Seroepidemiology of SARS-CoV-2 Virus in Healthcare Workers before Circulation of the Omicron Sublineages BA.4/BA.5 in Vojvodina, Serbia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Laboratory Testing
2.2.1. Anti-SARS-CoV-2 QuantiVac ELISA Test
2.2.2. LIAISON® SARS-CoV-2 TrimericS Test
2.3. Data Analysis
2.4. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, S.E.; Deeks, S.L.; Hatchette, T.F.; Crowcroft, N.S. The Role of Seroepidemiology in the Comprehensive Surveillance of Vaccine-Preventable Diseases. CMAJ 2012, 184, E70–E76. [Google Scholar] [CrossRef] [PubMed]
- Ristić, M.; Milosavljević, B.; Vapa, S.; Marković, M.; Petrović, V. Seroprevalence of Antibodies against SARS-CoV-2 Virus in Northern Serbia (Vojvodina): A Four Consecutive Sentinel Population-Based Survey Study. PLoS ONE 2021, 16, e0254516. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Wang, J.-H.; Hsueh, P.-R. Population-Based Seroprevalence Surveys of Anti-SARS-CoV-2 Antibody: An up-to-Date Review. Int. J. Infect. Dis. 2020, 101, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Shields, A.; Faustini, S.E.; Perez-Toledo, M.; Jossi, S.; Aldera, E.; Allen, J.D.; Al-Taei, S.; Backhouse, C.; Bosworth, A.; Dunbar, L.A.; et al. SARS-CoV-2 Seroprevalence and Asymptomatic Viral Carriage in Healthcare Workers: A Cross-Sectional Study. Thorax 2020, 75, 1089–1094. [Google Scholar] [CrossRef]
- Chen, W.-Q.; Lu, C.-Y.; Wong, T.-W.; Ling, W.-H.; Lin, Z.-N.; Hao, Y.-T.; Liu, Q.; Fang, J.-Q.; He, Y.; Luo, F.-T.; et al. Anti-SARS-CoV Immunoglobulin G in Healthcare Workers, Guangzhou, China. Emerg. Infect. Dis. 2005, 11, 89–94. [Google Scholar] [CrossRef]
- Ministry of Health of the Republic of Serbia Coronavirus COVID-19. Available online: https://covid19.rs/eng-coronavirus-analytics/ (accessed on 26 June 2022).
- Pustahija, T.; Ristić, M.; Medić, S.; Vuković, V.; Štrbac, M.; Rajčević, S.; Patić, A.; Petrović, V. Epidemiological Characteristics of COVID-19 Travel-Associated Cases in Vojvodina, Serbia, during 2020. PLoS ONE 2021, 16, e0261840. [Google Scholar] [CrossRef]
- Petrović, V.; Vuković, V.; Marković, M.; Ristić, M. Early Effectiveness of Four SARS-CoV-2 Vaccines in Preventing COVID-19 among Adults Aged ≥ 60 Years in Vojvodina, Serbia. Vaccines 2022, 10, 389. [Google Scholar] [CrossRef]
- The Government of the Republic of Serbia COVID-19. Available online: https://www.srbija.gov.rs/sekcija/en/151926/covid-19.php (accessed on 9 June 2021).
- Euroimmun Medizinische Labordiagnostika AG Anti-SARS-CoV-2 QuantiVac ELISA IgG. Lübeck, Germany: EuroimmunMedizinischeLabordiagnostika AG. Available online: https://www.coronavirus-diagnostics.com/documents/Indications/Infections/Coronavirus/EI_2606_D_UK_E.pdf (accessed on 27 July 2022).
- WHO. First WHO International Standard for Anti-SARS-CoV-2 Immunoglobulin (Human). NIBSC Code: 20/136. Instructions for Use. Version 2.0. Available online: https://www.nibsc.org/documents/ifu/20-136.pdf (accessed on 27 July 2022).
- Diasorin, S.p.A. LIAISON ® SARS-CoV-2 TrimericS IgG. Saluggia, Italy: Diasorin S.p.A. Available online: https://www.diasorin.com/sites/default/files/allegati_prodotti/liaisonr_sars-cov-2_trimerics_igg_assay_m0870004408_a_lr_0.pdf (accessed on 27 July 2022).
