Humoral and Cellular Responses to COVID-19 Vaccines in SARS-CoV-2 Infection-Naïve and -Recovered Korean Individuals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Anti-SARS-CoV-2 IgG Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Pseudotyped Virus Neutralization Test (pVNT)
2.4. Enzyme-Linked Immunospot (ELISpot) Assay
2.5. Statistical Analysis
3. Results
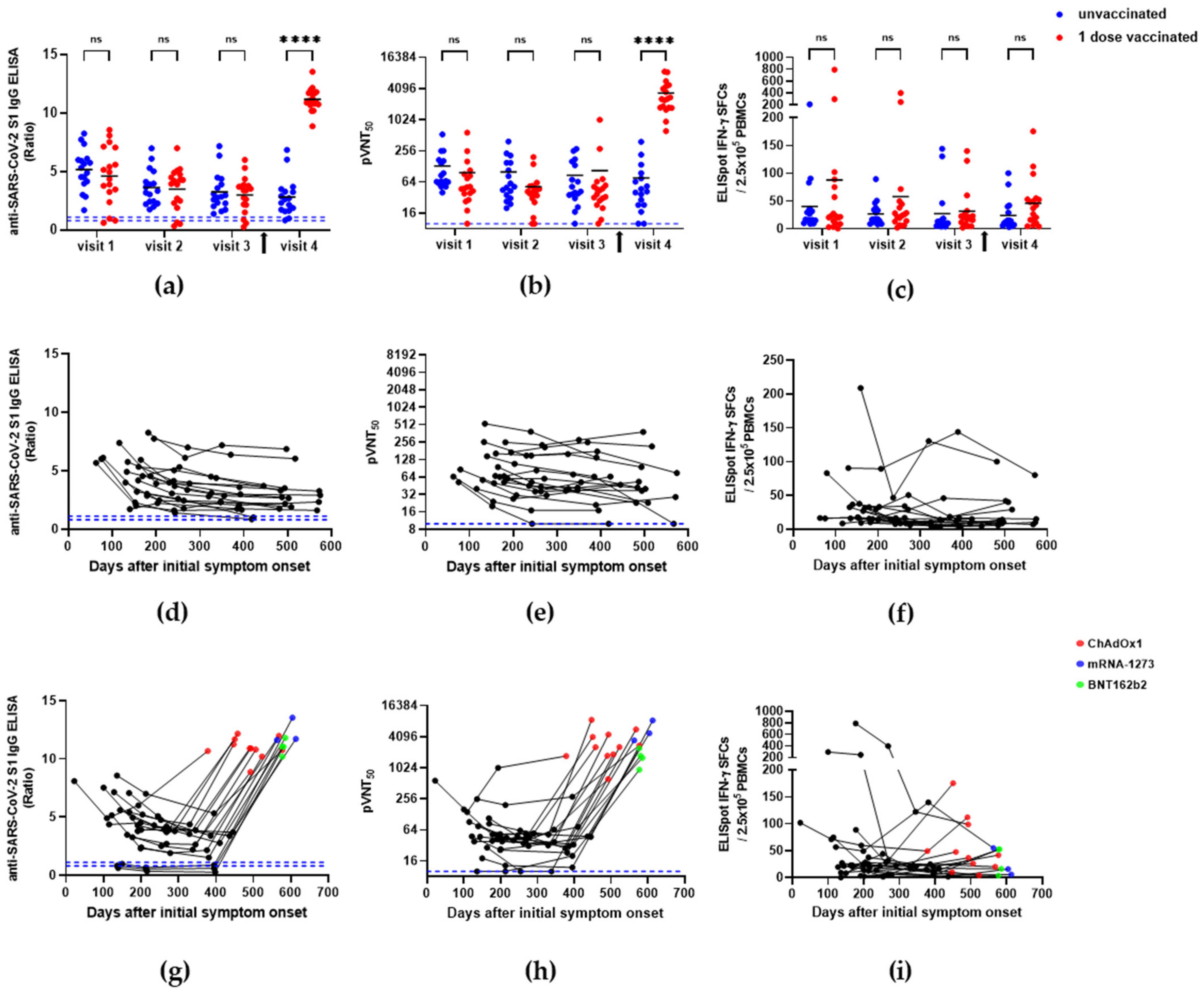
3.1. Immune Responses in SARS-CoV-2-Recovered Individuals
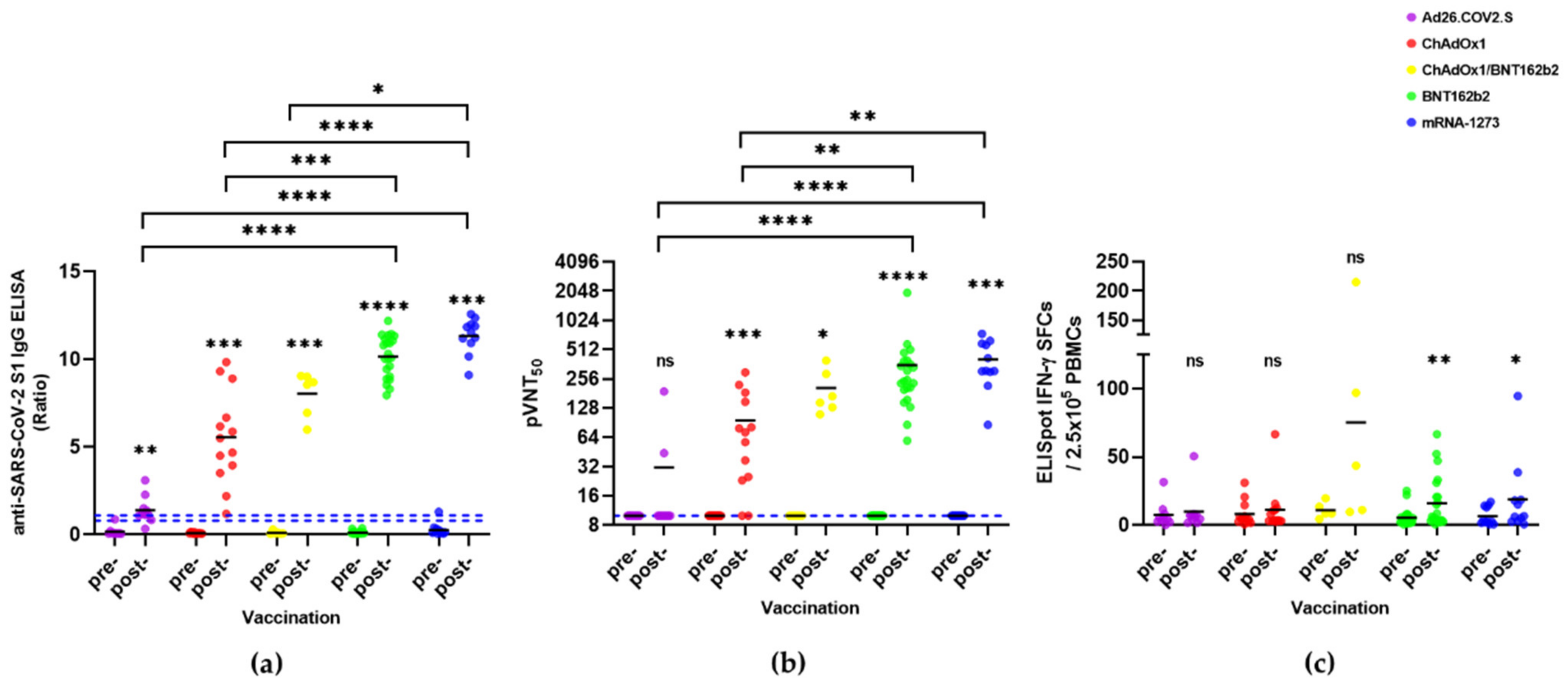
3.2. Immune Responses in SARS-CoV-2-Naïve Individuals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed consent statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 17 January 2022).
