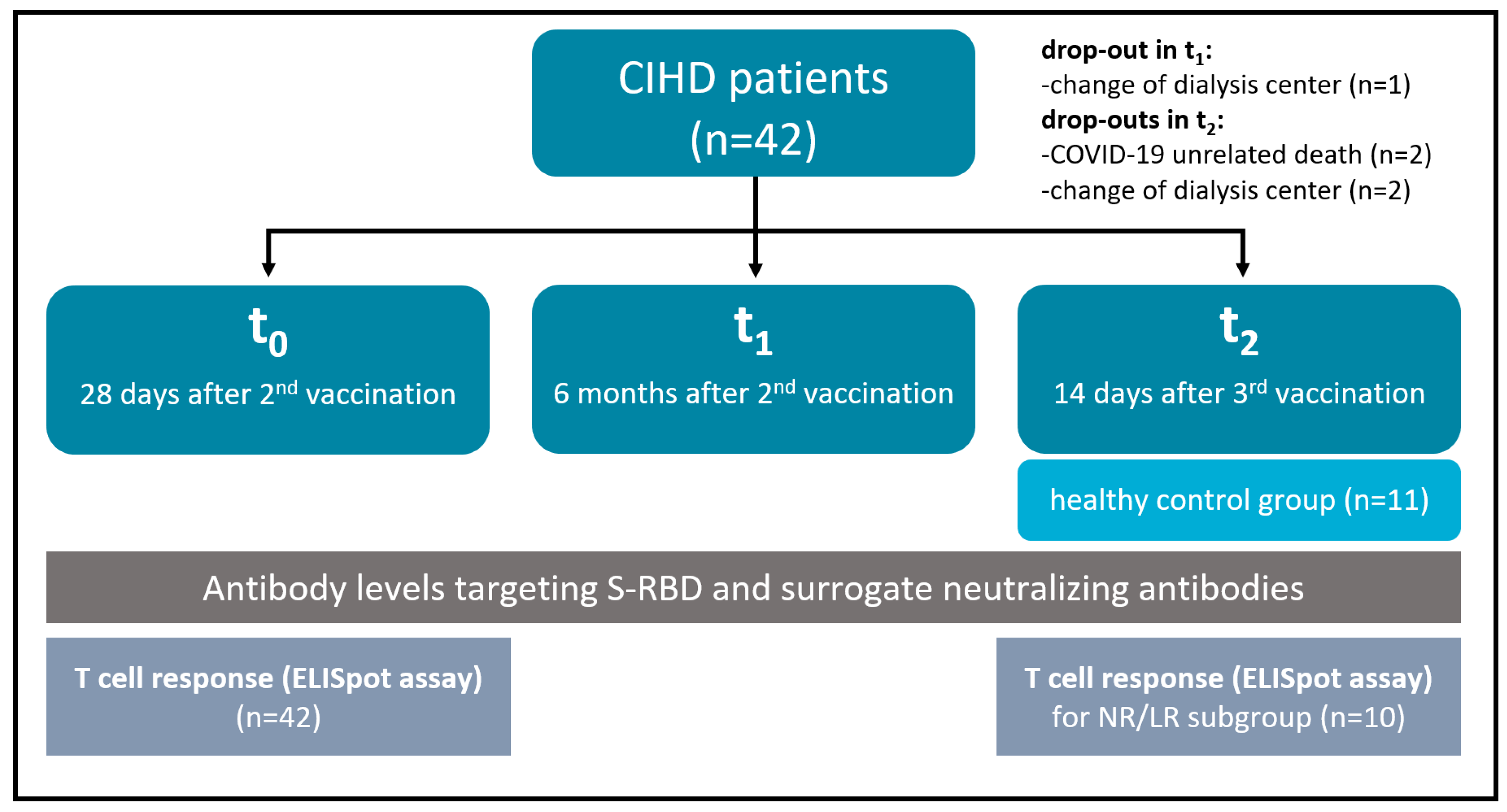
2.1. Study Design and Cohorts
2.2. Assessment of SARS-CoV-2-Specific Antibodies
2.3. Assessment of SARS-CoV-2-Specific Surrogate Neutralizing Antibodies (SNA)
2.4. Assessment of Cytokine-Producing SARS-CoV-2-Reactive T Cell Responses
2.5. Statistics
3. Results
3.1. Basic Study Cohort Characteristics
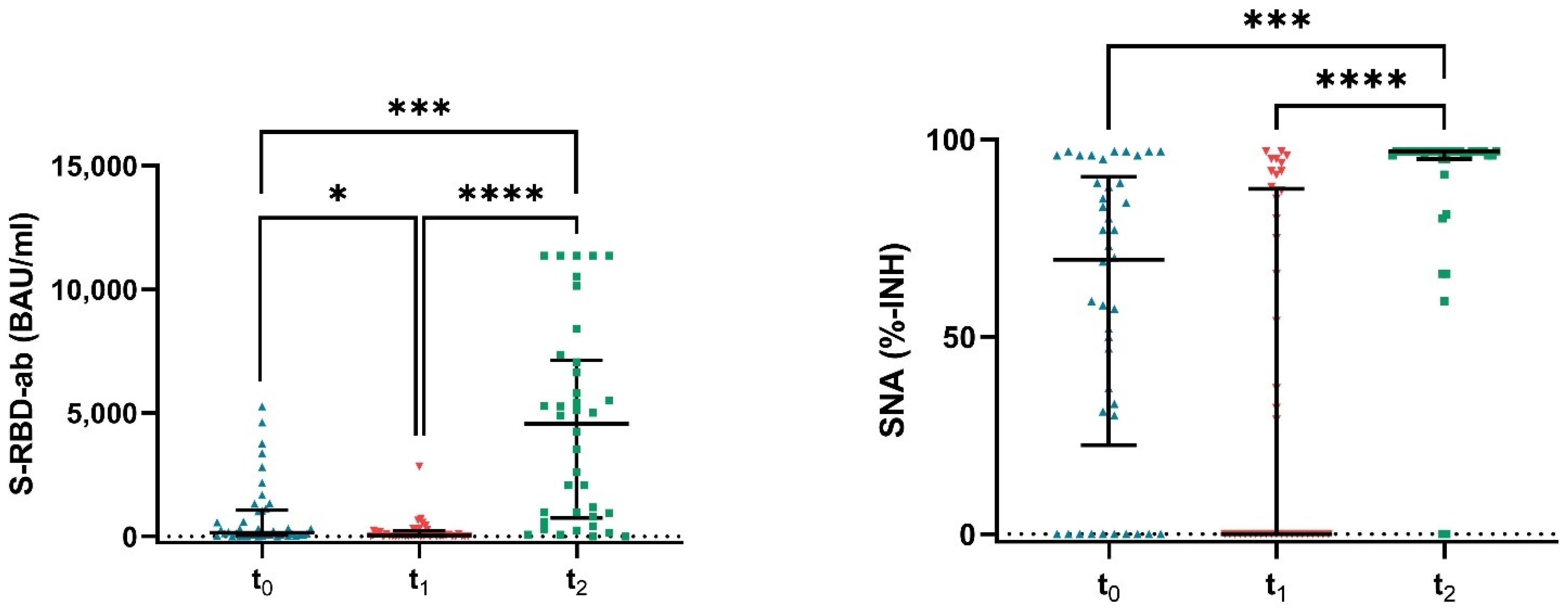
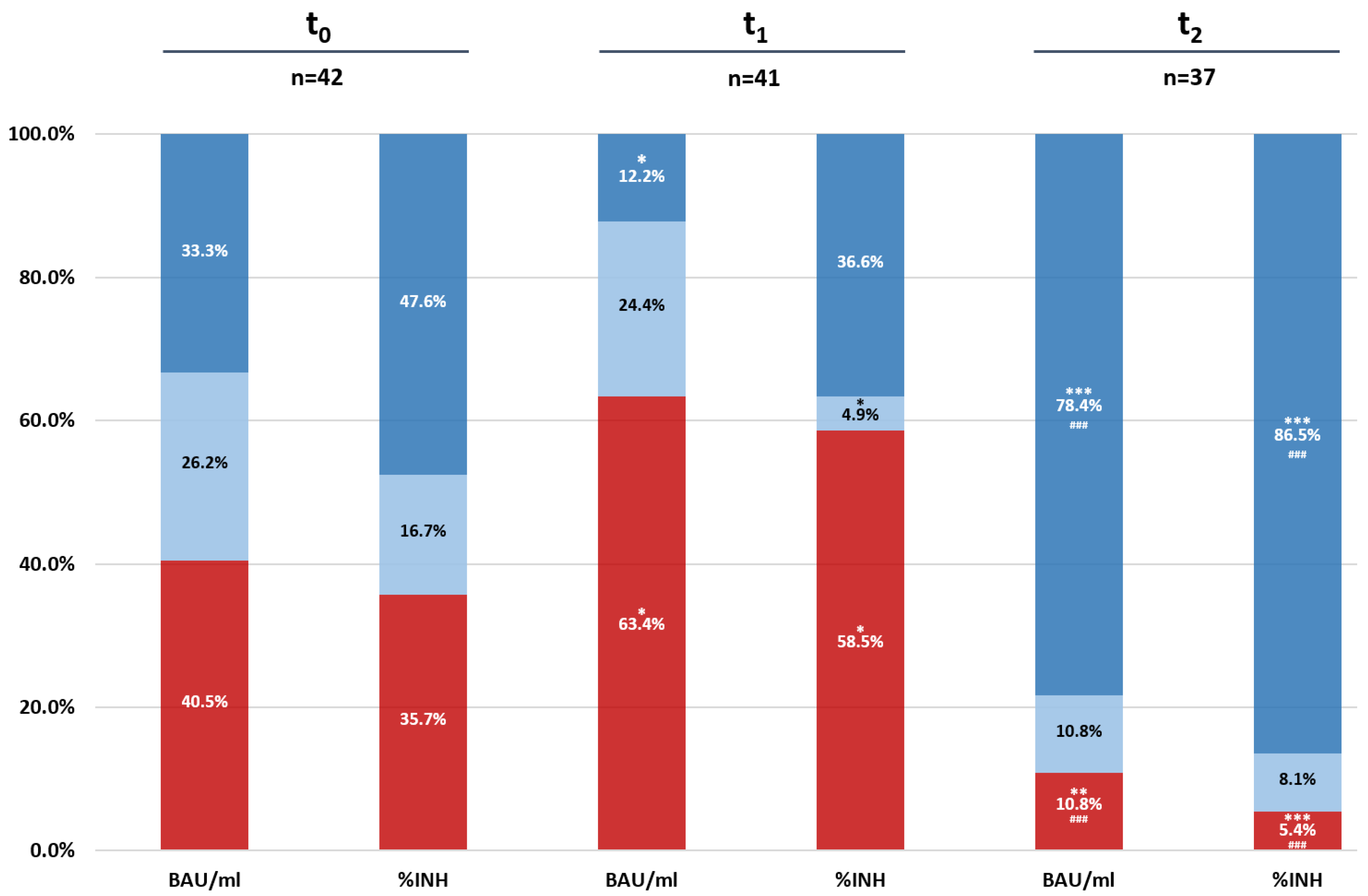
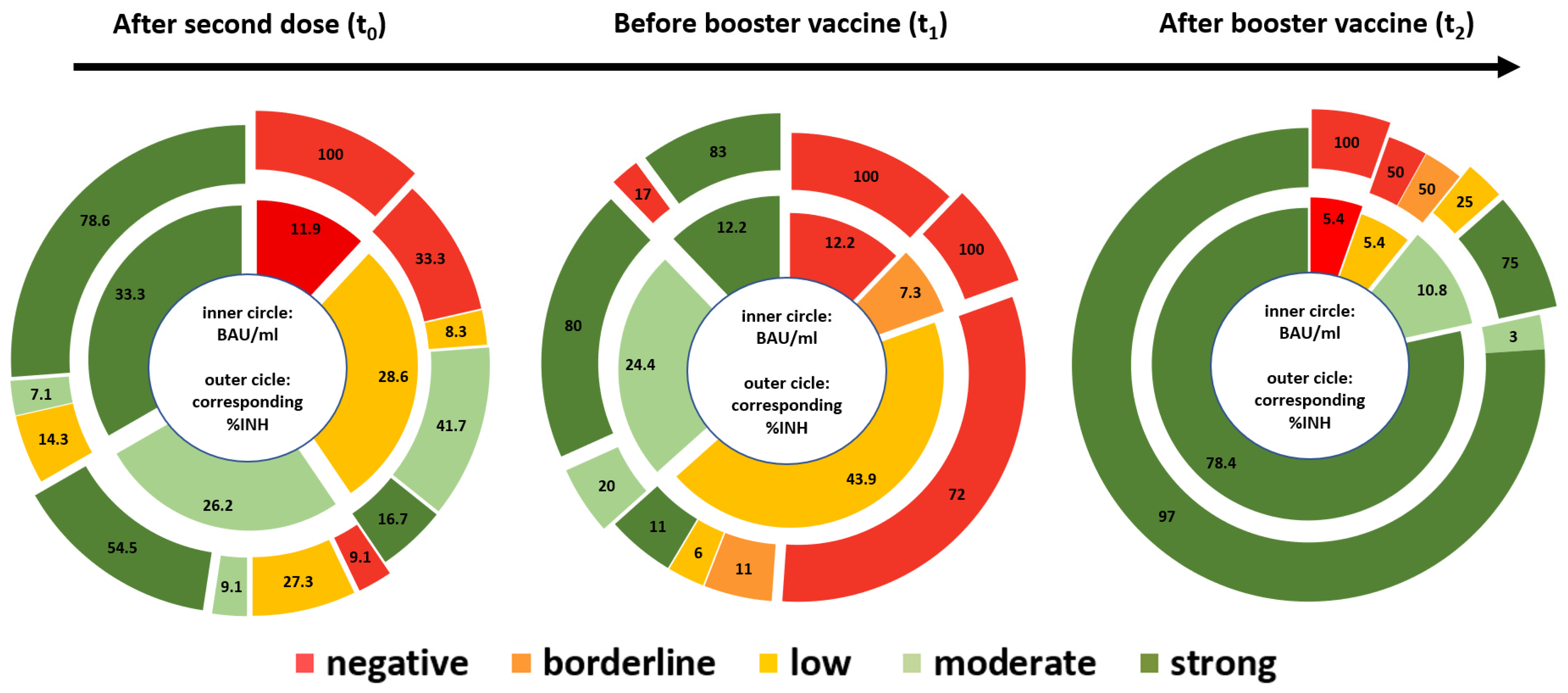
3.2. Dynamics of Antibody Levels in Vaccinated Dialysis Patients
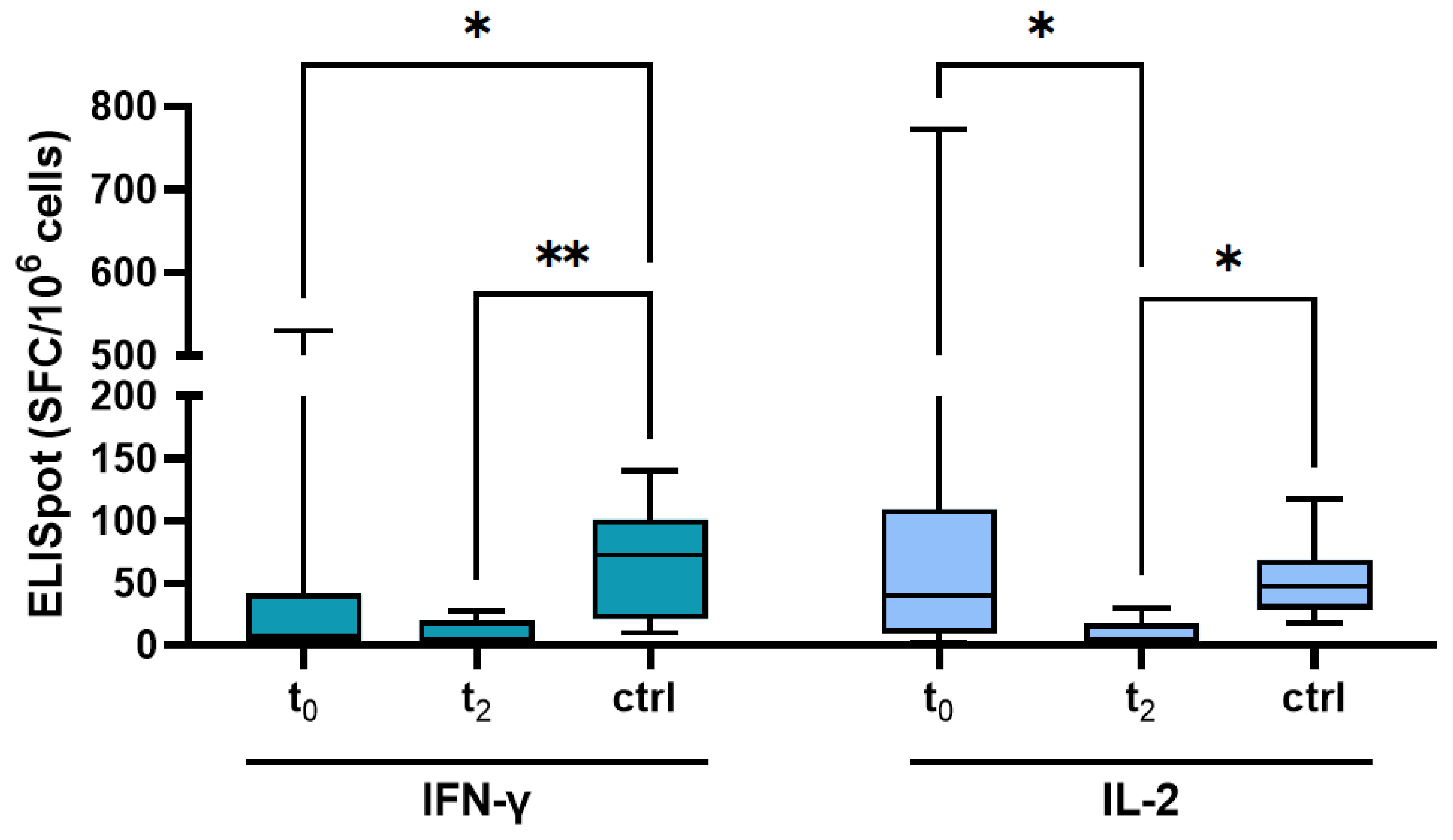
3.3. T Cell Response after Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Losappio, V.; Franzin, R.; Infante, B.; Godeas, G.; Gesualdo, L.; Fersini, A.; Castellano, G.; Stallone, G. Molecular Mechanisms of Premature Aging in Hemodialysis: The Complex Interplay Between Innate and Adaptive Immune Dysfunction. Int. J. Mol. Sci. 2020, 21, 3422. [Google Scholar] [CrossRef] [PubMed]
