Liver Injury in Patients Hospitalized for COVID-19: Possible Role of Therapy
Abstract
:1. Introduction
- to review the potential liver injury associated with pharmacological and non-pharmacological treatments currently in use for hospitalized COVID-19 patients;
- to assess a reasoned approach to the diagnosis and management of DILI in COVID-19 patients.
2. Drugs and Liver Injury
2.1. Systemic Corticosteroids
2.2. Other Immunomodulant Therapies: Anti-IL6, Anti-IL1, Anti-JAK
2.3. Antivirals: Remdesivir
2.4. Low-Molecular-Weight Heparins
2.5. Symptomatic Medications: NSAIDs and Paracetamol
2.6. Antibiotics
3. Non-Pharmacological Treatments: Non-Invasive Ventilation
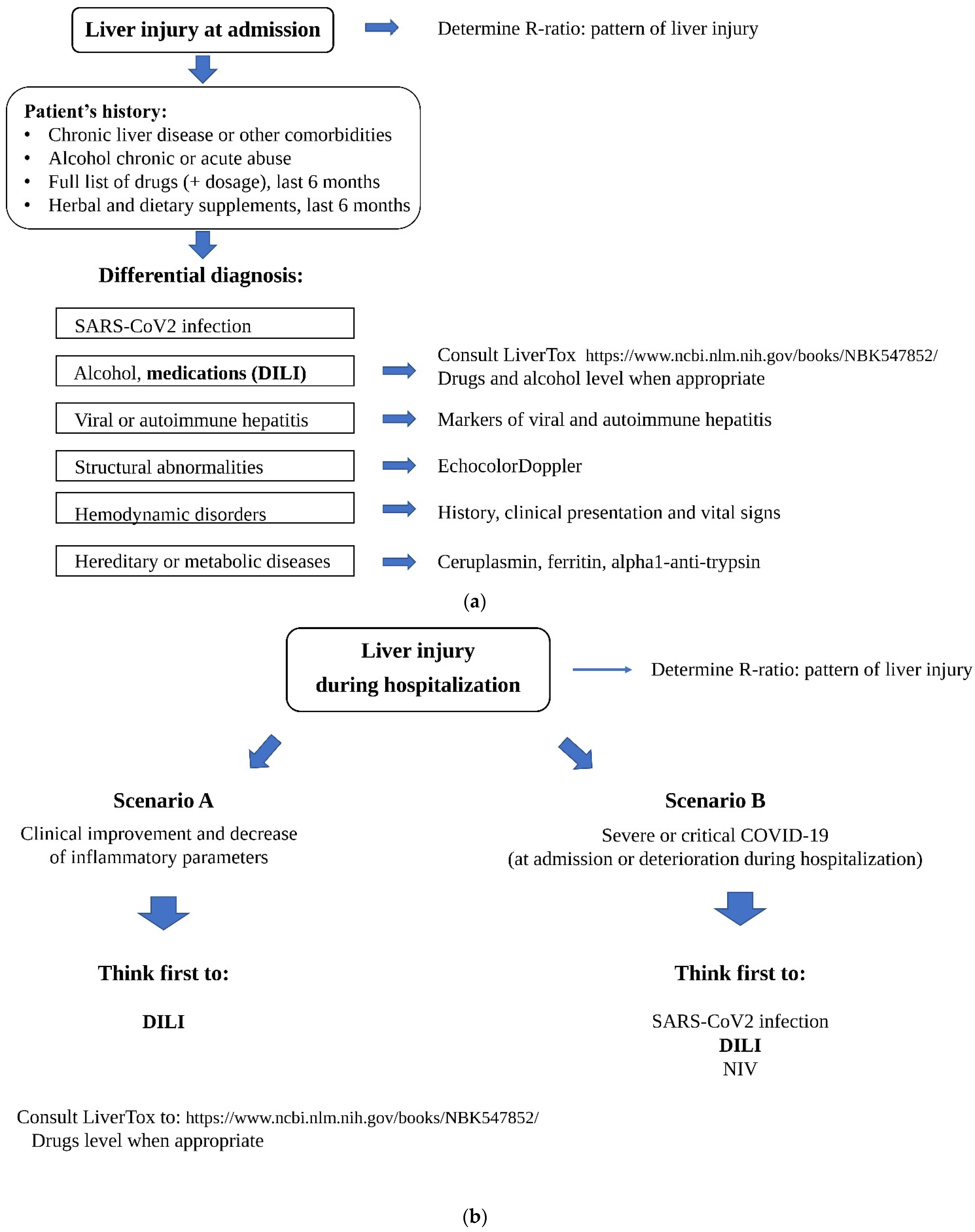
4. Diagnosis and Management of DILI in COVID-19 Patients
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pollard, C.A.; Morran, M.P.; Nestor-Kalinoski, A.L. The COVID-19 pandemic: A global health crisis. Physiol. Genom. 2020, 52, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, X. Acute respiratory failure in COVID-19: Is it “typical” ARDS? Crit. Care 2020, 24, 198. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, M.; Pandey, N.; Shukla, A.; Singh, S.K. SARS coronavirus 2: From genome to infectome. Respir. Res. 2020, 21, 318. [Google Scholar] [CrossRef]
- Lai, C.-C.; Ko, W.-C.; Lee, P.-I.; Jean, S.-S.; Hsueh, P.-R. Extra-respiratory manifestations of COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106024. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Rizwan, T.; Malik, F.; Akhter, R.; Malik, M.; Ahmad, J.; Khan, A.W.; Chaudhary, M.A.; Usman, M.S. COVID-19 and Liver Injury: A Systematic Review and Meta-Analysis. Cureus 2020, 12, e9424. [Google Scholar] [CrossRef] [PubMed]
- Kumar-M, P.; Mishra, S.; Jha, D.K.; Shukla, J.; Choudhury, A.; Mohindra, R.; Mandavdhare, H.S.; Dutta, U.; Sharma, V. Coronavirus disease (COVID-19) and the liver: A comprehensive systematic review and meta-analysis. Hepatol. Int. 2020, 14, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Deng, M.; Wang, X.; Yang, F. Predictor of poor prognosis of COVID-19 patients–liver injury. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 873–876. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, L.; Wang, F.-S. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol. Hepatol. 2020, 5, 428–430. [Google Scholar] [CrossRef]
- Chai, X.; Hu, L.; Zhang, Y.; Han, W.; Lu, Z.; Ke, A.; Zhou, J.; Shi, G.; Fang, N.; Fan, J.; et al. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020, 526, 135–140. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Liu, H.; Li, W.; Lin, F.; Jiang, L.; Li, X.; Xu, P.; Zhang, L.; Zhao, L.; et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020, 73, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Paizis, G.; Tikellis, C.; Cooper, M.E.; Schembri, J.M.; Lew, R.A.; Smith, I.A.; Shaw, T.; Warner, F.J.; Zuilli, A.; Burrell, L.M.; et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut 2005, 54, 1790–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testino, G.; DI Biagio, A.; Fagoonee, S.; Pellicano, R. SARS-CoV-2, alcohol consumption and liver