- Petrović, V.; Vuković, V.; Patić, A.; Marković, M.; Ristić, M. Immunogenicity of BNT162b2, BBIBP-CorV and Gam-COVID-Vac Vaccines and Immunity after Natural SARS-CoV-2 Infection-A Comparative Study from Novi Sad, Serbia. PLoS ONE 2022, 17, e0263468. [Google Scholar] [CrossRef]
- Markovic-Denic, L.; Zdravkovic, M.; Ercegovac, M.; Djukic, V.; Nikolic, V.; Cujic, D.; Micic, D.; Pekmezovic, T.; Marusic, V.; Popadic, V.; et al. Seroprevalence in Health Care Workers during the Later Phase of the Second Wave: Results of Three Hospitals in Serbia, Prior to Vaccine Administration. J. Infect. Public Health 2022, 15, 739–745. [Google Scholar] [CrossRef]
- Iruretagoyena, M.; Vial, M.R.; Spencer-Sandino, M.; Gaete, P.; Peters, A.; Delgado, I.; Perez, I.; Calderon, C.; Porte, L.; Legarraga, P.; et al. Longitudinal Assessment of SARS-CoV-2 IgG Seroconversionamong Front-Line Healthcare Workers during the First Wave of the COVID-19 Pandemic at a Tertiary-Care Hospital in Chile. BMC Infect. Dis. 2021, 21, 478. [Google Scholar] [CrossRef]
- Pagen, D.M.E.; Brinkhues, S.; Dukers-Muijrers, N.H.T.M.; den Heijer, C.D.J.; Bouwmeester-Vincken, N.; Hanssen, D.A.T.; van Loo, I.H.M.; Savelkoul, P.H.M.; Hoebe, C.J.P.A. Exposure Factors Associated with SARS-CoV-2 Seroprevalence during the First Eight Months of the COVID-19 Pandemic in the Netherlands: A Cross-Sectional Study. PLoS ONE 2022, 17, e0268057. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Pandit, S.; Pokhrel, S.; Chhusyabaga, M.; Bista, P.; Bhatt, M.P.; Subedi, D.D.; Rijal, B.P. Anti-SARS-CoV-2 Antibody Screening in Healthcare Workers and Its Correlation with Clinical Presentation in Tertiary Care Hospital, Kathmandu, Nepal, from November 2020 to January 2021. Interdiscip. Perspect. Infect. Dis. 2022, 2022, 8515051. [Google Scholar] [CrossRef] [PubMed]
- El-Sokkary, R.H.; Daef, E.; El-Korashi, L.A.; Khedr, E.M.; Gad, D.; Mohamed-Hussein, A.; Zayed, N.E.; Mostafa, E.F.; Bahgat, S.M.; Hassany, S.M.; et al. Sero-Prevalence of Anti-SARS-CoV-2 Antibodies among Healthcare Workers: A Multicenter Study from Egypt. J. Infect. Public Health 2021, 14, 1474–1480. [Google Scholar] [CrossRef]
- Gelanew, T.; Seyoum, B.; Mulu, A.; Mihret, A.; Abebe, M.; Wassie, L.; Gelaw, B.; Sorsa, A.; Merid, Y.; Muchie, Y.; et al. High Seroprevalence of Anti-SARS-CoV-2 Antibodies among Ethiopian Healthcare Workers. BMC Infect. Dis. 2022, 22, 261. [Google Scholar] [CrossRef]