- Taji, L.; Thomas, D.; Oliver, M.J.; Ip, J.; Tang, Y.; Yeung, A.; Cooper, R.; House, A.A.; McFarlane, P.; Blake, P.G. COVID-19 in patients undergoing long-term dialysis in Ontario. Can. Med Assoc. J. 2021, 193, E278–E284. [Google Scholar] [CrossRef] [PubMed]
- Hilbrands, L.B.; Duivenvoorden, R.; Vart, P.; Franssen, C.F.; Hemmelder, M.H.; Jager, K.J.; Kieneker, L.M.; Noordzij, M.; Pena, M.J.; Gansevoort, R.T.; et al. COVID-19-related mortality in kidney transplant and dialysis patients: Results of the ERACODA collaboration. Nephrol. Dial. Transplant. 2020, 35, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Weinhandl, E.D.; Wetmore, J.B.; Peng, Y.; Liu, J.; Gilbertson, D.T.; Johansen, K.L. Initial Effects of COVID-19 on Patients with ESKD. J. Am. Soc. Nephrol. 2021, 32, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Türeci, Ö.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Türeci, Ö.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.; Rubey, H.; Treipl, A.; Gromann, M.; Hemedi, B.; Zehetmayer, S.; Kirsch, B. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared to healthy controls. Nephrol. Dial. Transplant. 