injury. Minerva Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, M.; Grander, C.; Grabherr, F.; Griesmacher, A.; Ploner, T.; Hartig, F.; Bellmann-Weiler, R.; Joannidis, M.; Zoller, H.; Weiss, G.; et al. Systemic inflammation as fuel for acute liver injury in COVID-19. Dig. Liver Dis. 2021, 53, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Ferron, P.J.; Gicquel, T.; Mégarbane, B.; Clément, B.; Fromenty, B. Treatments in Covid-19 patients with pre-existing metabolic dysfunction-associated fatty liver disease: A potential threat for drug-induced liver injury? Biochimie 2020, 179, 266–274. [Google Scholar] [CrossRef]
- Benichou, C.; Danan, G.; Flahault, A. Causality assessment of adverse reactions to drugs—II. An original model for validation of drug causality assessment methods: Case reports with positive rechallenge. J. Clin. Epidemiol. 1993, 46, 1331–1336. [Google Scholar] [CrossRef]
- Sandhu, N.; Navarro, V. Drug-Induced Liver Injury in GI Practice. Hepatol. Commun. 2020, 4, 631–645. [Google Scholar] [CrossRef]
- Katarey, D.; Verma, S. Drug-induced liver injury. Clin. Med. 2016, 16, s104–s109. [Google Scholar] [CrossRef]
- Davern, T.J. Drug-induced liver disease. Clin. Liver Dis. 2012, 16, 231–245. [Google Scholar] [CrossRef]
- Teschke, R. Idiosyncratic DILI: Analysis of 46,266 Cases Assessed for Causality by RUCAM and Published From 2014 to Early 2019. Front. Pharmacol. 2019, 10, 730. [Google Scholar] [CrossRef]
- Kuna, L.; Bozic, I.; Kizivat, T.; Bojanic, K.; Mrso, M.; Kralj, E.; Smolic, R.; Wu, G.Y.; Smolic, M. Models of Drug Induced Liver Injury (DILI)–Current Issues and Future Perspectives. Curr. Drug Metab. 2018, 19, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Bonkovsky, H.L.; Fontana, R.; Lee, W.; Stolz, A.; Talwalkar, J.; Reddy, K.R.; Watkins, P.B.; Navarro, V.; Barnhart, H.; et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology 2015, 148, 1340–1352.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, A.M. Acetaminophen hepatotoxicity. Clin. Liver Dis. 2007, 11, 525–548. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; Aithal, G.P.; Björnsson, E.S.; Kaplowitz, N.; Kullak-Ublick, G.A.; Larrey, D.; Karlsen, T.H.; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Jeronimo, C.M.P.; Farias, M.E.L.; Val, F.F.A.; Sampaio, V.S.; Alexandre, M.A.A.; Melo, G.C.; Safe, I.P.; Borba, M.G.S.; Netto, R.L.A.; Maciel, A.B.S.; et al. Methylprednisolone as Adjunctive Therapy for Patients Hospitalized With Coronavirus Disease 2019 (COVID-19; Metcovid): A Randomized, Double-blind, Phase IIb, Placebo-controlled Trial. Clin. Infect. Dis. 2020, 72, e373–e381. [Google Scholar] [CrossRef]
- Sterne, J.A.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.; Berwanger, O.; Cavalcanti, A.B.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar]
- Chalmers, J.; Abo-Leyah, H.; Loftus, H.; Spears, M. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar]
- Cortes, M.G.; Robles-Diaz, M.; Stephens, C.; Ortega-Alonso, A.; Lucena, M.I.; Andrade, R.J. Drug induced liver injury: An update. Arch. Toxicol. 2020, 94, 3381–3407. [Google Scholar] [CrossRef]
