Gender Differences in Adverse Events Following the Pfizer-BioNTech COVID-19 Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analyses
3. Results
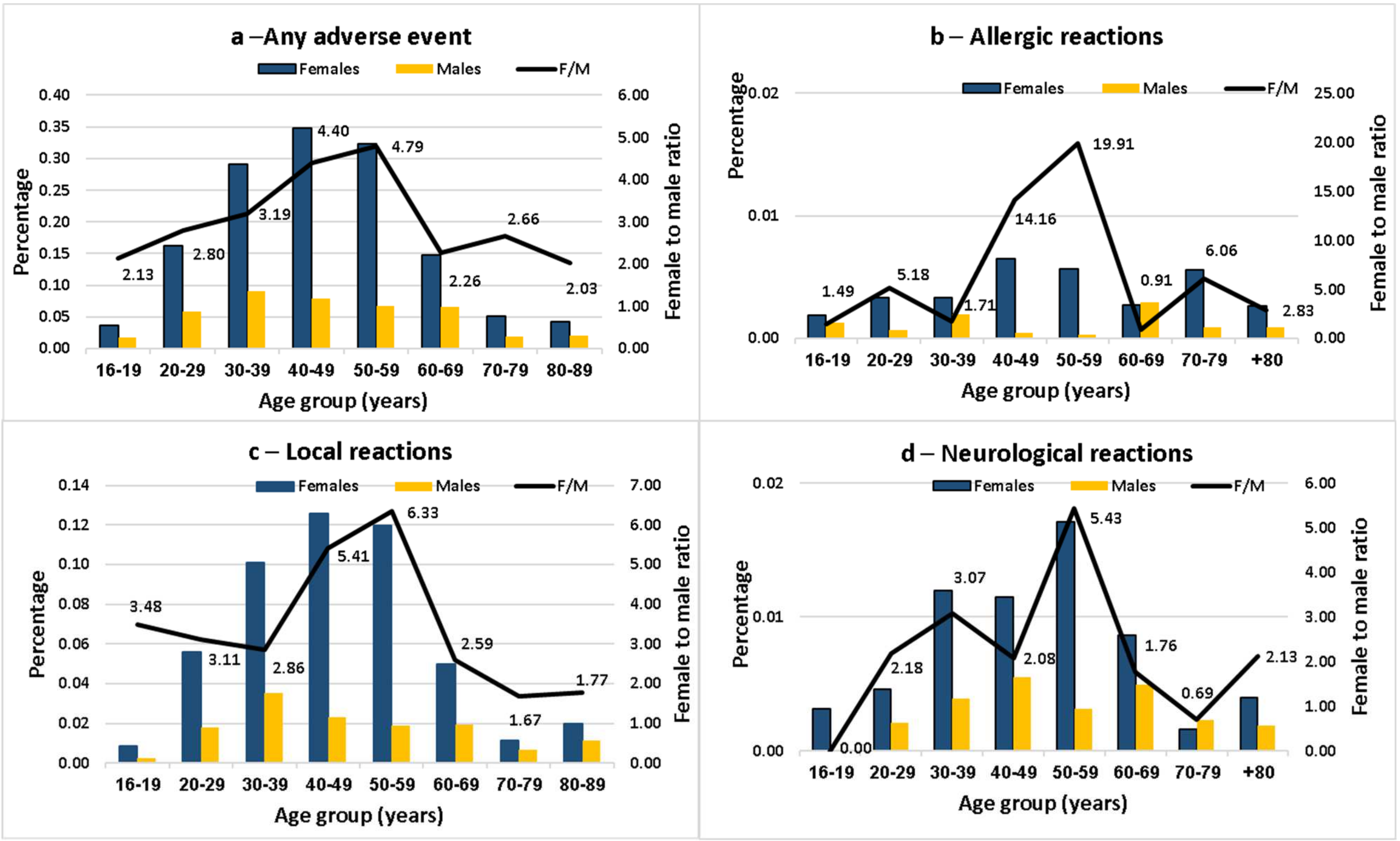
Adverse Events in the Workplace Survey
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Cole, M.; Su, J.R. Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US—December 14, 2020–January 18, 2021. JAMA 2021, 325, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Robinson, L.B.; Camargo, C.A.; Shenoy, E.S.; Banerji, A.; Landman, A.B.; Wickner, P. Acute Allergic Reactions to mRNA COVID-19 Vaccines. JAMA 2021, 325, 1562–1565. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine -United States, December 21, 2020–January 10, 2021. MMWR Morb. Mortal Wkly Rep. 2021, 70, 125–129. [Google Scholar] [CrossRef]
- Gee, J.; Marquez, P.; Su, J. First Month of COVID-19 Vaccine Safety Monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morb. Mortal Wkly Rep. 2021, 70, 283–288. [Google Scholar] [CrossRef]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Xiong, X.; Yuan, J.; Li, M.; Jiang, B.; Lu, Z.K. Age and Gender Disparities in Adverse Events Following COVID-19 Vaccination: Real-World Evidence Based on Big Data for Risk Management. Front. Med. 2021, 8, 700014. [Google Scholar] [CrossRef]
- Klein, S.L.; Marriott, I.; Fish, E.N. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.M.; Lim, J.; Choe, K.-W.; Lee, K.-D.; Jo, D.H.; Kim, M.J.; Kim, J.M.; Kim, K.N. Reactogenicity after the first and second doses of BNT162b2 mRNA coronavirus disease vaccine: A single-center study. Clin. Exp. Vaccine Res. 2021, 10, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.S.; Zhang, M.X.; Chien, C.W.; Yang, W.Y.; Shi, G.F.; Qiu, S.; Tung, T.H.; Chen, H.X. Sex Differences in Adverse Reactions to an Inactivated SARS-CoV-2 Vaccine Among Medical Staff in China. Front. Med. 2021, 8, 731593. [Google Scholar] [CrossRef] [PubMed]
- Heidari, S.; Palmer-Ross, A.; Goodman, T. A Systematic Review of the Sex and Gender Reporting in COVID-19 Clinical Trials. Vaccines 2021, 9, 1322. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, A.; Shajahan, S.; Harris, K.; Hallam, L.; Hockham, C.; Womersley, K.; Woodward, M.; Sheel, M. Sex and Gender in COVID-19 Vaccine Research: Substantial Evidence Gaps Remain. Front. Glob. Women’s Health 2021, 2, 761511. [Google Scholar] [CrossRef]
