Homologous and Heterologous Anti-COVID-19 Vaccination Does Not Induce New-Onset Formation of Autoantibodies Typically Accompanying Lupus Erythematodes, Rheumatoid Arthritis, Celiac Disease and Antiphospholipid Syndrome
Abstract
:1. Introduction
2. Materials and Methods
3. Results
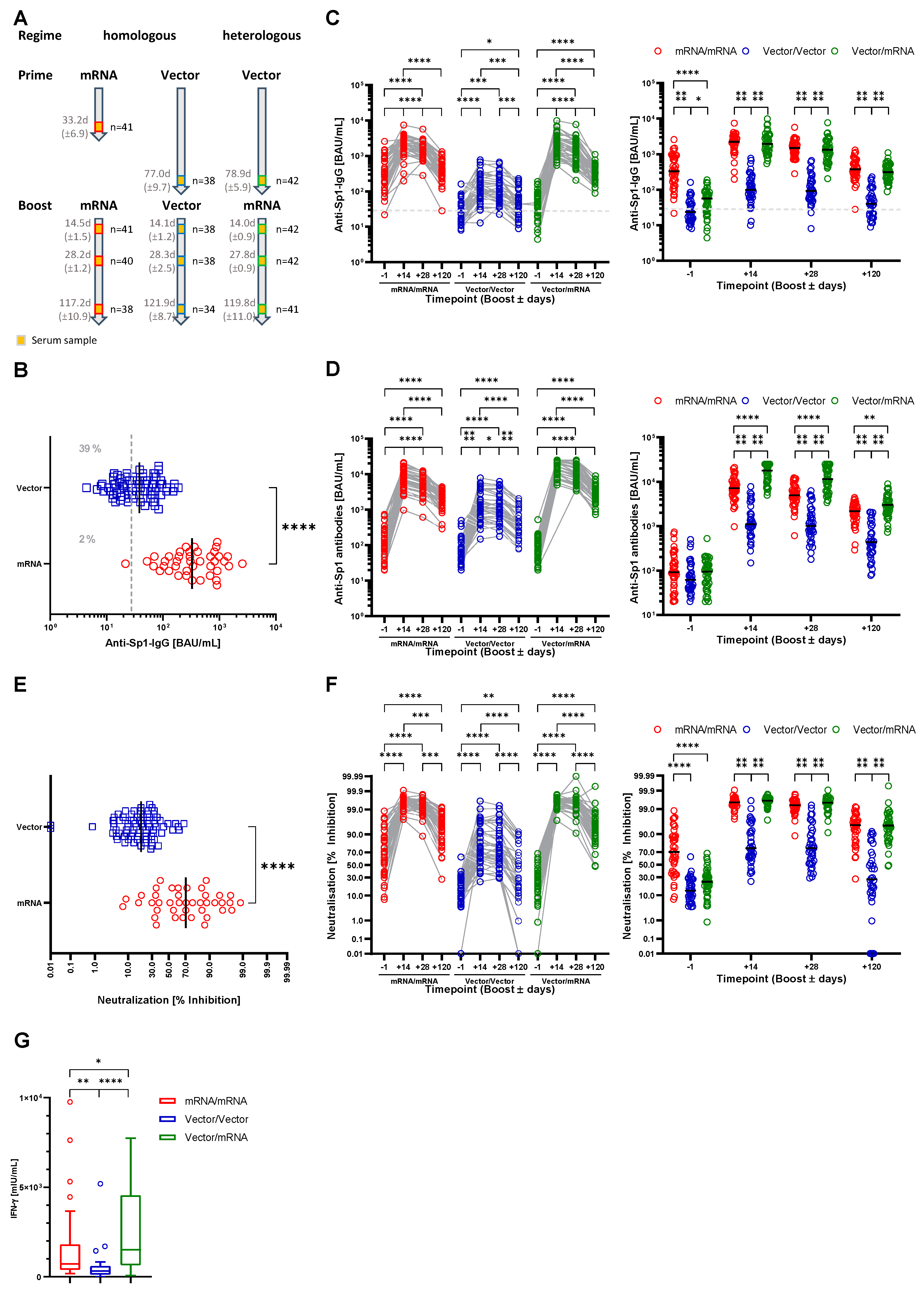