- Hu, P.-F.; Xie, W.-F. Corticosteroid therapy in drug-induced liver injury: Pros and cons. J. Dig. Dis. 2018, 20, 122–126. [Google Scholar] [CrossRef]
- Hori, T.; Onishi, Y.; Kamei, H.; Kurata, N.; Ishigami, M.; Ishizu, Y.; Ogura, Y. Fibrosing cholestatic hepatitis C in post-transplant adult recipients of liver transplantation. Ann. Gastroenterol. 2016, 29, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.Y.; Patel, S.; Kurji, K.H.; Sarnicola, E.; Eslani, M.; Govil, A.; Holland, E.J. Ocular Surface Stem Cell Transplantation for Treatment of Keratitis–Ichthyosis–Deafness Syndrome. Cornea 2018, 38, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Uchida, T.; Yamaguchi, H.; Kudo, Y.; Yonekawa, T.; Nakazato, M. Fulminant hepatitis and elevated levels of sIL-2R in thyroid storm. Endocrinol. Diabetes Metab. Case Rep. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Yip, T.C.-F.; Lui, G.C.-Y.; Wong, V.W.-S.; Chow, V.C.-Y.; Ho, T.H.-Y.; Li, T.C.-M.; Tse, Y.-K.; Hui, D.S.-C.; Chan, H.L.-Y.; Wong, G.L.-H. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut 2020, 70, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.; Hazlehurst, J.M.; Tomlinson, J.W. Glucocorticoids and non-alcoholic fatty liver disease. J. Steroid Biochem. Mol. Biol. 2015, 154, 94–103. [Google Scholar] [CrossRef]
- Farrell, G.C. Drugs and Steatohepatitis. Semin. Liver Dis. 2002, 22, 185–194. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Quirch, M.; Lee, J.; Rehman, S. Hazards of the Cytokine Storm and Cytokine-Targeted Therapy in Patients with COVID-19: Review. J. Med Internet Res. 2020, 22, e20193. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.; Ntritsos, G.; Gogali, A.; Milionis, H.; Evangelou, E.; Kostikas, K. Tocilizumab administration for the treatment of hospitalized patients with COVID-19: A systematic review and meta-analysis. Respirology 2021, 26, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, M.; Laskou, F.; Stapleton, P.P.; Hadavi, S.; Dasgupta, B. Tocilizumab (Actemra). Hum. Vaccines Immunother. 2017, 13, 1972–1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvarani, C.; Dolci, G.; Massari, M.; Merlo, D.F.; Cavuto, S.; Savoldi, L.; Bruzzi, P.; Boni, F.; Braglia, L.; Turrà, C.; et al. Effect of Tocilizumab vs. Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ngamprasertchai, T.; Kajeekul, R.; Sivakorn, C.; Ruenroegnboon, N.; Luvira, V.; Siripoon, T.; Luangasanatip, N. Efficacy and Safety of Immunomodulators in Patients with COVID-19: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Infect. Dis. Ther. 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kyriakoulis, K.G.; Kollias, A.; Poulakou, G.; Kyriakoulis, I.G.; Trontzas, I.P.; Charpidou, A.; Syrigos, K. The Effect of Anakinra in Hospitalized Patients with COVID-19: An Updated Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 4462. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.M.; Schechtman, J.; Bennett, R.; Handel, M.L.; Burmester, G.R.; Tesser, J.; Modafferi, D.; Poulakos, J.; Sun, G. Anakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis: A large, international, multicenter, placebo-controlled trial. Arthritis Rheum. 2003, 48, 927–934. [Google Scholar] [CrossRef]