- Al-Naamani, K.; Al-Jahdhami, I.; Al-Tamtami, W.; Al-Amri, K.; Al-Khabori, M.; Al Sinani, S.; Said, E.A.; Omer, H.; Al-Bahluli, H.; Al-Ryiami, S.; et al. Prevalence and Persistence of SARS-CoV2 Antibodies among Healthcare Workers in Oman. J. Infect. Public Health 2021, 14, 1578–1584. [Google Scholar] [CrossRef]
- Haq, I.; Qurieshi, M.A.; Khan, M.S.; Majid, S.; Bhat, A.A.; Kousar, R.; Chowdri, I.N.; Qazi, T.B.; Lone, A.A.; Sabah, I.; et al. The Burden of SARS-CoV-2 among Healthcare Workers across 16 Hospitals of Kashmir, India-A Seroepidemiological Study. PLoS ONE 2021, 16, e0259893. [Google Scholar] [CrossRef]
- Wiggen, T.D.; Bohn, B.; Ulrich, A.K.; Stovitz, S.D.; Strickland, A.J.; Naumchik, B.M.; Walsh, S.; Smith, S.; Baumgartner, B.; Kline, S.; et al. SARS-CoV-2 Seroprevalence among Healthcare Workers. PLoS ONE 2022, 17, e0266410. [Google Scholar] [CrossRef]
- Kasztelewicz, B.; Janiszewska, K.; Burzyńska, J.; Szydłowska, E.; Migdał, M.; Dzierżanowska-Fangrat, K. Prevalence of IgG Antibodies against SARS-CoV-2 among Healthcare Workers in a Tertiary Pediatric Hospital in Poland. PLoS ONE 2021, 16, e0249550. [Google Scholar] [CrossRef] [PubMed]
- Alkurt, G.; Murt, A.; Aydin, Z.; Tatli, O.; Agaoglu, N.B.; Irvem, A.; Aydin, M.; Karaali, R.; Gunes, M.; Yesilyurt, B.; et al. Seroprevalence of Coronavirus Disease 2019 (COVID-19) among Health Care Workers from Three Pandemic Hospitals of Turkey. PLoS ONE 2021, 16, e0247865. [Google Scholar] [CrossRef]
- Dávila-Conn, V.; Soto-Nava, M.; Caro-Vega, Y.N.; Paz-Juárez, H.E.; García-Esparza, P.; Tapia-Trejo, D.; Pérez-García, M.; Belaunzarán-Zamudio, P.F.; Reyes-Terán, G.; Sierra-Madero, J.G.; et al. Seroepidemiology of SARS-CoV-2 in Healthcare Personnel Working at the Largest Tertiary COVID-19 Referral Hospitals in Mexico City. PLoS ONE 2022, 17, e0264964. [Google Scholar] [CrossRef]
- Houlihan, C.F.; Vora, N.; Byrne, T.; Lewer, D.; Heaney, J.; Moore, D.A.; Matthews, R.; Adam, S.; Enfield, L.; Severn, A.; et al. SARS-CoV-2 Virus and Antibodies in Front-Line Health Care Workers in an Acute Hospital in London: Preliminary Results from a Longitudinal Study. medRxiv 2020. [Google Scholar] [CrossRef]
- Eyre, D.W.; Lumley, S.F.; O’Donnell, D.; Campbell, M.; Sims, E.; Lawson, E.; Warren, F.; James, T.; Cox, S.; Howarth, A.; et al. Differential Occupational Risks to Healthcare Workers from SARS-CoV-2 Observed during a Prospective Observational Study. eLife 2020, 9, e60675. [Google Scholar] [CrossRef] [PubMed]