2021, 36, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Speer, C.; Schaier, M.; Nusshag, C.; Töllner, M.; Buylaert, M.; Kälble, F.; Reichel, P.; Grenz, J.; Süsal, C.; Benning, L.; et al. Longitudinal Humoral Responses after COVID-19 Vaccination in Peritoneal and Hemodialysis Patients over Twelve Weeks. Vaccines 2021, 9, 1130. [Google Scholar] [CrossRef] [PubMed]
- Ducloux, D.; Colladant, M.; Chabannes, M.; Yannaraki, M.; Courivaud, C. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021, 100, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Kalimuddin, S.; Tham, C.Y.; Qui, M.; de Alwis, R.; Sim, J.X.; Lim, J.M.; Tan, H.-C.; Syenina, A.; Zhang, S.L.; Low, J.G.; et al. Early T cell and binding antibody responses are associated with COVID-19 RNA vaccine efficacy onset. Med 2021, 2, 682–688.e4. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Kohmer, N.; Rühl, C.; Ciesek, S.; Rabenau, H.F. Utility of Different Surrogate Enzyme-Linked Immunosorbent Assays (sELISAs) for Detection of SARS-CoV-2 Neutralizing Antibodies. J. Clin. Med. 2021, 10, 2128. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, P.V.; Zhang, W. Unique strengths of ELISPOT for T cell diagnostics. Methods Mol. Biol. 2012, 792, 3–23. [Google Scholar] [PubMed]