3.1. Characterization of the Study Cohort
3.2. Both Heterologous and Homologous Prime-Boost Immunization Strategies Provide Humoral and Cellular Immunity to SARS-CoV-2
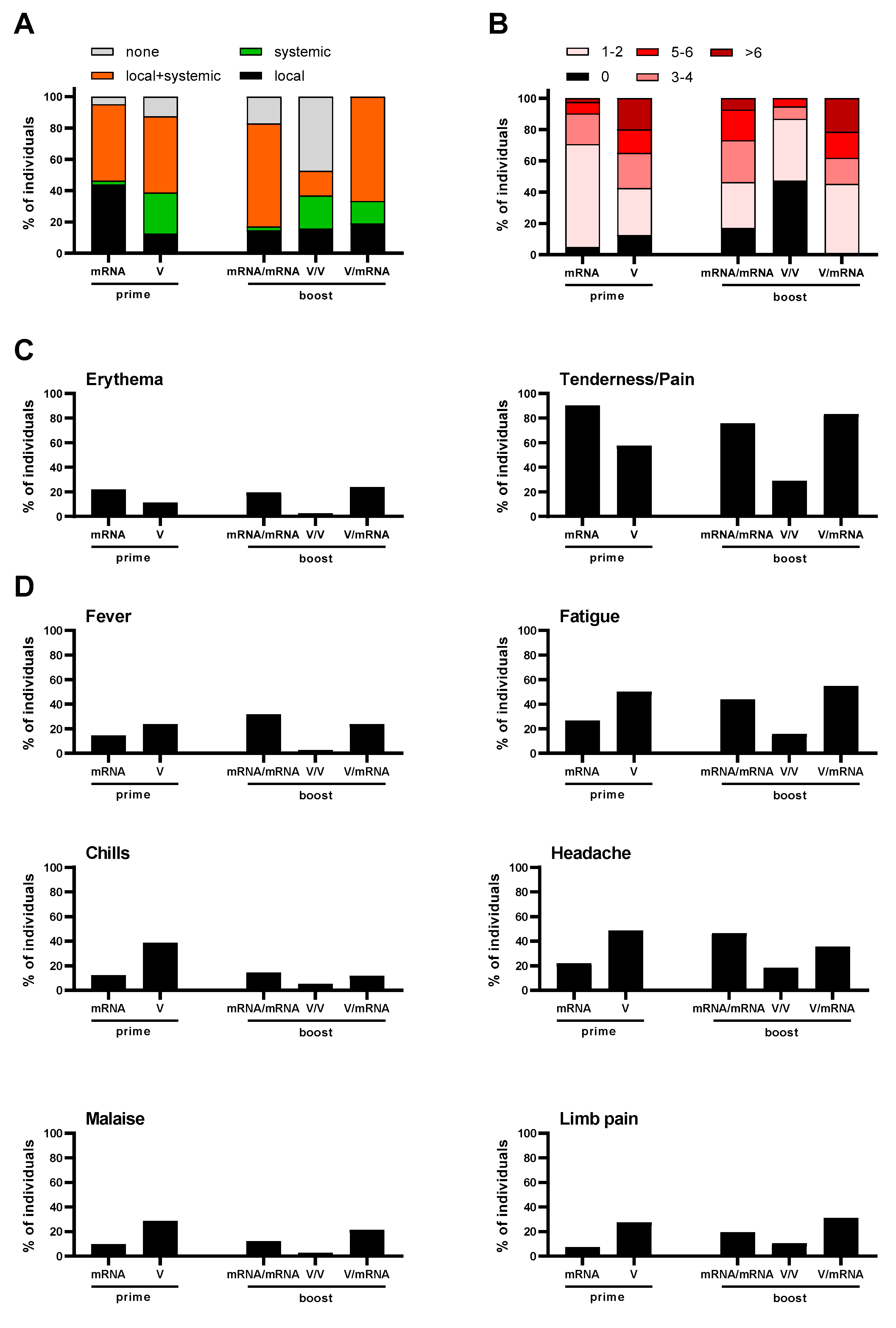
3.3. Reactogenicity
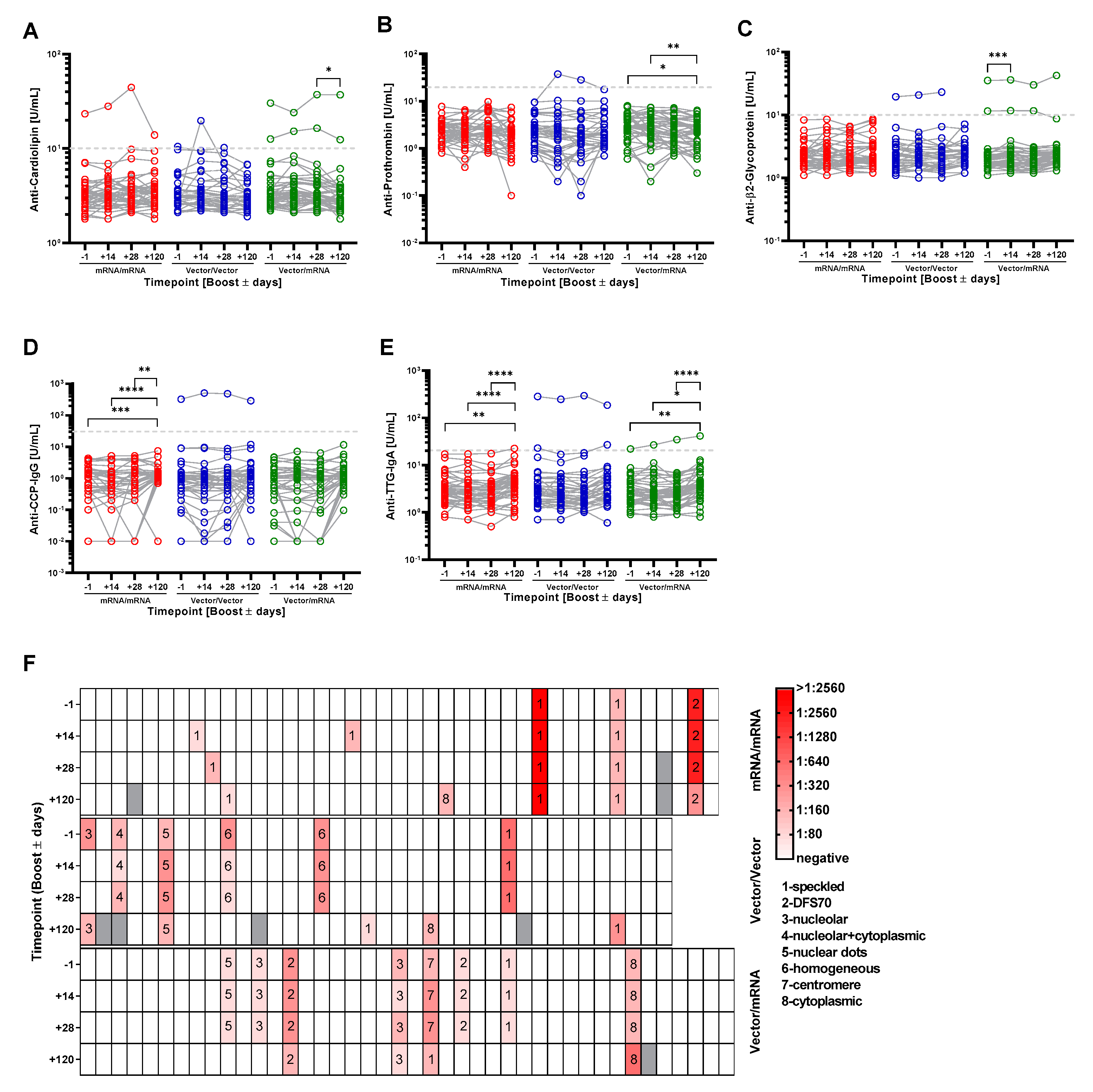
3.4. Homologous and Heterologous Prime-Boost Immunization Strategies Do Not Induce the Generation of Autoantibodies That Typically Accompany Lupus Erythematodes, Rheumatoid Arthritis, Celiac Disease and Antiphospholipid Syndrome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- WHO. COVID-19 Dashboard. Available online: https://covid19.who.int/ (accessed on 14 February 2022).