- Institute of Public Health of Serbia “Dr Milan Jovanović Batut”. Imunizacija [In Serbian]. Available online: https://www.batut.org.rs/index.php?category_id=186 (accessed on 5 December 2022).
- Andreano, E.; Paciello, I.; Piccini, G.; Manganaro, N.; Pileri, P.; Hyseni, I.; Leonardi, M.; Pantano, E.; Abbiento, V.; Benincasa, L.; et al. Hybrid Immunity Improves B Cells and Antibodies against SARS-CoV-2 Variants. Nature 2021, 600, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P.A. SARS-CoV-2 Reinfections: Overview of Efficacy and Duration of Natural and Hybrid Immunity. Environ. Res. 2022, 209, 112911. [Google Scholar] [CrossRef] [PubMed]
- Visci, G.; Zunarelli, C.; Mansour, I.; Porru, S.; De Palma, G.; Duval, X.; Monaco, M.G.L.; Spiteri, G.; Carta, A.; Lippi, G.; et al. Serological Response after SARS-CoV2 Vaccination in Healthcare Workers: A Multicenter Study. Med. Lav. 2022, 113, e2022022. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody Responses to SARS-CoV-2 in Patients with Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef]
- Ara, J.; Islam, M.S.; Quader, M.T.U.; Das, A.; Hasib, F.M.Y.; Islam, M.S.; Rahman, T.; Das, S.; Chowdhury, M.A.H.; Das, G.B.; et al. Sero-Prevalence of Anti-SARS-CoV-2 Antibodies in Chattogram Metropolitan Area, Bangladesh. medRxiv 2022. [Google Scholar] [CrossRef]
- Medić, S.; Anastassopoulou, C.; Lozanov-Crvenković, Z.; Vuković, V.; Dragnić, N.; Petrović, V.; Ristić, M.; Pustahija, T.; Gojković, Z.; Tsakris, A.; et al. Risk and Severity of SARS-CoV-2 Reinfections during 2020-2022 in Vojvodina, Serbia: A Population-Level Observational Study. Lancet Reg. Health Eur. 2022, 20, 100453. [Google Scholar] [CrossRef]
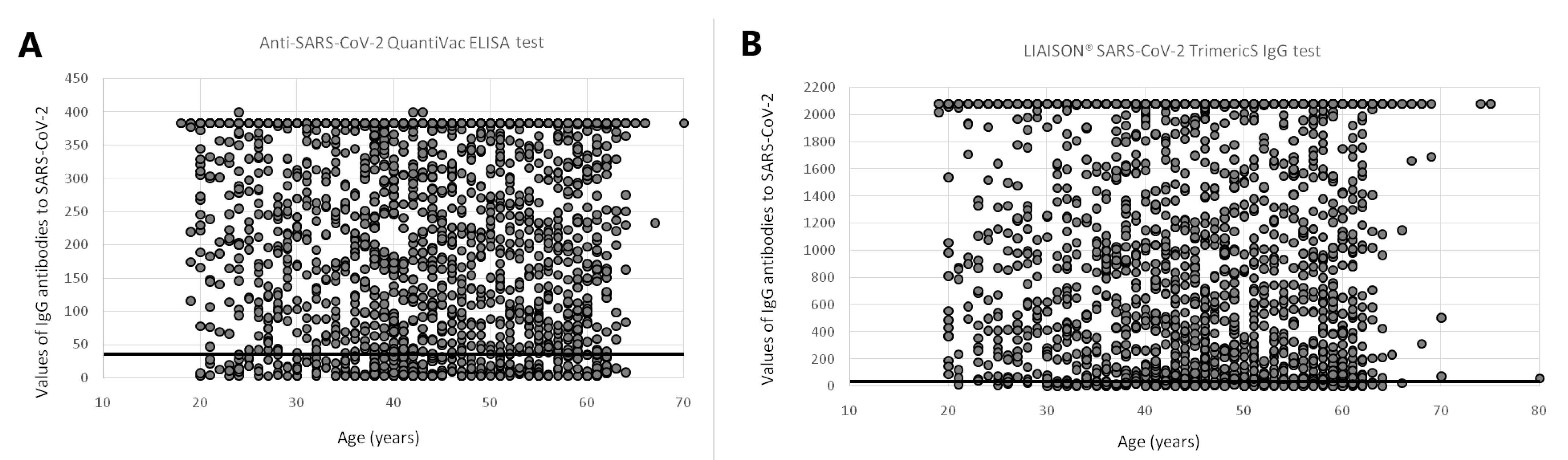

| All Participants | Anti-SARS-CoV-2 QuantiVac ELISA | LIAISON® SARS-CoV-2 TrimericS | p-Value 1 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Seroposi-tive | Seronegative | Seroprevalence % (95% CI) | Total | Seroposi-tive | Seronega-tive | Seroprevalence % (95% CI) | |||||||||||||
| n = 6936 | n = 3835 | n = 3537 | n = 298 | n = 3101 | n = 2911 | n = 190 | ||||||||||||||
| n | % | (95% CI) | n | % | n | % | n | % | 92.23 | 91.34–93.06 | n | % | n | % | n | % | 93.87 | 92.97–94.69 | 0.0079 | |
| Gender | ||||||||||||||||||||
| Male | 1143 | 16.48 | 15.56–17.32 | 606 | 15.80 | 561 | 15.86 | 45 | 15.10 | 92.57 | 90.18–94.53 | 537 | 17.32 | 502 | 17.24 | 35 | 18.42 | 93.48 | 91.05–95.42 | 0.5475 |