- Jdiaa, S.S.; Mansour, R.; El Alayli, A.; Gautam, A.; Thomas, P.; Mustafa, R.A. COVID–19 and chronic kidney disease: An updated overview of reviews. J. Nephrol. 2022, 35, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-P.; Wang, N.-C.; Chang, Y.-H.; Tian, X.-Y.; Na, D.-Y.; Zhang, L.-Y.; Zheng, L.; Lan, T.; Wang, L.-F.; Liang, G.-D. Duration of Antibody Responses after Severe Acute Respiratory Syndrome. Emerg. Infect. Dis. 2007, 13, 1562–1564. [Google Scholar] [CrossRef] [PubMed]
- Kohmer, N.; Rabenau, H.F.; Ciesek, S.; Krämer, B.K.; Göttmann, U.; Keller, C.; Rose, D.; Blume, C.; Thomas, M.; Lammert, A.; et al. Heterologous immunization with BNT162b2 followed by mRNA-1273 in dialysis patients: Seroconversion and presence of neutralizing antibodies. Nephrol. Dial. Transplant. 2022, gfac144. [Google Scholar] [CrossRef] [PubMed]
- Espi, M.; Charmetant, X.; Barba, T.; Mathieu, C.; Pelletier, C.; Koppe, L.; Chalencon, E.; Kalbacher, E.; Mathias, V.; Ovize, A.; et al. A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney Int. 2022, 101, 390–402. [Google Scholar] [CrossRef] [PubMed]
| All Patients | NR/LR Group | p-Value | ||||
|---|---|---|---|---|---|---|
| n = 42 | n = 10 | |||||
| Age (years) | med (IQR) | 62 | (52–72.5) | 74.5 | (67.3–79.3) | 0.0041 |
| Gender (male) | n (%) | 29 | (69) | 6 | (60) | 0.7109 |
| Vintage on dialysis (years) | med (IQR) | 2.4 | (1–7.2) | 6.2 | (0.9–12) | 0.3893 |
| Days after 2nd vaccination (days) | med (IQR) | 34.5 | (30.5–47.8) | 34 | (29–47) | 0.8189 |
| Ethnicity | ||||||
| Caucasian | n (%) | 31 | (73.8) | 8 | (80) | 1.0000 |
| Black | n (%) | 6 | (14.3) | 2 | (20) | 0.6415 |
| Other | n (%) | 5 | (11.9) | / | ||
| Reason for HD | ||||||
| Diabetic nephropathy | n (%) | 9 | (21.4) | 3 | (30) | 0.6792 |
| Hypertensive nephropathy | n (%) | 4 | (9.5) | 1 | (10) | 1.0000 |
| ADPKD | n (%) | 6 | (14.3) | 2 | (20) | 0.6415 |
| GN | n (%) | 3 | (7.1) | 1 | (10) | 1.0000 |
| Amyloidosis | n (%) | 5 | (11.9) | / | ||
| Chronic interstitial nephritis | n (%) | 6 | (14.3) | / | ||