- Kyriazopoulou, E.; Poulakou, G.; Milionis, H.; Metallidis, S.; Adamis, G.; Tsiakos, K. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial. Nat. Med. 2021, 27, 1752–1760. [Google Scholar] [CrossRef]
- Limen, R.Y.; Sedono, R.; Sugiarto, A.; Hariyanto, T.I. Janus kinase (JAK)-inhibitors and coronavirus disease 2019 (Covid-19) outcomes: A systematic review and meta-analysis. Expert Rev. Anti-Infect. Ther. 2021, 29, 1–10. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Tse, C.L.Y.; Burry, L.; Dresser, L.D. Baricitinib: A Review of Pharmacology, Safety, and Emerging Clinical Experience in COVID-19. Pharmacotherapy 2020, 40, 843–856. [Google Scholar] [CrossRef]
- Marconi, V.C.; Ramanan, A.V.; de Bono, S.; E Kartman, C.; Krishnan, V.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Alatorre-Alexander, J.; Pellegrini, R.D.C.; et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021, 9, 1407–1418. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Preliminary report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Angamo, M.T.; Mohammed, M.A.; Peterson, G.M. Efficacy and safety of remdesivir in hospitalised COVID-19 patients: A systematic review and meta-analysis. Infection 2021, 31, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Luzum, J.A. Pharmacogenomics of COVID-19 therapies. NPJ Genom. Med. 2020, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.K.; Gleason, P.P.; Sen, S. Elevation of Hepatic Transaminases after Enoxaparin Use: Case Report and Review of Unfractionated and Low-Molecular-Weight Heparin-Induced Hepatotoxicity. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2001, 21, 108–113. [Google Scholar] [CrossRef]
- Yang, X.; Li, N.; Guo, T.; Guan, X.; Tan, J.; Gao, X.; Wu, Y.; Jia, L.; Gu, M.; Hua, L.; et al. Comparison of the Effects of Low-Molecular-Weight Heparin and Fondaparinux on Liver Function in Patients With Pulmonary Embolism. J. Clin. Pharmacol. 2020, 60, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Hahn, K.J.; Morales, S.J.; Lewis, J.H. Enoxaparin-Induced Liver Injury: Case Report and Review of the Literature and FDA Adverse Event Reporting System (FAERS). Drug Saf.-Case Rep. 2015, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Harrill, A.; Roach, J.; Fier, I.; Eaddy, J.S.; Kurtz, C.L.; Antoine, D.J.; Spencer, D.M.; Kishimoto, T.K.; Pisetsky, D.S.; Park, B.K.; et al. The Effects of Heparins on the Liver: Application of Mechanistic Serum Biomarkers in a Randomized Study in Healthy Volunteers. Clin. Pharmacol. Ther. 2012, 92, 214–220. [Google Scholar] [CrossRef]
- Arora, N.; Goldhaber, S.Z. Anticoagulants and transaminase elevation. Circulation 2006, 113, e698–e702. [Google Scholar] [CrossRef] [Green Version]
- Lawler, P.R.; Goligher, E.C.; Berger, J.S.; Neal, M.D. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 385, 790–802. [Google Scholar]
- Goligher, E.C.; Bradbury, C.A. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021, 385, 777–789. [Google Scholar]
- Mazloomzadeh, S.; Khaleghparast, S.; Ghadrdoost, B.; Mousavizadeh, M.; Baay, M.R.; Noohi, F.; Sharifnia, H.; Ahmadi, A.; Tavan, S.; Alamdari, N.M.; et al. Effect of Intermediate-Dose vs. Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA 2021, 325, 1620–1630. [Google Scholar]