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-X.; Xu, Y.-H.; Wang, X.; Sun, C.; Guo, Y.; Qiu, S.; Ma, K.-W. Advances in SARS-CoV-2: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9208–9215. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.-Z.; Hong, W.-D.; Mao, L.; Lin, C.; Fang, Z.; Pan, J.-Y. Clinical Characteristics and Outcomes of Severe and Critical Patients with 2019 Novel Coronavirus Disease (COVID-19) in Wenzhou: A Retrospective Study. Front. Med. 2020, 7, 552002. [Google Scholar] [CrossRef]
- Liu, Y.; Sawalha, A.H.; Lu, Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2021, 33, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Amezcua-Guerra, L.M.; Rojas-Velasco, G.; Brianza-Padilla, M.; Vázquez-Rangel, A.; Márquez-Velasco, R.; Baranda-Tovar, F.; Springall, R.; Gonzalez-Pacheco, H.; Juárez-Vicuña, Y.; Tavera-Alonso, C.; et al. Presence of antiphospholipid antibodies in COVID-19: A case series study. Ann. Rheum. Dis. 2021, 80, e73. [Google Scholar] [CrossRef]
- Fujii, H.; Tsuji, T.; Yuba, T.; Tanaka, S.; Suga, Y.; Matsuyama, A.; Omura, A.; Shiotsu, S.; Takumi, C.; Ono, S.; et al. High levels of anti-SSA/Ro antibodies in COVID-19 patients with severe respiratory failure: A case-based review. Clin. Rheumatol. 2020, 39, 3171–3175. [Google Scholar] [CrossRef]
- Pascolini, S.; Vannini, A.; Deleonardi, G.; Ciordinik, M.; Sensoli, A.; Carletti, I.; Veronesi, L.; Ricci, C.; Pronesti, A.; Mazzanti, L.; et al. COVID-19 and Immunological Dysregulation: Can Autoantibodies be Useful? Clin. Transl. Sci. 2021, 14, 502–508. [Google Scholar] [CrossRef]
- Gomes, C.; Zuniga, M.; Crotty, K.A.; Qian, K.; Tovar, N.C.; Lin, L.H.; Argyropoulos, K.V.; Clancy, R.; Izmirly, P.; Buyon, J.; et al. Autoimmune anti-DNA and anti-phosphatidylserine antibodies predict development of severe COVID-19. Life Sci. Alliance 2021, 4, e202101180. [Google Scholar] [CrossRef]
- Lingel, H.; Meltendorf, S.; Billing, U.; Thurm, C.; Vogel, K.; Majer, C.; Prätsch, F.; Roggenbuck, D.; Heuft, H.-G.; Hachenberg, T.; et al. Unique autoantibody prevalence in long-term recovered SARS-CoV-2-infected individuals. J. Autoimmun. 2021, 122, 102682. [Google Scholar] [CrossRef]
- Izda, V.; Jeffries, M.A.; Sawalha, A.H. COVID-19: A review of therapeutic strategies and vaccine candidates. Clin. Immunol. 2021, 222, 108634. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El, S.H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA Vaccine against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef]
- Verma, A.K.; Lavine, K.J.; Lin, C.-Y. Myocarditis after COVID-19 mRNA Vaccination. N. Engl. J. Med. 2021, 385, 1332–1334. [Google Scholar] [CrossRef]
- Witberg, G.; Barda, N.; Hoss, S.; Richter, I.; Wiessman, M.; Aviv, Y.; Grinberg, T.; Auster, O.; Dagan, N.; Balicer, R.D.; et al. Myocarditis after COVID-19 Vaccination in a Large Health Care Organization. N. Engl. J. Med. 2021, 385, 2132–2139. [Google Scholar] [CrossRef]