| Nephrosclerosis | n (%) | 6 | (14.3) | / | ||
| Vasculitis | n (%) | 1 | (2.4) | / | ||
| Others/unknown | n (%) | 2 | (4.8) | 3 | (30) | 0.0432 |
| Comorbidities | ||||||
| Diabetes mellitus | n (%) | 13 | (31.0) | 6 | (60) | 0.1424 |
| Lung disease | n (%) | 5 | (11.9) | 2 | (20) | 0.6079 |
| Smoker | n (%) | 14 | (33.3) | 3 | (30) | 1.0000 |
| Cancer | n (%) | 5 | (11.9) | / | ||
| Severe cardiovascular disease | n (%) | 23 | (54.8) | 6 | (60) | 1.0000 |
| Hypertension | n (%) | 38 | (90.5) | 9 | (90) | 1.0000 |
| Kidney or liver-Tx | n (%) | 6 | (14.3) | 4 | (40) | 0.0847 |
| Immunosupression | n (%) | 9 | (21.4) | 6 | (60) | 0.0243 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patyna, S.; Eckes, T.; Koch, B.F.; Sudowe, S.; Oftring, A.; Kohmer, N.; Rabenau, H.F.; Ciesek, S.; Avaniadi, D.; Steiner, R.; et al. Impact of Moderna mRNA-1273 Booster Vaccine on Fully Vaccinated High-Risk Chronic Dialysis Patients after Loss of Humoral Response. Vaccines 2022, 10, 585. https://doi.org/10.3390/vaccines10040585