- Sholzberg, M.; Tang, G.H.; Rahhal, H.; AlHamzah, M.; Kreuziger, L.B.; Áinle, F.N.; Alomran, F.; Alayed, K.; Alsheef, M.; AlSumait, F.; et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ 2021, 375, n2400. [Google Scholar] [CrossRef] [PubMed]
- Team, D.T. EMA advice on the use of NSAIDs for COVID-19. Drug Ther. Bull. 2020, 58, 69. [Google Scholar]
- Micallef, J.; Soeiro, T.; Jonville-Béra, A.P. Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection. Therapie 2020, 75, 355–362. [Google Scholar] [CrossRef]
- Licata, A.; Minissale, M.G.; Calvaruso, V.; Craxì, A. A focus on epidemiology of drug-induced liver injury: Analysis of a prospective cohort. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 112–121. [Google Scholar]
- Przybyła, G.W.; Szychowski, K.A.; Gmiński, J. Paracetamol–An old drug with new mechanisms of action. Clin. Exp. Pharmacol. Physiol. 2020, 48, 3–19. [Google Scholar] [CrossRef]
- Fisher, E.S.; Curry, S.C. Evaluation and treatment of acetaminophen toxicity. Adv. Pharmacol. 2019, 85, 263–272. [Google Scholar] [CrossRef]
- Gulmez, S.E.; Unal, U.S.; Lassalle, R.; Chartier, A.; Grolleau, A.; Moore, N. Risk of hospital admission for liver injury in users of NSAIDs and nonoverdose paracetamol: Preliminary results from the EPIHAM study. Pharmacoepidemiol. Drug Saf. 2018, 27, 1174–1181. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.P.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Chang, C.Y.; Schiano, T.D. Review article: Drug hepatotoxicity. Aliment. Pharmacol. Ther. 2007, 25, 1135–1151. [Google Scholar] [CrossRef]
- Pani, A.; Lauriola, M.; Romandini, A.; Scaglione, F. Macrolides and viral infections: Focus on azithromycin in COVID-19 pathology. Int. J. Antimicrob. Agents 2020, 56, 106053. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-J.; Kuo, M.-L.; Hsiao, H.-S.; Lee, P.-T. Azithromycin modulates immune response of human monocyte-derived dendritic cells and CD4+ T cells. Int. Immunopharmacol. 2016, 40, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, A.B.; Zampieri, F.G.; Rosa, R.G.; Azevedo, L.C.; Veiga, V.C.; Avezum, A.; Damiani, L.P.; Marcadenti, A.; Kawano-Dourado, L.; Lisboa, T.; et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate COVID-19. N. Engl. J. Med. 2020, 383, 2041–2052. [Google Scholar] [CrossRef]
- Molina, J.; Delaugerre, C.; Le Goff, J.; Mela-Lima, B.; Ponscarme, D.; Goldwirt, L.; de Castro, N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal. Infect. 2020, 50, 384. [Google Scholar] [CrossRef] [PubMed]
- Sultana, J.; Cutroneo, P.M.; Crisafulli, S.; Puglisi, G.; Caramori, G.; Trifirò, G. Azithromycin in COVID-19 Patients: Pharmacological Mechanism, Clinical Evidence and Prescribing Guidelines. Drug Saf. 2020, 43, 691–698. [Google Scholar] [CrossRef]
- AIFA. Azitromicina Nella Terapia dei Pazienti Adulti Con COVID-19. 2020. Available online: https://www.aifa.gov.it/documents/20142/1123276/azitromicina_08.04.2020.pdf/951fa605-0bf9-3882-ae2f-15128fe97a1b (accessed on 23 November 2021).