- Vygen-Bonnet, S.; Koch, J.; Bogdan, C.; Harder, T.; Heininger, U.; Kling, K.; Littmann, M.; Meerpohl, J.; Meyer, H.; Mertens, T.; et al. Beschlussentwurf der STIKO zur 4. Aktualisierung der COVID-19-Impfempfehlung und die dazugehörige wissenschaftliche Begründung. Epid. Bull. 2021, 16, 3–8. [Google Scholar] [CrossRef]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir. Med. 2021, 9, 1255–1265. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Tenbusch, M.; Schumacher, S.; Vogel, E.; Priller, A.; Held, J.; Steininger, P.; Beileke, S.; Irrgang, P.; Brockhoff, R.; Salmanton-García, J.; et al. Heterologous prime–boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect. Dis. 2021, 21, 1212–1213. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; de Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.E. Measles Vaccine. Viral Immunol. 2018, 31, 86–95. [Google Scholar] [CrossRef]
- Kapil, P.; Merkel, T.J. Pertussis vaccines and protective immunity. Curr. Opin. Immunol. 2019, 59, 72–78. [Google Scholar] [CrossRef]
- Panchanathan, V.; Chaudhri, G.; Karupiah, G. Protective Immunity against Secondary Poxvirus Infection Is Dependent on Antibody but Not on CD4 or CD8 T-Cell Function. J. Virol. 2006, 80, 6333–6338. [Google Scholar] [CrossRef] [Green Version]
- Pinto, L.A.; Dillner, J.; Beddows, S.; Unger, E.R. Immunogenicity of HPV prophylactic vaccines: Serology assays and their use in HPV vaccine evaluation and development. Vaccine 2018, 36, 4792–4799. [Google Scholar] [CrossRef]
- Ponticelli, D.; Madotto, F.; Conti, S.; Antonazzo, I.C.; Vitale, A.; Della Ragione, G.; Romano, M.L.; Borrelli, M.; Schiavone, B.; Polosa, R.; et al. Response to BNT162b2 mRNA COVID-19 vaccine among healthcare workers in Italy: A 3-month follow-up. Intern. Emerg. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Normark, J.; Vikström, L.; Gwon, Y.-D.; Persson, I.-L.; Edin, A.; Björsell, T.; Dernstedt, A.; Christ, W.; Tevell, S.; Evander, M.; et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination. N. Engl. J. Med. 2021, 385, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Pozzetto, B.; Legros, V.; Djebali, S.; Barateau, V.; Guibert, N.; Villard, M.; Peyrot, L.; Allatif, O.; Fassier, J.-B.; Massardier-Pilonchéry, A.; et al. Immunogenicity and efficacy of heterologous ChAdOx1-BNT162b2 vaccination. Nature 2021, 600, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, D.; Antonazzo, I.C.; Caci, G.; Vitale, A.; Della Ragione, G.; Romano, M.L.; Borrelli, M.; Schiavone, B.; Polosa, R.; Ferrara, P. Dynamics of antibody response to BNT162b2 mRNA COVID-19 vaccine after 6 months. J. Travel Med. 2021, 28, taab173. [Google Scholar] [CrossRef]
- Sokal, A.; Barba-Spaeth, G.; Fernández, I.; Broketa, M.; Azzaoui, I.; de La Selle, A.; Vandenberghe, A.; Fourati, S.; Roeser, A.; Meola, A.; et al. mRNA vaccination of naive and COVID-19-recovered individuals elicits potent memory B cells that recognize SARS-CoV-2 variants. Immunity 2021, 54, 2893–2907.e5. [Google Scholar] [CrossRef] [PubMed]