Patyna S, Eckes T, Koch BF, Sudowe S, Oftring A, Kohmer N, Rabenau HF, Ciesek S, Avaniadi D, Steiner R, et al. Impact of Moderna mRNA-1273 Booster Vaccine on Fully Vaccinated High-Risk Chronic Dialysis Patients after Loss of Humoral Response. Vaccines. 2022; 10(4):585. https://doi.org/10.3390/vaccines10040585
Chicago/Turabian StylePatyna, Sammy, Timon Eckes, Benjamin F. Koch, Stephan Sudowe, Anke Oftring, Niko Kohmer, Holger F. Rabenau, Sandra Ciesek, Despina Avaniadi, Rahel Steiner, and et al. 2022. "Impact of Moderna mRNA-1273 Booster Vaccine on Fully Vaccinated High-Risk Chronic Dialysis Patients after Loss of Humoral Response" Vaccines 10, no. 4: 585. https://doi.org/10.3390/vaccines10040585
APA StylePatyna, S., Eckes, T., Koch, B. F., Sudowe, S., Oftring, A., Kohmer, N., Rabenau, H. F., Ciesek, S., Avaniadi, D., Steiner, R., Hauser, I. A., Pfeilschifter, J. M., & Betz, C. (2022). Impact of Moderna mRNA-1273 Booster Vaccine on Fully Vaccinated High-Risk Chronic Dialysis Patients after Loss of Humoral Response. Vaccines, 10(4), 585. https://doi.org/10.3390/vaccines10040585