- Signal Assessment Report on Embolic and Thrombotic Events (SMQ) with COVID-19 Vaccine (ChAdOx1-S [Recombinant])—COVID-19 Vaccine AstraZeneca (Other Viral Vaccines) EMA/PRAC/157045/2021. Available online: https://www.ema.europa.eu/en/documents/prac-recommendation/signal-assessment-report-embolic-thrombotic-events-smq-covid-19-vaccine-chadox1-s-recombinant-covid_en.pdf (accessed on 1 December 2021).
- Denly, L. The effect of sex on responses to influenza vaccines. Hum. Vaccines Immunother. 2020, 17, 1396–1402. [Google Scholar] [CrossRef]
- Clothier, H.J.; Lawrie, J.; Lewis, G.; Russell, M.; Crawford, N.W.; Buttery, J.P. SAEFVIC: Surveillance of adverse events following immunisation (AEFI) in Victoria, Australia, 2018. Commun. Dis. Intell. 2020, 44, 1–27. [Google Scholar] [CrossRef]
- Esposito, D.; Titievsky, L.; Beachler, D.C.; Hawes, J.C.; Isturiz, R.; Scott, D.A.; Gangemi, K.; Maroko, R.; Hall-Murray, C.K.; Lanes, S. Incidence of outcomes relevant to vaccine safety monitoring in a US commercially-insured population. Vaccine 2018, 36, 8084–8093. [Google Scholar] [CrossRef]
- Shohat, T.; Green, M.S.; Nakar, O.; Ballin, A.; Duvdevani, P.; Cohen, A.; Shohat, M. Gender differences in the reactogenicity of measles-mumps-rubella vaccine. Isr. Med. Assoc. J. 2000, 2, 192–195. [Google Scholar]
- Harris, T.; Nair, J.; Fediurek, J.; Deeks, S.L. Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012–2015. Vaccine 2017, 35, 2600–2604. [Google Scholar] [CrossRef]
- Alguacil-Ramos, A.M.; Muelas-Tirado, J.; Garrigues-Pelufo, T.M.; Portero-Alonso, A.; Diez-Domingo, J.; Pastor-Villalba, E.; Lluch- Rodrigo, J.A. Surveillance for adverse events following immunization (AEFI) for 7 years using a computerised vaccination system. Public Health 2016, 135, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Tadount, F.; Doyon-Plourde, P.; Rafferty, E.; MacDonald, S.; Sadarangani, M.; Quach, C. Is there a difference in the immune response, efficacy, effectiveness and safety of seasonal influenza vaccine in males and females?—A systematic review. Vaccine 2020, 38, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Green, M.S.; Nitzan, D.; Schwartz, N.; Niv, Y.; Peer, V. Sex differences in the case-fatality rates for COVID-19—A comparison of the age-related differences and consistency over seven countries. PLoS ONE 2021, 16, e0250523. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Balzano-Nogueira, L.; Lleo, A.; Conesa, A. Transcriptional Differences for COVID-19 Disease Map Genes between Males and Females Indicate a Different Basal Immunophenotype Relevant to the Disease. Genes 2020, 11, E1447. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, H.; Klein, S.L. Sex Differences in Immunity to Viral Infections. Front. Immunol. 2021, 12, 720952. [Google Scholar] [CrossRef]