| Female | 5793 | 83.52 | 82.63–84.39 | 3229 | 84.20 | 2976 | 84.14 | 253 | 84.90 | 92.16 | 91.18–93.06 | 2564 | 82.68 | 2409 | 82.76 | 155 | 81.58 | 93.95 | 92.96–94.84 | 0.0082 |
| Age (year) | ||||||||||||||||||||
| 18–29 | 707 | 10.19 | 9.49–10.93 | 450 | 11.73 | 416 | 11.76 | 34 | 11.41 | 92.44 | 89.60–94.71 | 257 | 8.29 | 253 | 8.69 | 4 | 2.11 | 98.44 | 96.06–99.57 | 0.0007 |
| 30–39 | 1347 | 19.42 | 18.49–20.37 | 798 | 20.81 | 734 | 20.75 | 64 | 21.48 | 91.98 | 89.87–93.77 | 549 | 17.70 | 501 | 17.21 | 48 | 25.26 | 91.26 | 88.58–93.49 | 0.6383 |
| 40–49 | 2083 | 30.03 | 28.95–31.12 | 1099 | 28.66 | 1002 | 28.33 | 97 | 32.55 | 91.17 | 89.33–92.78 | 984 | 31.73 | 928 | 31.88 | 56 | 29.47 | 94.31 | 92.67–95.67 | 0.0061 |
| 50–59 | 2043 | 29.46 | 28.39–30.55 | 1098 | 28.63 | 1025 | 28.98 | 73 | 24.50 | 93.35 | 91.71–94.75 | 945 | 30.47 | 887 | 30.47 | 58 | 30.53 | 93.86 | 92.13–95.30 | 0.6391 |
| ≥60 | 756 | 10.90 | 10.18–11.66 | 390 | 10.17 | 360 | 10.18 | 30 | 10.07 | 92.31 | 89.20–94.75 | 366 | 11.80 | 342 | 11.75 | 24 | 12.63 | 93.44 | 90.40–95.75 | 0.5468 |
| Occupation | ||||||||||||||||||||
| Physician | 1241 | 17.89 | 16.99–18.81 | 617 | 16.09 | 588 | 16.62 | 29 | 9.73 | 95.30 | 93.32–96.83 | 624 | 20.12 | 603 | 20.71 | 21 | 11.05 | 96.63 | 94.90–97.90 | 0.2338 |
| Nurse | 3253 | 46.90 | 45.72–48.08 | 1917 | 49.99 | 1766 | 49.93 | 151 | 50.67 | 92.12 | 90.82–93.29 | 1336 | 43.08 | 1253 | 43.04 | 83 | 43.68 | 93.79 | 92.36–95.02 | 0.0698 |
| Pharmacist | 70 | 1.01 | 0.79–1.27 | 42 | 1.10 | 37 | 1.05 | 5 | 1.68 | 88.10 | 74.37–96.02 | 28 | 0.90 | 28 | 0.96 | 0 | 0.00 | 100 | 87.66–100 | 0.0600 |
| Dentist | 93 | 1.34 | 1.08–1.64 | 29 | 0.76 | 29 | 0.82 | 0 | 0.00 | 100 | 88.06–100 | 64 | 2.06 | 62 | 2.13 | 2 | 1.05 | 96.88 | 89.17–99.62 | 0.3389 |
| Laboratory technician | 411 | 5.93 | 5.39–6.51 | 207 | 5.40 | 192 | 5.43 | 15 | 5.03 | 92.75 | 88.33–95.88 | 204 | 6.58 | 186 | 6.39 | 18 | 9.47 | 91.18 | 86.42–94.69 | 0.5586 |
| Other medical staff | 221 | 3.19 | 2.79–3.63 | 111 | 2.89 | 107 | 3.03 | 4 | 1.34 | 96.40 | 91.04-99.01 | 110 | 3.55 | 102 | 3.50 | 8 | 4.21 | 92.73 | 86.18-96.81 | 0.2296 |
| Support non-medical staff | 1647 | 23.75 | 22.75-24.77 | 912 | 23.78 | 818 | 23.13 | 94 | 31.54 | 89.69 | 87.53-91.59 | 735 | 23.70 | 677 | 23.26 | 58 | 30.53 | 92.11 | 89.92-93.95 | 0.0918 |
| Healthcare level | ||||||||||||||||||||