- Angileri, F.; Legare, S.; Marino Gammazza, A.; Conway de Macario, E.; JL Macario, A.; Cappello, F. Molecular mimicry may explain multi-organ damage in COVID-19. Autoimmun. Rev. 2020, 19, 102591. [Google Scholar] [CrossRef]
- Kanduc, D.; Shoenfeld, Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: Implications for the vaccine. Immunol. Res. 2020, 68, 310–313. [Google Scholar] [CrossRef]
- Lucchese, G.; Flöel, A. SARS-CoV-2 and Guillain-Barré syndrome: Molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell Stress Chaperones 2020, 25, 731–735. [Google Scholar] [CrossRef]
- Marino Gammazza, A.; Légaré, S.; Lo Bosco, G.; Fucarino, A.; Angileri, F.; Conway de Macario, E.; Macario, A.J.L.; Cappello, F. Human molecular chaperones share with SARS-CoV-2 antigenic epitopes potentially capable of eliciting autoimmunity against endothelial cells: Possible role of molecular mimicry in COVID-19. Cell Stress Chaperones 2020, 25, 737–741. [Google Scholar] [CrossRef]
- Bowles, L.; Platton, S.; Yartey, N.; Dave, M.; Lee, K.; Hart, D.P.; MacDonald, V.; Green, L.; Sivapalaratnam, S.; Pasi, K.J.; et al. Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with COVID-19. N. Engl. J. Med. 2020, 383, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Danda, D.; Kavadichanda, C.; Das, S.; Adarsh, M.B.; Negi, V.S. Autoimmune and rheumatic musculoskeletal diseases as a consequence of SARS-CoV-2 infection and its treatment. Rheumatol. Int. 2020, 40, 1539–1554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with COVID-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. COVID-19 and autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Nesher, G.; Shoenfeld, Y. Predicting post-vaccination autoimmunity: Who might be at risk? Pharmacol. Res. 2015, 92, 18–22. [Google Scholar] [CrossRef]
- Cruz-Tapias, P.; Blank, M.; Anaya, J.-M.; Shoenfeld, Y. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr. Opin. Rheumatol. 2012, 24, 389–393. [Google Scholar] [CrossRef]
| mRNA/mRNA n = 41 | Vector/Vector | Vector/mRNA | ||
|---|---|---|---|---|
| Subgroup | Spx/Spx n = 25 | Com/Com n = 16 | n = 38 | n = 42 |
| Age (years) | 35.9 (±13.3) | 47.2 (±13.5) | 37.9 (±14.1) | |
| Sex | ||||
| Male | 16 (39%) | 15 (39%) | 13 (31%) | |
| Female | 25 (61%) | 23 (61%) | 29 (69%) | |
| Boost (Prime + xx days) | 34.2 (±6.8) | 78.0 (±10.0) | 80.3 (±5.8) | |
| 1st serum sample (Prime + xx days) | 33.2 (±6.9) | 77.0 (±9.7) | 78.9 (±5.9) | |
| 2nd serum sample (Boost + xx days) | 14.5 (±1.5) | 14.1 (±1.2) | 14.0 (±0.9) | |
| 3rd serum sample (Boost + xx days) | 28.2 (±1.2) | 28.3 (±2.5) | 27.8 (±0.9) | |
| 4th serum sample (Boost + xx days) | 117.2 (±10.9) | 121.9 (±8.7) | 119.8 (±11.0) | |
| Boost − 1 days | n = 41 | n = 38 | n = 42 | |
| Boost + 14 days | n = 41 | n = 38 | n = 42 | |
| Boost + 28 days | n = 40 | n = 38 | n = 42 | |
| Boost + 120 days | n = 38 | n = 34 | n = 41 | |
| Participant No. | Study Group | Autoantibody 1 | Autoantibody 2 | ||||
|---|---|---|---|---|---|---|---|
| Autoantibody | Time Point | Result | Autoantibody | Time Point | Result | ||
| 6 | mRNA/mRNA | Cardiolipin | −1 | 23. U/mL | |||