- Trigunaite, A.; Dimo, J.; Jørgensen, T.N. Suppressive effects of androgens on the immune system. Cell. Immunol. 2015, 294, 87–94. [Google Scholar] [CrossRef]
- Bupp, G.M.R.; Jorgensen, T.N. Androgen-Induced Immunosuppression. Front. Immunol. 2018, 9, 794. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Merz, B.N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.; DeMeo, D.L.; De Vries, G.J.; Epperson, N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Ursin, R.L.; Shapiro, J.R.; Klein, S.L. Sex-biased Immune Responses Following SARS-CoV-2 Infection. Trends Microbiol. 2020, 28, 952–954. [Google Scholar] [CrossRef]
- Foresta, C.; Rocca, M.S.; Di Nisio, A. Gender susceptibility to COVID-19: A review of the putative role of sex hormones and X chromosome. J. Endocrinol. Investig. 2021, 44, 951–956. [Google Scholar] [CrossRef]
- Mackey, E.; Thelen, K.M.; Bali, V.; Fardisi, M.; Trowbridge, M.; Jordan, C.L.; Moeser, A.J. Perinatal androgens organize sex differences in mast cells and attenuate anaphylaxis severity into adulthood. Proc. Natl. Acad. Sci. USA 2020, 117, 23751–23761. [Google Scholar] [CrossRef] [PubMed]


| First Dose | Second Dose | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (M) (N = 414) | Female (F) (N = 509) | F:M Ratio | 95% CI | p-Value | Male (M) (N = 414) | Female (F) (N = 509) | F:M Ratio | 95% CI | p-Value | |||||
| Age | n | % | n | % | n | % | n | % | ||||||
| 20–29 | 2 | 5.4 | 18 | 29.0 | 5.37 | 1.32–21.85 | 0.0046 | 13 | 35.1 | 28 | 45.2 | 1.29 | 0.77–2.16 | 0.3272 |
| 30–39 | 18 | 16.2 | 40 | 27.0 | 1.67 | 1.01–2.75 | 0.0498 | 35 | 31.5 | 69 | 46.6 | 1.48 | 1.07–2.04 | 0.0142 |
| 40–49 | 10 | 12.7 | 21 | 20.8 | 1.64 | 0.82–3.29 | 0.1515 | 14 | 17.7 | 39 | 38.6 | 2.18 | 1.28–3.72 | 0.0023 |
| 50–59 | 6 | 8.8 | 13 | 14.6 | 1.66 | 0.66–4.13 | 0.2709 | 9 | 13.2 | 23 | 25.8 | 1.95 | 0.97–3.94 | 0.0520 |
| 60+ | 10 | 8.4 | 15 | 13.8 | 1.64 | 0.77–3.49 | 0.1959 | 11 | 9.2 | 24 | 22.0 | 2.38 | 1.23–4.63 | 0.0075 |
| Total | 46 | 11.1 | 107 | 21.0 | 1.89 | 1.37–2.61 | <0.0001 | 82 | 19.8 | 183 | 36.0 | 1.82 | 1.45–2.28 | <0.0001 |
| Sought medical care | ||||||||||||||
| Age | n | % | n | % | F:M | 95% CI | p-value | n | % | n | % | F:M | 95% CI | p-value |
| 20–29 | 5 | 13.5 | 2 | 3.2 | 0.24 | 0.05–1.17 | 0.0988 | 9 | 24.3 | 8 | 12.9 | 0.53 | 0.22–1.26 | 0.1499 |
| 30–39 | 3 | 2.7 | 14 | 9.5 | 3.50 | 1.03–11.88 | 0.0299 | 12 | 10.8 | 18 | 12.2 | 1.13 | 0.57–2.24 | 0.7366 |