| Primary | 2823 | 40.70 | 39.54-41.87 | 1158 | 30.20 | 1060 | 29.97 | 98 | 32.89 | 91.54 | 89.79-93.08 | 1665 | 53.69 | 1566 | 53.80 | 99 | 52.11 | 94.05 | 92.80-95.14 | 0.0101 |
| Secondary | 2026 | 29.21 | 28.14-30.30 | 1443 | 37.63 | 1334 | 37.72 | 109 | 36.58 | 92.45 | 90.96-93.76 | 583 | 18.80 | 541 | 18.58 | 42 | 22.11 | 92.80 | 90.39-94.76 | 0.7860 |
| Tertiary | 2087 | 30.09 | 29.01-31.18 | 1234 | 32.18 | 1143 | 32.32 | 91 | 30.54 | 92.63 | 91.03-94.02 | 853 | 27.51 | 804 | 27.62 | 49 | 25.79 | 94.26 | 92.48-95.72 | 0.1434 |
| Comorbidities | ||||||||||||||||||||
| Hypertension | 186 | 2.68 | 2.31-3.09 | 81 | 2.11 | 80 | 2.26 | 1 | 0.34 | 98.77 | 93.32-99.97 | 105 | 3.39 | 101 | 3.47 | 4 | 2.11 | 96.19 | 90.53-98.95 | 0.2819 |
| Chronic pulmonary disease | 210 | 3.03 | 2.64-3.46 | 119 | 3.10 | 106 | 3.00 | 13 | 4.36 | 89.08 | 82.05-94.06 | 91 | 2.93 | 87 | 2.99 | 4 | 2.11 | 95.60 | 89.12-98.79 | 0.0868 |
| Cardiovascular disease | 441 | 6.36 | 5.80-6.96 | 253 | 6.60 | 230 | 6.50 | 23 | 7.72 | 90.91 | 86.67-94.15 | 188 | 6.06 | 179 | 6.15 | 9 | 4.74 | 95.21 | 91.10–97.79 | 0.0855 |
| Diabetes | 192 | 2.77 | 2.40–3.18 | 106 | 2.76 | 101 | 2.86 | 5 | 1.68 | 95.28 | 89.33–98.45 | 86 | 2.77 | 78 | 2.68 | 8 | 4.21 | 90.70 | 82.49–95.90 | 0.2103 |
| Obesity | 32 | 0.46 | 0.31–0.65 | 13 | 0.34 | 12 | 0.34 | 1 | 0.34 | 92.31 | 63.97–99.81 | 19 | 0.61 | 19 | 0.65 | 0 | 0.00 | 100 | 82.35–100 | 0.2268 |
| Malignancy | 90 | 1.30 | 1.05–1.60 | 50 | 1.30 | 44 | 1.24 | 6 | 2.01 | 88.00 | 75.69–95.47 | 40 | 1.29 | 38 | 1.31 | 2 | 1.05 | 95.00 | 83.08–99.39 | 0.2489 |
| Other chronic disease | 389 | 5.61 | 5.08–6.18 | 214 | 5.58 | 196 | 5.54 | 18 | 6.04 | 91.59 | 87.03–94.94 | 175 | 5.64 | 162 | 5.57 | 13 | 6.84 | 92.57 | 87.63–95.98 | 0.7229 |
| Without comorbidity | 5396 | 77.80 | 76.80–78.77 | 2999 | 78.20 | 2768 | 78.26 | 231 | 77.52 | 92.30 | 91.29–93.23 | 2397 | 77.30 | 2247 | 77.19 | 150 | 78.95 | 93.74 | 92.69–94.68 | 0.3382 |
| Contact with COVID-19 patients at workplace | ||||||||||||||||||||
| Yes | 2812 | 40.54 | 39.38–41.71 | 1550 | 40.42 | 1448 | 40.94 | 102 | 34.23 | 93.42 | 92.07–94.60 | 1262 | 40.70 | 1196 | 41.09 | 66 | 34.74 | 94.77 | 93.39–95.93 | 0.1331 |
| No | 4124 | 59.46 | 58.29–60.62 | 2285 | 59.58 | 2089 | 59.06 | 196 | 65.77 | 91.42 | 90.20–92.54 | 1839 | 59.30 | 1715 | 58.91 | 124 | 65.26 | 93.26 | 92.02–94.36 | 0.0282 |
| Previously having laboratory-confirmed COVID-19 | ||||||||||||||||||||