- World Health Organization (WHO). COVID19 Vaccine Tracker. Available online: https://covid19.trackvaccines.org/agency/who/ (accessed on 6 January 2022).
- Cohn, B.A.; Cirillo, P.M.; Murphy, C.C.; Krigbaum, N.Y.; Wallace, A.W. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science 2021, 375, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Robertson, C.; Taylor, B. BNT162b2 and ChAdOx1 nCoV-19 Vaccine Effectiveness against Death from the Delta Variant. N. Engl. J. Med. 2021, 385, 2195–2197. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Hasan, M.R.; Chemaitelly, H.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; AlMukdad, S.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat. Med. 2021, 27, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Holder, J. New York Times. Tracking Coronavirus Vaccinations around the World. Available online: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html (accessed on 5 January 2022).
- Angyal, A.; Longet, S.; Moore, S.C.; Payne, R.P.; Harding, A.; Tipton, T.; Rongkard, P.; Ali, M.; Hering, L.M.; Meardon, N.; et al. T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: A multicentre prospective cohort study. Lancet Microbe 2021, 3, e21–e31. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- Manisty, C.; Otter, A.D.; Treibel, T.A.; McKnight, Á.; Altmann, D.M.; Brooks, T.; Noursadeghi, M.; Boyton, R.J.; Semper, A.; Moon, J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 2021, 397, 1057–1058. [Google Scholar] [CrossRef]
- Prendecki, M.; Clarke, C.; Brown, J.; Cox, A.; Gleeson, S.; Guckian, M.; Randell, P.; Pria, A.D.; Lightstone, L.; Xu, X.-N.; et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet 2021, 397, 1178–1181. [Google Scholar] [CrossRef]
- Saadat, S.; Rikhtegaran Tehrani, Z.; Logue, J.; Newman, M.; Frieman, M.B.; Harris, A.D.; Sajadi, M.M. Binding and Neutralization Antibody Titers After a Single Vaccine Dose in Health Care Workers Previously Infected With SARS-CoV-2. JAMA 2021, 325, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Vicenti, I.; Gatti, F.; Scaggiante, R.; Boccuto, A.; Zago, D.; Basso, M.; Dragoni, F.; Zazzi, M.; Parisi, S.G. Single-dose BNT162b2 mRNA