| 40–49 | 7 | 8.9 | 6 | 5.9 | 0.67 | 0.24–1.92 | 0.4526 | 5 | 6.3 | 4 | 4.0 | 0.63 | 0.17–2.25 | 0.5086 |
| 50–59 | 2 | 2.9 | 5 | 5.6 | 1.91 | 0.38–9.55 | 0.6996 | 2 | 2.9 | 9 | 10.1 | 3.44 | 0.77–15.40 | 0.1156 |
| 60+ | 6 | 5.0 | 2 | 1.8 | 0.36 | 0.08–1.77 | 0.2842 | 5 | 4.2 | 7 | 6.4 | 1.53 | 0.50–4.67 | 0.4533 |
| Total | 23 | 5.6 | 29 | 5.7 | 1.03 | 0.60–1.75 | 0.9259 | 33 | 8.0 | 46 | 9.0 | 1.13 | 0.74–1.74 | 0.5647 |
| Adverse Events | First Dose | Second Dose | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (N = 152) | Female (N = 114) | F to M Ratio | 95% CI | p-Value | Male (N = 152) | Female (N = 114) | F to M Ratio | 95% CI | p-Value | |||||
| n | % | n | % | n | % | n | % | |||||||
| Local | ||||||||||||||
| Swelling in the injection area | 8 | 5.3 | 22 | 19.3 | 3.64 | 1.58–8.54 | 0.0001 | 8 | 5.3 | 21 | 18.4 | 3.47 | 1.42–6.80 | 0.0001 |
| Pain in the injection area | 41 | 27.0 | 69 | 60.0 | 2.22 | 1.42–3.54 | 0.0001 | 26 | 17.1 | 63 | 55.3 | 3.23 | 1.62–3.66 | 0.0001 |
| Pain all over the injected hand | 6 | 3.0 | 24 | 21.1 | 7.03 | 1.93–10.88 | 0.0001 | 8 | 5.3 | 25 | 21.9 | 4.13 | 1.68–7.72 | 0.0001 |
| Rash or redness in the injection area | 1 | 0.7 | 3 | 2.6 | 3.71 | 0.41–37.23 | 0.212 | 1 | 0.7 | 4 | 3.5 | 5.00 | 0.59–45.79 | 0.109 |
| Systemic | ||||||||||||||
| Fever | 0 | 0 | 3 | 2.6 | NA | NA | 0.078 | 15 | 9.9 | 19 | 16.7 | 1.69 | 0.84–3.01 | 0.073 |
| Shivering | 2 | 1.3 | 13 | 11.4 | 8.77 | 1.81–34.28 | 0.0001 | 13 | 8.6 | 38 | 33.3 | 3.87 | 1.76–5.72 | 0.0001 |
| Muscle pains | 5 | 3.3 | 19 | 16.7 | 5.06 | 1.72–11.79 | 0.0001 | 21 | 13.8 | 41 | 36.0 | 2.61 | 1.35–3.51 | 0.0001 |
| Joint pains | 0 | 0 | 6 | 5.3 | NA | NA | 0.006 | 7 | 4.6 | 18 | 15.8 | 3.43 | 1.34–7.19 | 0.002 |
| Headaches | 3 | 2.0 | 21 | 18.3 | 9.15 | 2.45–26.35 | 0.0001 | 15 | 9.9 | 37 | 32.5 | 3.28 | 1.56–4.77 | 0.0001 |
| Fatigue | 10 | 6.6 | 25 | 21.9 | 3.32 | 1.45–5.85 | 0.0001 | 30 | 19.7 | 51 | 44.7 | 2.27 | 1.26–2.79 | 0.0001 |
| Lack of ability to stand | 0 | 0 | 10 | 8.8 | NA | NA | 0.0001 | 1 | 0.7 | 19 | 16.7 | 23.86 | 2.97–161.09 | 0.0001 |
| General weakness | 1 | 0.7 | 23 | 20.2 | 30.67 | 3.52–187.68 | 0.0001 | 19 | 12.5 | 37 | 32.5 | 2.60 | 1.33–3.67 | 0.0001 |
| Allergy | 0 | 0 | 3 | 2.6 | NA | NA | 0.078 | 0 | 0 | 1 0.9) | 0.9 | NA | NA | 0.429 |
| Anaphylaxis | 0 | 0 | 0 | 0 | NA | NA | NA | 0 | 0 | 0 | 0 | NA | NA | NA |
| Sensory | ||||||||||||||
| Paresthesia in the injected hand | 0 | 0 | 6 | 5.3 | NA | NA | 0.006 | 0 | 0 | 6 | 5.3 | NA | NA | 0.006 |
| Facial paralysis | 0 | 0 | 1 | 0.9 | NA | NA | 0.3 | 0 | 0 | 1 | 0.9 | NA | NA | 0.429 |