| Yes | 3905 | 56.30 | 55.12–57.47 | 2170 | 56.58 | 2054 | 58.07 | 116 | 38.93 | 94.65 | 93.62–95.56 | 1735 | 55.95 | 1674 | 57.51 | 61 | 32.11 | 96.48 | 95.50–97.30 | 0.0063 |
| No | 3031 | 43.70 | 42.53–44.88 | 1665 | 43.42 | 1483 | 41.93 | 182 | 61.07 | 89.07 | 87.47–90.53 | 1366 | 44.05 | 1237 | 42.49 | 129 | 67.89 | 90.56 | 88.88–92.06 | 0.1786 |
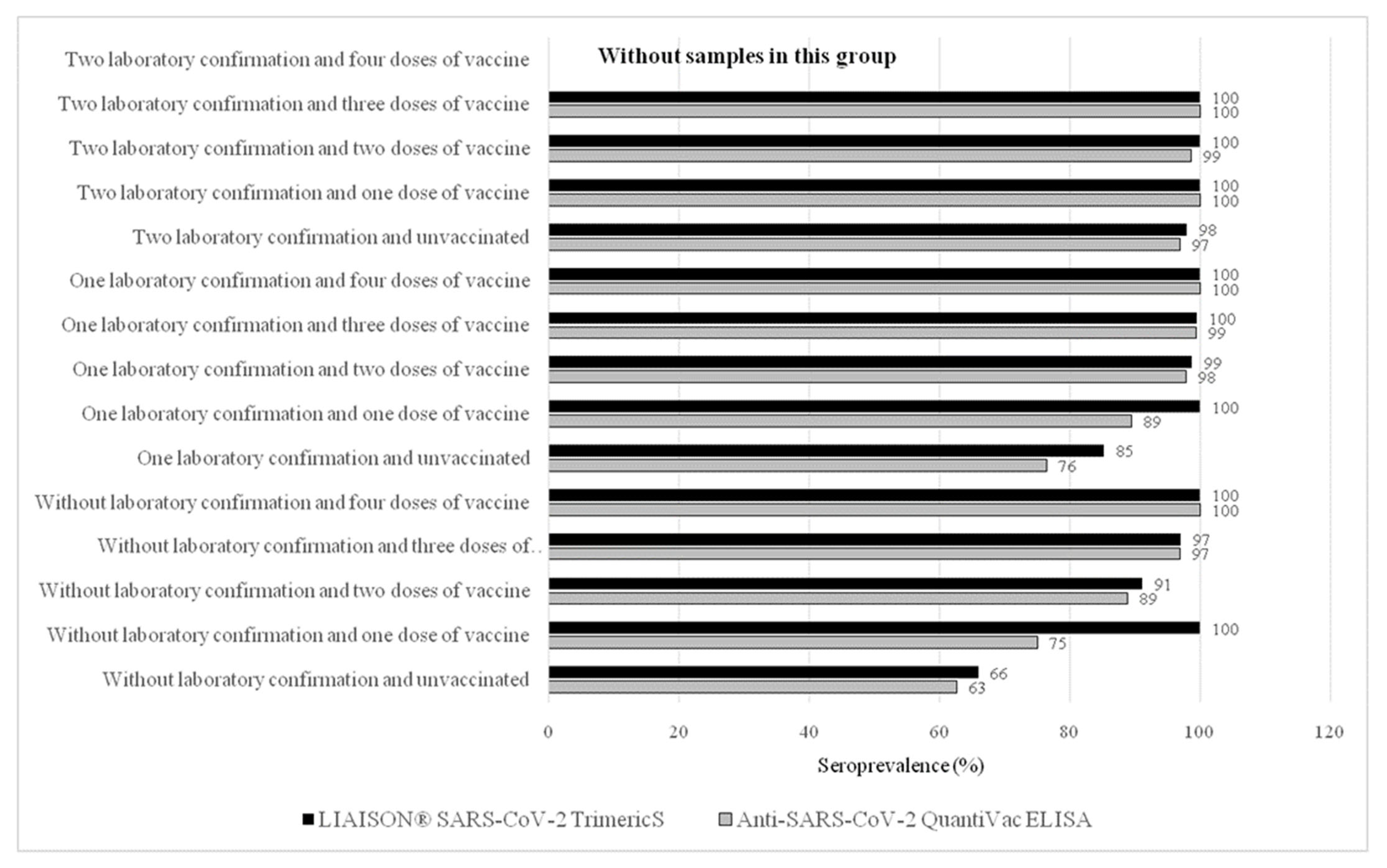
| Vaccinated against COVID-19 with at least one dose of vaccine | ||||||||||||||||||||
| Yes | 5485 | 79.08 | 78.10–80.03 | 3063 | 79.87 | 2965 | 83.83 | 98 | 32.89 | 96.80 | 96.11–97.39 | 2422 | 78.10 | 2365 | 81.24 | 57 | 30.00 | 97.65 | 96.97–98.22 | 0.0592 |
| No | 1451 | 20.92 | 19.97–21.90 | 772 | 20.13 | 572 | 16.17 | 200 | 67.11 | 74.09 | 70.85–77.15 | 679 | 21.90 | 546 | 18.76 | 133 | 70.00 | 80.41 | 77.22–83.33 | 0.0043 |
| Anti-SARS-CoV-2 QuantiVac ELISA | LIAISON® SARS-CoV-2 TrimericS | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude OR (95% CI) | p-Value | Adjusted OR a,b (95% CI) | p-Value b | Crude OR (95% CI) | p-Value | Adjusted OR a,b (95% CI) | p-Value b | |
| Gender | ||||||||
| Male | 1.06 (0.76–1.47) | 0.7297 | Referent | |||||
| Female | Referent | 1.08 (0.74–1.58) | 0.6781 | |||||
| Age (year) | ||||||||
| 18–29 | 1.18 (0.79–1.78) | 0.4150 | 6.06 (2.16–16.99) | 0.0006 | 6.22 (2.21–17.47) | 0.0005 | ||
| 30–39 | 1.11 (0.80–1.54) | 0.5341 | Referent | |||||
| 40–49 | Referent | 1.59 (1.06–2.34) | 0.0237 | 1.57 (1.05–2.36) | 0.0274 | |||
| 50–59 | 1.36 (0.99–1.86) | 0.0569 | 1.47 (0.98–2.18) | 0.0598 | ||||
| ≥60 | 1.17 (0.76–1.78) | 0.4913 | 1.367 (0.82–2.27) | 0.2305 | ||||
| Occupation | ||||||||
| Physician | 2.74 (1.00–7.49) | 0.0495 | 2.78 (1.45–5.33) | 0.0021 | 2.72 (1.39–5.34) | 0.0036 | ||
| Nurse | 1.58 (0.61–4.08) | 0.3443 | 1.46 (0.86–2.49) | 0.1628 | ||||
| Pharmacist | Referent | 5.65 (0.33–96.45) | 0.2313 | |||||
| Dentist | 8.65 (0.46–162.87) | 0.1496 | 3.00 (0.68–13.30) | 0.1481 | ||||
| Laboratory technician | 1.73 (0.59–5.05) | 0.3162 | Referent | |||||
| Other medical staff | 3.61 (0.92–14.18) | 0.0654 | 1.23 (0.52–2.94) | 0.6348 | ||||
| Support non-medical staff | 1.18 (0.45–3.07) | 0.7402 | 1.13 (0.65–1.96) | 0.6659 | ||||
| Healthcare level | ||||||||
| Primary | Referent | 1.23 (0.84–1.78) | 0.2816 | |||||
| Secondary | 1.13 (0.85–1.50) | 0.3948 | Referent | |||||
| Tertiary | 1.16 (0.86–1.56) | 0.3244 | 1.27 (0.83–1.95) | 0.2658 | ||||
| Comorbidities | ||||||||