COVID-19 vaccine significantly boosts neutralizing antibody response in health care workers recovering from asymptomatic or mild natural SARS-CoV-2 infection. Int. J. Infect. Dis. 2021, 108, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.J.; Pade, C.; Gibbons, J.M.; Butler, D.K.; Otter, A.D.; Menacho, K.; Fontana, M.; Smit, A.; Sackville-West, J.E.; Cutino-Moguel, T.; et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science 2021, 372, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Havervall, S.; Marking, U.; Greilert-Norin, N.; Ng, H.; Gordon, M.; Salomonsson, A.C.; Hellström, C.; Pin, E.; Blom, K.; Mangsbo, S.; et al. Antibody responses after a single dose of ChAdOx1 nCoV-19 vaccine in healthcare workers previously infected with SARS-CoV-2. EBioMedicine 2021, 70, 103523. [Google Scholar] [CrossRef] [PubMed]
- Jeewandara, C.; Kamaladasa, A.; Pushpakumara, P.D.; Jayathilaka, D.; Aberathna, I.S.; Danasekara, D.; Guruge, D.; Ranasinghe, T.; Dayarathna, S.; Pathmanathan, T.; et al. Immune responses to a single dose of the AZD1222/Covishield vaccine in health care workers. Nat. Commun. 2021, 12, 4617. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, M.; Shashidhar, J.; Deepika, G.; Ravikanth, V.; Krishna, V.V.; Sadhana, Y.; Pragathi, K.; Reddy, D.N. Immunological memory and neutralizing activity to a single dose of COVID-19 vaccine in previously infected individuals. Int. J. Infect. Dis. 2021, 108, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Park, A.K.; Kim, I.H.; Kim, J.; Kim, J.M.; Kim, H.M.; Lee, C.Y.; Han, M.G.; Rhie, G.E.; Kwon, D.; Nam, J.G.; et al. Genomic Surveillance of SARS-CoV-2: Distribution of Clades in the Republic of Korea in 2020. Osong Public Health Res. Perspect. 2021, 12, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Garcia-Beltran, W.F.; Chang, C.C.; Mairena, C.B.; Thierauf, J.C.; Kirkpatrick, G.; Onozato, M.L.; Cheng, J.; St Denis, K.J.; Lam, E.C.; et al. Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2 and Ad26.COV2.S COVID-19 vaccines. J. Infect. Dis. 2021, jiab593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, W.; Zhou, X.; Zhao, Q.; Wang, Q.; Jia, B. Seroprevalence of neutralizing antibodies to human adenoviruses type-5 and type-26 and chimpanzee adenovirus type-68 in healthy Chinese adults. J. Med. Virol. 