| Facial paresthesia | 0 | 0 | 2 | 1.8 | NA | NA | 0.183 | 0 | 0 | 2 | 1.8 | NA | NA | 0.183 |
| Herpes zoster | 0 | 1 | 0.9 | NA | NA | 0.429 | 1 | 0.7 | 0 | 0 | NA | NA | 0.571 | |
| Adverse Event | First Dose | Second Dose | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male (N = 152), n (%) | Female (N = 114), n (%) | F to M Ratio | 95% CI | p-Value | Male (N = 152), n (%) | Female (N = 114), n (%) | F to M Ratio | 95% CI | p-Value | |
| Local | ||||||||||
| Swelling in the injection area | 29 (19.1) | 44 (38.6) | 2.02 | 1.15–2.64 | 0.0001 | 24 (15.8) | 33(28.9) | 1.83 | 0.99–2.60 | 0.008 |
| Pain in the injection area | 74 (48.7) | 80 (70.2) | 1.44 | 0.98–1.62 | 0.0001 | 49 (32.2) | 69(60.5) | 1.88 | 1.14–2.10 | 0.0001 |
| Pain all over the injected hand | 8 (5.3) | 24 (21.1) | 3.98 | 1.62–7.49 | 0.0001 | 9 (5.9) | 24(21.1) | 3.58 | 1.50–6.47 | 0.0001 |
| Rash or redness in the injection area | 2 (1.3) | 5 (4.4) | 3.38 | 0.64–16.39 | 0.123 | 1 (0.7) | 4(3.5) | 5.00 | 0.59–45.79 | 0.109 |
| Systemic | ||||||||||
| Fever | 0 (0) | 1 (0.9) | NA | NA | 0.429 | 10 (6.6) | 16(14.0) | 2.12 | 0.94–4.25 | 0.035 |
| Shivering | 3 (2.0) | 12 (10.5) | 5.25 | 1.42–17.06 | 0.003 | 11 (7.2) | 35(30.7) | 4.26 | 1.84–6.60 | 0.0001 |
| Muscle pains | 8 (5.3) | 17 (14.9) | 2.81 | 1.16–5.82 | 0.007 | 16 (10.5) | 38(33.3) | 3.17 | 1.53–4.51 | 0.0001 |
| Joint pains | 1 (0.7) | 3 (2.6) | 3.71 | 0.41–37.23 | 0.212 | 5 (3.3) | 15(13.2) | 4.00 | 1.36–9.78 | 0.003 |
| Headaches | 3 (2.0) | 18 (15.8) | 7.90 | 2.12–23.39 | 0.0001 | 11 (7.2) | 33(28.9) | 4.01 | 1.75–6.34 | 0.0001 |
| Fatigue | 9 (5.9) | 21 (18.4) | 3.12 | 1.32–5.87 | 0.0001 | 28 (18.4) | 45(39.5) | 2.15 | 1.19–2.77 | 0.0001 |
| Lack of ability to stand | 1 (0.7) | 7 (6.1) | 8.71 | 1.10–70.97 | 0.012 | 3 (2.0) | 15(13.2) | 6.60 | 1.78–20.30 | 0.0001 |
| General weakness | 2 (1.3) | 21 (18.4) | 14.15 | 2.86–50.15 | 0.0001 | 15 (9.9) | 30(26.3) | 2.66 | 1.30–4.14 | 0.0001 |
| Allergy | 0 (0) | 2 (1.8) | NA | NA | 0.183 | 1 (0.7) | 1 (0.9) | 1.29 | 0.08–21.05 | 0.674 |
| Anaphylaxis | 0 | 0 | NA | NA | NA | 0 | 0 | NA | NA | NA |
| Sensory | ||||||||||
| Paresthesia in the injected hand | 0 (0) | 8 (7.0) | NA | NA | 0.001 | 0 (0) | 6(5.5) | NA | NA | 0.006 |
| Facial paralysis | 1 (0.7) | 0 (0) | NA | NA | 0.571 | 1 (0.7) | 1 (0.9) | 1.29 | 0.08–21.05 | 0.674 |
| Facial paresthesia | 0 (0) | 2 (1.8) | NA | NA | 0.183 | 1 (0.7) | 1 (0.9) | 1.29 | 0.08–21.05 | 0.674 |
| Herpes zoster | 0 (0) | 1 (0.9) | NA | NA | 0.429 | 1 (0.7) | 0(0) | NA | NA | 0.571 |