| Hypertension | 10.91 (1.27–93.54) | 0.0293 | 3.98 (0.41–38.53) | 0.2336 | 2.59 (0.75–8.91) | 0.1313 | ||
| Chronic pulmonary disease | 1.11 (0.40–3.11) | 0.8399 | 2.23 (0.65–7.70) | 0.2042 | ||||
| Cardiovascular disease | 1.36 (0.52–3.54) | 0.5243 | 2.04 (0.76–5.48) | 0.1576 | ||||
| Diabetes | 2.75 (0.80–9.50) | 0.1088 | Referent | |||||
| Obesity | 1.64 (0.18–14.93) | 0.6624 | 4.22 (0.23–76.37) | 0.3294 | ||||
| Malignancy | Referent | 1.95 (0.39–9.63) | 0.4130 | |||||
| Other chronic disease | 1.48 (0.56–3.96) | 0.4292 | 1.28 (0.51–3.21) | 0.6016 | ||||
| Without comorbidity | 1.63 (0.69–3.87) | 0.2650 | 1.54 (0.73–3.24) | 0.2593 | ||||
| Contact with COVID-19 patients at workplace | ||||||||
| Yes | 1.33 (1.04–1.71) | 0.0238 | 1.46 (1.13–1.90) | 0.0041 | 1.31 (0.96–1.78) | 0.0852 | ||
| No | Referent | Referent | ||||||
| Previously having laboratory-confirmed COVID-19 | ||||||||
| Yes | 2.17 (1.71–2.77) | <0.0001 | 2.18 (1.71–2.78) | <0.0001 | 2.86 (2.09–3.91) | <0.0001 | 2.90 (2.12–3.97) | <0.0001 |
| No | Referent | Referent | ||||||
| Vaccinated against COVID-19 with at least one dose of vaccine | ||||||||
| Yes | 10.58 (8.18–13.69) | <0.0001 | 11.34 (8.70–14.78) | <0.0001 | 10.11 (7.31–13.97) | <0.0001 | 11.05 (7.92–15.40) | <0.0001 |
| No | Referent | Referent | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ristić, M.; Vuković, V.; Patić, A.; Marković, M.; Petrović, V. Seroepidemiology of SARS-CoV-2 Virus in Healthcare Workers before Circulation of the Omicron Sublineages BA.4/BA.5 in Vojvodina, Serbia. Vaccines 2022, 10, 2168. https://doi.org/10.3390/vaccines10122168
Ristić M, Vuković V, Patić A, Marković M, Petrović V. Seroepidemiology of SARS-CoV-2 Virus in Healthcare Workers before Circulation of the Omicron Sublineages BA.4/BA.5 in Vojvodina, Serbia. Vaccines. 2022; 10(12):2168. https://doi.org/10.3390/vaccines10122168
Chicago/Turabian StyleRistić, Mioljub, Vladimir Vuković, Aleksandra Patić, Miloš Marković, and Vladimir Petrović. 2022. "Seroepidemiology of SARS-CoV-2 Virus in Healthcare Workers before Circulation of the Omicron Sublineages BA.4/BA.5 in Vojvodina, Serbia" Vaccines 10, no. 12: 2168. https://doi.org/10.3390/vaccines10122168
APA StyleRistić, M., Vuković, V., Patić, A., Marković, M., & Petrović, V. (2022). Seroepidemiology of SARS-CoV-2 Virus in Healthcare Workers before Circulation of the Omicron Sublineages BA.4/BA.5 in Vojvodina, Serbia. Vaccines, 10(12), 2168. https://doi.org/10.3390/vaccines10122168