| +14 | 28 U/mL | ||||||
| +28 | 44.3 U/mL | ||||||
| +120 | 13.9 U/mL | ||||||
| 9 | mRNA/mRNA | ANA | −1 | negative | |||
| +14 | 1:80 sp | ||||||
| +28 | negative | ||||||
| +120 | negative | ||||||
| 10 | mRNA/mRNA | ANA | −1 | negative | |||
| +14 | negative | ||||||
| +28 | 1:160 sp | ||||||
| +120 | negative | ||||||
| 20 | mRNA/mRNA | ANA | −1 | negative | |||
| +14 | 1:160 sp | ||||||
| +28 | negative | ||||||
| +120 | negative | ||||||
| 39 | Vector/mRNA | Cardiolipin | −1 | 30.3 U/mL | β2-Glycoprotein | −1 | 35.2 U/mL |
| +14 | 24 U/mL | +14 | 36 U/mL | ||||
| +28 | 37.2 U/mL | +28 | 29.9 U/mL | ||||
| +120 | 37 U/mL | +120 | 42.3 U/mL | ||||
| 52 | Vector/Vector | Prothrombin | −1 | 9.4 U/mL | ANA | −1 | 1:160 Dots |
| +14 | 37.4 U/mL | +14 | 1:320 Dots | ||||
| +28 | 28.3 U/mL | +28 | 1:320 Dots | ||||
| +120 | 17.7 U/mL | +120 | 1:160 Dots | ||||
| 70 | Vector/mRNA | TTG-IgA | −1 | 21.9 U/mL | |||
| +14 | 26.7 U/mL | ||||||
| +28 | 34.6 U/mL | ||||||
| +120 | 41.2 U/mL | ||||||
| 81 | Vector/Vector | CCP | −1 | 325 U/mL | ANA | −1 | 1:320 ho |
| +14 | 510 U/mL | +14 | 1:320 ho | ||||
| +28 | 482 U/mL | +28 | 1:320 ho | ||||
| +120 | 289 U/mL | +120 | negative | ||||
| 86 | Vector/Vector | Cardiolipin | −1 | 2.8 U/mL | |||
| +14 | 19.7 U/mL | ||||||
| +28 | 6.9 U/mL | ||||||
| +120 | 3.4 U/mL | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thurm, C.; Reinhold, A.; Borucki, K.; Kahlfuss, S.; Feist, E.; Schreiber, J.; Reinhold, D.; Schraven, B. Homologous and Heterologous Anti-COVID-19 Vaccination Does Not Induce New-Onset Formation of Autoantibodies Typically Accompanying Lupus Erythematodes, Rheumatoid Arthritis, Celiac Disease and Antiphospholipid Syndrome. Vaccines 2022, 10, 333. https://doi.org/10.3390/vaccines10020333
Thurm C, Reinhold A, Borucki K, Kahlfuss S, Feist E, Schreiber J, Reinhold D, Schraven B. Homologous and Heterologous Anti-COVID-19 Vaccination Does Not Induce New-Onset Formation of Autoantibodies Typically Accompanying Lupus Erythematodes, Rheumatoid Arthritis, Celiac Disease and Antiphospholipid Syndrome. Vaccines. 2022; 10(2):333. https://doi.org/10.3390/vaccines10020333
Chicago/Turabian StyleThurm, Christoph, Annegret Reinhold, Katrin Borucki, Sascha Kahlfuss, Eugen Feist, Jens Schreiber, Dirk Reinhold, and Burkhart Schraven. 2022. "Homologous and Heterologous Anti-COVID-19 Vaccination Does Not Induce New-Onset Formation of Autoantibodies Typically Accompanying Lupus Erythematodes, Rheumatoid Arthritis, Celiac Disease and Antiphospholipid Syndrome" Vaccines 10, no. 2: 333. https://doi.org/10.3390/vaccines10020333
APA StyleThurm, C., Reinhold, A., Borucki, K., Kahlfuss, S., Feist, E., Schreiber, J., Reinhold, D., & Schraven, B. (2022). Homologous and Heterologous Anti-COVID-19 Vaccination Does Not Induce New-Onset Formation of Autoantibodies Typically Accompanying Lupus Erythematodes, Rheumatoid Arthritis, Celiac Disease and Antiphospholipid Syndrome. Vaccines, 10(2), 333. https://doi.org/10.3390/vaccines10020333