- Sekhavati, E.; Jafari, F.; SeyedAlinaghi, S.; Jamalimoghadamsiahkali, S.; Sadr, S.; Tabarestani, M.; Pirhayati, M.; Zendehdel, A.; Manafi, N.; Hajiabdolbaghi, M.; et al. Safety and effectiveness of azithromycin in patients with COVID-19: An open-label randomised trial. Int. J. Antimicrob. Agents 2020, 56, 106143. [Google Scholar] [CrossRef]
- Martinez, M.A.; Vuppalanchi, R.; Fontana, R.J.; Stolz, A.; Kleiner, D.E.; Hayashi, P.H.; Gu, J.; Hoofnagle, J.H.; Chalasani, N. Clinical and histologic features of azithromycin-induced liver injury. Clin. Gastroenterol. Hepatol. 2015, 13, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Azithromycin. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Cammarota, G.; Esposito, T.; Azzolina, D.; Cosentini, R.; Menzella, F.; Aliberti, S.; Coppadoro, A.; Bellani, G.; Foti, G.; Grasselli, G.; et al. Noninvasive respiratory support outside the intensive care unit for acute respiratory failure related to coronavirus-19 disease: A systematic review and meta-analysis. Crit. Care 2021, 25, 268. [Google Scholar] [CrossRef]
- Grieco, D.L.; Menga, L.S.; Cesarano, M.; Rosà, T.; Spadaro, S.; Bitondo, M.M.; Montomoli, J.; Falò, G.; Tonetti, T.; Cutuli, S.L.; et al. Effect of Helmet Noninvasive Ventilation vs. High-Flow Nasal Oxygen on Days Free of Respiratory Support in Patients With COVID-19 and Moderate to Severe Hypoxemic Respiratory Failure: The HENIVOT Randomized Clinical Trial. JAMA 2021, 325, 1731–1743. [Google Scholar] [CrossRef]
- Gattinoni, L.; Gattarello, S.; Steinberg, I.; Busana, M.; Palermo, P.; Lazzari, S.; Romitti, F.; Quintel, M.; Meissner, K.; Marini, J.J.; et al. COVID-19 pneumonia: Pathophysiology and management. Eur. Respir. Rev. 2021, 30, 210138. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radovanovic, D.; Pini, S.; Franceschi, E.; Pecis, M.; Airoldi, A.; Rizzi, M.; Santus, P. Characteristics and outcomes in hospitalized COVID-19 patients during the first 28 days of the spring and autumn pandemic waves in Milan: An observational prospective study. Respir. Med. 2021, 178, 106323. [Google Scholar] [CrossRef] [PubMed]
- Matuschak, G.M. Liver-lung interactions in critical illness. New horizons 1994, 2, 488–504. [Google Scholar]
- Kredel, M.; Muellenbach, R.M.; Brock, R.W.; Wilckens, H.-H.; Brederlau, J.; Roewer, N.; Wunder, C. Liver dysfunction after lung recruitment manoeuvres during pressure-controlled ventilation in experimental acute respiratory distress. Crit. Care 2007, 11, R13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Träger, K.; Radermacher, P.; Georgieff, M. PEEP and hepatic metabolic performance in septic shock. Intensive care medicine. Intensive Care Med. 1996, 22, 1274–1275. [Google Scholar] [CrossRef]
- Brienza, N.; Dalfino, L.; Cinnella, G.; Diele, C.; Bruno, F.; Fiore, T. Jaundice in critical illness: Promoting factors of a concealed reality. Intensiv. Care Med. 2006, 32, 267–274. [Google Scholar] [CrossRef]
- Morris, C.A.; Avorn, J. Internet marketing of herbal products. JAMA 2003, 290, 1505–1509. [Google Scholar] [CrossRef]