2013, 85, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Mast, T.C.; Kierstead, L.; Gupta, S.B.; Nikas, A.A.; Kallas, E.G.; Novitsky, V.; Mbewe, B.; Pitisuttithum, P.; Schechter, M.; Vardas, E.; et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: Correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 2010, 28, 950–957. [Google Scholar] [CrossRef] [PubMed]
| Categories | Unvaccinated Group | 1-Dose-Vaccinated Group |
|---|---|---|
| Number | 17 | 18 |
| Age (range) | 53.3 (34–70) | 56.4 (40–71) |
| Sex | ||
| Female | 15 | 16 |
| Male | 2 | 2 |
| Visit (Mean days after ISO 1) | ||
| Visit 1 | 143.9 ± 40.8 | 146.6 ± 42.6 |
| Visit 2 | 226.4 ± 41.2 | 227.6 ± 41.6 |
| Visit 3 | 331.9 ± 51.3 | 358.7 ± 62.3 |
| Visit 4 | 495.7 ± 54.8 | 526.7 ± 65.7 |
| Mean days after ISO at vaccination (range) | NA 2 | 497.9 (311–594) |
| Mean number of days after vaccination at time of blood collection (range) | NA | 30.7 (9–66) |
| Visit | SARS-CoV-2 S1 IgG ELISA (Ratio) | pVNT (pVNT50) | IFN-γ ELISpot (SFCs/2.5 × 105 Cells) | |||
|---|---|---|---|---|---|---|
| Unvaccinated (n = 17) | Vaccinated (n = 18) | Unvaccinated (n = 17) | Vaccinated (n = 18) | Unvaccinated (n = 17) | Vaccinated (n = 18) | |
| V1 | 5.2 ± 1.8 | 4.6 ± 2.5 | 131 ± 124 | 97 ± 133 | 39.9 ± 49.5 | 87.9 ± 188.1 |
| V2 | 3.6 ± 1.5 | 3.5 ± 1.8 | 100 ± 98 | 52 ± 46 | 26.7 ± 21.0 | 57.8 ± 101.0 |
| V3 | 3.3 ± 1.6 | 3.0 ± 1.5 | 86 ± 84 | 106 ± 235 | 27.2 ± 42.6 | 31.6 ± 38.6 |
| V4 | 2.8 ± 1.6 | 11.2 ± 1.0 | 77 ± 95 | 3333 ± 2322 | 23.8 ± 27.6 | 46.0 ± 44.3 |
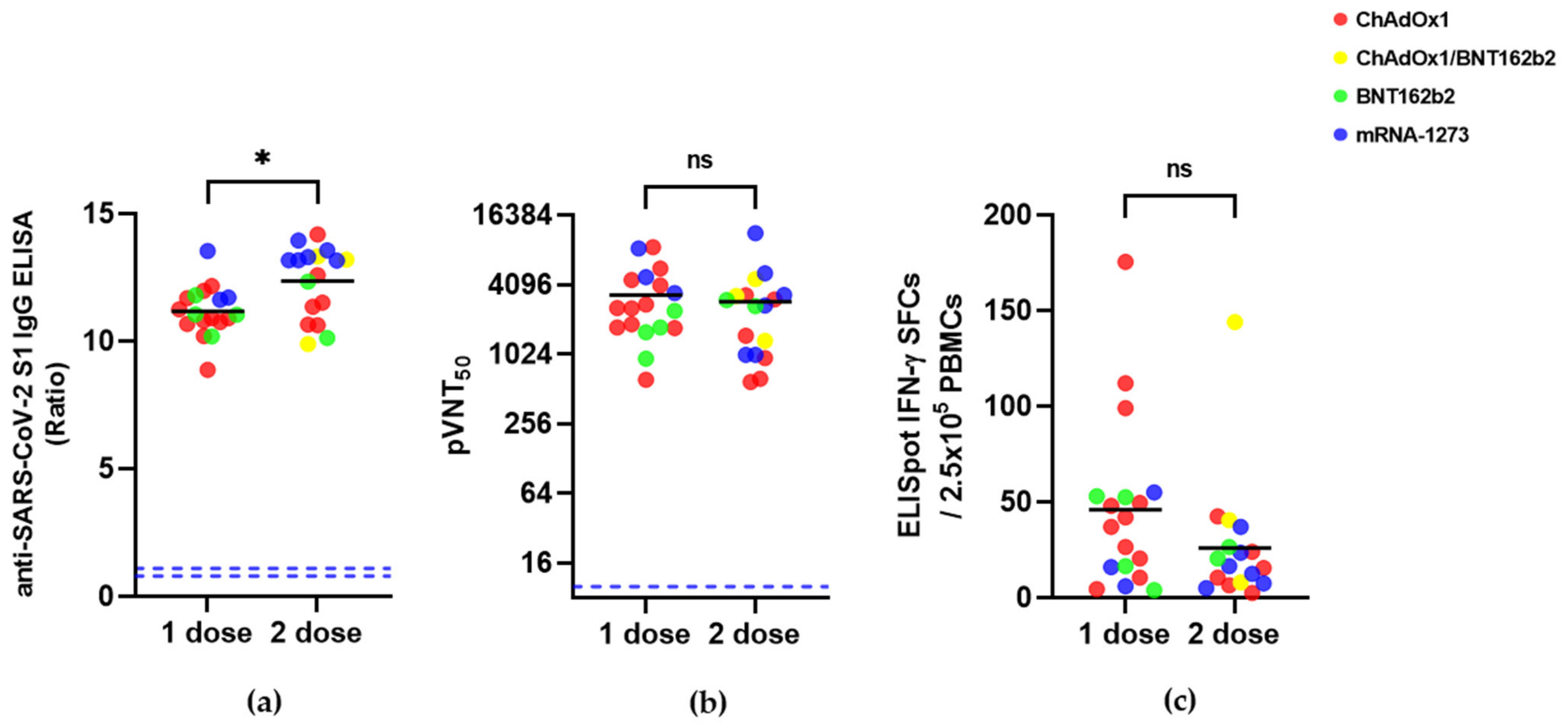
| Measurements | 1 Dose (n = 18) | 2 Dose (n = 17) |
|---|---|---|
| SARS-CoV-2 S1 IgG ELISA (Ratio) | 11.2 ± 1.0 | 12.4 ± 1.4 |
| pVNT (pVNT50) | 3333 ± 2322 | 2914 ± 2591 |
| IFN-γ ELISpot (SFCs/2.5 × 105 cells) | 46.0 ± 44.3 | 26.1 ± 32.8 |
| Characters | Vaccine Type | |||||
|---|---|---|---|---|---|---|
| Ad26.CoV2.S | ChAdOX1 | ChAdOX1/BNT162b2 | BNT162b2 | mRNA-1273 | Total | |
| Number | 10 | 13 | 6 | 22 | 11 | 62 |
| Age | 34.4 (29–39) | 61.4 (58–63) | 41.3 (29–48) | 40.4 (25–58) | 28.5 (24–57) | 41.8 (24–63) |