| Adverse Event | Third Dose | ||||||
|---|---|---|---|---|---|---|---|
| Male (N = 172) | Female (N = 122) | F to M Ratio | 95% CI | p-Value | |||
| n | % | n | % | ||||
| Local | |||||||
| Swelling in the injection area | 19 | 11.0 | 35 | 28.7 | 2.59 | 1.56-4.31 | 0.0001 |
| Pain in the injection area | 46 | 26.7 | 75 | 61.5 | 2.29 | 1.73–3.05 | <0.0001 |
| Pain all over the injected hand | 13 | 7.6 | 39 | 32.0 | 4.23 | 2.36–7.58 | 0.0008 |
| Rash or redness in the injection area | 4 | 2.3 | 10 | 8.2 | 3.52 | 0.47–10.98 | 0.0845 |
| Systemic | |||||||
| Fever | 30 | 17.4 | 35 | 28.7 | 1.64 | 1.07–2.52 | <0.0001 |
| Shivering | 21 | 12.2 | 33 | 27.1 | 2.21 | 1.35–3.63 | <0.0001 |
| Muscle pains | 23 | 13.4 | 53 | 43.4 | 3.25 | 2.11–4.99 | <0.0001 |
| Joint pains | 17 | 9.9 | 34 | 27.9 | 2.82 | 1.65–4.81 | 0.0002 |
| Headaches | 23 | 13.4 | 40 | 32.8 | 2.45 | 1.55–3.87 | <0.0001 |
| Fatigue | 48 | 27.9 | 72 | 59.0 | 2.11 | 1.59–2.80 | <0.0001 |
| Lack of ability to stand | 19 | 11.1 | 24 | 19.7 | 1.78 | 1.02–3.10 | 0.0004 |
| General weakness | 50 | 29.1 | 59 | 48.4 | 1.66 | 1.23–2.23 | <0.0001 |
| Allergy | 7 | 4.1 | 3 | 2.5 | 0.60 | 0.16–2.29 | 0.1413 |
| Anaphylaxis | 3 | 1.7 | 0 | 0.0 | - | – | - |
| Sensory | |||||||
| Paresthesia in the injected hand | 8 | 4.7 | 8 | 6.6 | 1.41 | 0.54–3.65 | 0.4797 |
| Facial paralysis | 3 | 1.7 | 1 | 0.8 | 0.47 | 0.05–4.46 | 0.3840 |
| Facial paresthesia | 3 | 1.7 | 1 | 0.8 | 0.47 | 0.05–4.46 | 0.3840 |
| Herpes zoster | 3 | 1.7 | 2 | 1.6 | 0.94 | 0.16–5.54 | 0.2693 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Green, M.S.; Peer, V.; Magid, A.; Hagani, N.; Anis, E.; Nitzan, D. Gender Differences in Adverse Events Following the Pfizer-BioNTech COVID-19 Vaccine. Vaccines 2022, 10, 233. https://doi.org/10.3390/vaccines10020233
Green MS, Peer V, Magid A, Hagani N, Anis E, Nitzan D. Gender Differences in Adverse Events Following the Pfizer-BioNTech COVID-19 Vaccine. Vaccines. 2022; 10(2):233. https://doi.org/10.3390/vaccines10020233
Chicago/Turabian StyleGreen, Manfred S, Victoria Peer, Avi Magid, Neta Hagani, Emilia Anis, and Dorit Nitzan. 2022. "Gender Differences in Adverse Events Following the Pfizer-BioNTech COVID-19 Vaccine" Vaccines 10, no. 2: 233. https://doi.org/10.3390/vaccines10020233
APA StyleGreen, M. S., Peer, V., Magid, A., Hagani, N., Anis, E., & Nitzan, D. (2022). Gender Differences in Adverse Events Following the Pfizer-BioNTech COVID-19 Vaccine. Vaccines, 10(2), 233. https://doi.org/10.3390/vaccines10020233