- Alyami, H.S.; Orabi, M.A.; Aldhabbah, F.M.; Alturki, H.N.; Aburas, W.I.; Alfayez, A.I.; Alharbi, A.S.; Almasuood, R.A.; Alsuhaibani, N.A. Knowledge about COVID-19 and beliefs about and use of herbal products during the COVID-19 pandemic: A cross-sectional study in Saudi Arabia. Saudi Pharm. J. 2020, 28, 1326–1332. [Google Scholar] [CrossRef]
- Plášek, J.; Gumulec, J.; Máca, J.; Škarda, J.; Procházka, V.; Grézl, T.; Václavík, J. COVID-19 Associated Coagulopathy: Mechanisms and Host-Directed Treatment. Am. J. Med Sci. 2021. [Google Scholar] [CrossRef]
- Chong, W.H.; Saha, B.K.; Ramani, A.; Chopra, A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection 2021, 49, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar] [PubMed]
| Drug | Liver Contraindications | Risk Level of DILI | DILI Pattern |
|---|---|---|---|
| systemic corticosteroids | Generic caution in liver failure | + | hepatocellular or mixed |
| tocilizumab | ALT > 5 times URL | ++ | hepatocellular |
| sarilumab | ALT > 1.5 times URL | ++ | hepatocellular |
| anakinra | Not recommended in severe liver failure (Child-Pugh C) | + | hepatocellular |
| baricitinib, tofacitinib, imatinib | Not recommended in severe liver failure (Child-Pugh C) | + | hepatocellular |
| remdesivir | ALT > 5 times URL | ++ | hepatocellular |
| low-molecular-weight heparins | Generic caution in liver failure | + | hepatocellular |
| NSAIDs | Contraindicated in severe liver failure (Child-Pugh C), caution in mild or moderate liver failure (indication to use low dosage) | ++ | hepatocellular |
| paracetamol | Contraindicated in severe liver failure (Child-Pugh C), caution in mild or moderate liver failure (indication to use low dosage) | ++++ | hepatocellular |
| antibiotics amoxicillin-clavulanate trimethoprim/sulfamethoxazole azitromicin | +++ ++ ++ | cholestatic cholestatic mixed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabrielli, M.; Franza, L.; Esperide, A.; Gasparrini, I.; Gasbarrini, A.; Franceschi, F.; on behalf of GEMELLI AGAINST COVID 2019. Liver Injury in Patients Hospitalized for COVID-19: Possible Role of Therapy. Vaccines 2022, 10, 192. https://doi.org/10.3390/vaccines10020192
Gabrielli M, Franza L, Esperide A, Gasparrini I, Gasbarrini A, Franceschi F, on behalf of GEMELLI AGAINST COVID 2019. Liver Injury in Patients Hospitalized for COVID-19: Possible Role of Therapy. Vaccines. 2022; 10(2):192. https://doi.org/10.3390/vaccines10020192
Chicago/Turabian StyleGabrielli, Maurizio, Laura Franza, Alessandra Esperide, Irene Gasparrini, Antonio Gasbarrini, Francesco Franceschi, and on behalf of GEMELLI AGAINST COVID 2019. 2022. "Liver Injury in Patients Hospitalized for COVID-19: Possible Role of Therapy" Vaccines 10, no. 2: 192. https://doi.org/10.3390/vaccines10020192
APA StyleGabrielli, M., Franza, L., Esperide, A., Gasparrini, I., Gasbarrini, A., Franceschi, F., & on behalf of GEMELLI AGAINST COVID 2019. (2022). Liver Injury in Patients Hospitalized for COVID-19: Possible Role of Therapy. Vaccines, 10(2), 192. https://doi.org/10.3390/vaccines10020192