| Sex | ||||||
| Female | 0 | 7 | 1 | 19 | 3 | 30 |
| Male | 10 | 6 | 5 | 3 | 8 | 32 |
| Days post-vaccination at time of blood collection | 33 (29–34) | 33 (28–35) | 31 (30–33) | 32 (26–36) | 30 (28–34) | 32 (26–36) |
| Vaccines | SARS-CoV-2 S1 IgG ELISA (Ratio) | pVNT (pVNT50) | IFN-γ ELISpot (SFC/2.5 × 105 Cells) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Ad26.COV2.S | 0.2 ± 0.3 | 1.4 ± 0.8 | 10 | 31 ± 56 | 7.4 ± 9.1 | 9.8 ± 14.5 |
| ChAdOx1 | 0.1 ± 0.0 | 5.6 ± 2.7 | 10 | 96 ± 90 | 8.0 ± 8.9 | 11.2 ± 17.2 |
| ChAdOx1/ BNT162b2 | 0.1 ± 0.1 | 8.0 ± 1.3 | 10 | 206 ± 111 | 10.9 ± 5.8 | 75.2 ± 85.8 |
| BNT162b2 | 0.1 ± 0.1 | 10.2 ± 1.2 | 10 | 355 ± 384 | 5.3 ± 6.4 | 15.9 ± 18.7 |
| mRNA-1273 | 0.3 ± 0.4 | 11.3 ± 1.0 | 10 | 406 ± 200 | 6.5 ± 6.6 | 18.9 ± 27.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.-Y.; Kim, Y.; Lee, K.-M.; Jang, E.-J.; Woo, C.-H.; Hong, C.-U.; Choi, S.-T.; Xayaheuang, S.; Jang, J.-G.; Ahn, J.-H.; et al. Humoral and Cellular Responses to COVID-19 Vaccines in SARS-CoV-2 Infection-Naïve and -Recovered Korean Individuals. Vaccines 2022, 10, 332. https://doi.org/10.3390/vaccines10020332
Hwang J-Y, Kim Y, Lee K-M, Jang E-J, Woo C-H, Hong C-U, Choi S-T, Xayaheuang S, Jang J-G, Ahn J-H, et al. Humoral and Cellular Responses to COVID-19 Vaccines in SARS-CoV-2 Infection-Naïve and -Recovered Korean Individuals. Vaccines. 2022; 10(2):332. https://doi.org/10.3390/vaccines10020332
Chicago/Turabian StyleHwang, Ji-Young, Yunhwa Kim, Kyung-Min Lee, Eun-Jeong Jang, Chang-Hoon Woo, Chang-Ui Hong, Seok-Tae Choi, Sivilay Xayaheuang, Jong-Geol Jang, June-Hong Ahn, and et al. 2022. "Humoral and Cellular Responses to COVID-19 Vaccines in SARS-CoV-2 Infection-Naïve and -Recovered Korean Individuals" Vaccines 10, no. 2: 332. https://doi.org/10.3390/vaccines10020332
APA StyleHwang, J.-Y., Kim, Y., Lee, K.-M., Jang, E.-J., Woo, C.-H., Hong, C.-U., Choi, S.-T., Xayaheuang, S., Jang, J.-G., Ahn, J.-H., & Park, H. (2022). Humoral and Cellular Responses to COVID-19 Vaccines in SARS-CoV-2 Infection-Naïve and -Recovered Korean Individuals. Vaccines, 10(2), 332. https://doi.org/10.3390/vaccines10020332






