Abstract
Between the first case of COVID-19 in January 2020 and the end of 2021, Thailand experienced four waves of the epidemic. The third and fourth waves were caused by the alpha and delta strains from April 2021 to November 2021. Serosurveillance studies provide snapshots of the true scale of the outbreak, including the asymptomatic infections that could not be fully captured by a hospital-based case detection system. We aimed to investigate the distribution of SARs-CoV-2 seroprevalence in unvaccinated adults after the delta wave outbreak. From November to December 2021, we conducted a cross-sectional survey study in 12 public health areas (PHAs) across Thailand. A total of 26,717 blood samples were collected and tested for SARs-CoV-2 antibodies (anti-S IgG) using a qualitative immunoassay. The results showed that seropositive prevalence in this cohort was 1.4% (95% CI: 1.24 to 1.52). The lowest prevalence was in the northern region (PHA 1) and in central Thailand (PHA 3) at 0.4% (95% CI: 0.15 to 0.95), while the highest was in the southern region of Thailand (PHA 12) at 5.8% (95% CI: 4.48 to 7.29). This seropositive prevalence was strikingly lower than the reports from other countries. Our serosurveillance results suggest that the vaccination of unvaccinated groups should be accelerated, especially in the public health areas with the lowest seroprevalence.
1. Introduction
The coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1,2]. This illness led to globally catastrophic outcomes, resulting in more than four million deaths as of September 2021 [3]. In Thailand, the first wave of COVID-19 hit in late March 2020, and the fourth wave began in the form of the delta variant during mid-2021 [4]. As a result, public health measures and movement restrictions, such as lockdowns, school closures, working from home, and social distancing, were implemented to control the transmission of the disease.
Several techniques are employed for COVID-19 diagnosis. Considering the breakthroughs in medical diagnosis, nucleic-acid-based approaches are destined to become a rapid and reliable method. Among these, real-time reverse-transcriptase PCR (real-time RT-PCR) is preferred as a gold standard due to its advantages as a specific and simple qualitative assay [5]. Samples from the upper respiratory tract, including nasopharyngeal swabs, can be tested using real-time RT-PCR.
The World Health Organization (WHO) estimated that around 80% of COVID-19 cases are asymptomatic or mild, 15% are severe, and 5% are critical, requiring a ventilator [6]. Pre-symptomatic or asymptomatic cases have a prolonged period of viral shedding and still transmit infections [7,8]; unfortunately, these cases do not undergo real-time RT-PCR testing. Therefore, the infection rate reported based on real-time RT-PCR testing of the population cannot fully reflect the COVID-19 outbreak situation. To investigate the true nature of an outbreak, serological surveys should be implemented to assess the population infected with SARS-CoV-2, including asymptomatic and symptomatic cases [9].
In Thailand, serological studies were conducted before the delta wave. A study among non-healthcare workers with a high potential of exposure to SARS-CoV-2 in the northern regions (the Chiang Mai and Lamphun provinces) during the second wave of the outbreak (November 2020–January 2021) reported 0.9% (n = 1651) positive results for antibody tests [10]. Another study in healthcare workers in the Bangkok metropolitan area, and the western and eastern regions of Thailand from January to March 2021 reported that the positive rate for SARS-Cov-2 IgG-spike antibodies was 0.2% (n = 600) [11]. However, no data from serological survey studies conducted after the outbreak of the fourth wave in Thailand are available, while several countries reported various prevalence rates of COVID-19 seropositivity after the delta outbreak. In the United States, the seroprevalence based on blood donation testing reached 94.7% (n = 2,408,093) by December 2021 [12]. In Canada, the antibody-positive rate among blood donors between November 13 and 24, 2021 was 100% (n = 9018) [13]. In Scotland, the seroprevalence in people attending community healthcare settings from 15 November to 19 December 2021 was estimated to be 87.4% (n = 2816) [14]. In South Africa, a study on blood donors in eight provinces from 8 to 12 November 2021 found that the antibody-positive rate was about 71.1% (n = 3395) [15].
To investigate the true number of COVID-19 infection cases after the delta outbreak in Thailand, a study was performed among unvaccinated Thais to determine SARS-CoV-2 seroprevalence between November and December 2021. This survey, by determining the prevalence of natural infections, could aid the government in preparing effective strategies for COVID-19 prevention in the next waves of the pandemic.
2. Materials and Methods
2.1. Study Design and Setting
From November to December 2021, we conducted a cross-sectional survey study in 12 public health areas across Thailand. We approached subjects who visited COVID-19 vaccination centers regarding participation in this project. Enrolled subjects had to be aged between 18 and 60 years old, with no history of COVID-19 illness or vaccination. After providing written informed consent, the participants were bled and interviewed to obtain information on gender, occupation, place of residence, and number of household members. SARs-CoV-2 antibody status tests were administered to all the subjects individually.
2.2. Sampling Plan Strategies
People were sampled after the 4th pandemic wave in Thailand. Apparently, this was performed rather proportionally across the 13 health–administrative Thai regions. Subjects were enrolled based on voluntary participation at vaccination points: 40,694 people coming in were informed to participate in the project; in addition, requests for personnel data and blood donations were put forward if they met the basic criterion of not being vaccinated up to the point of participation, and not being clinically ill to the best of their knowledge with COVID-19. The summary for the sampling plane is show in Figure 1.
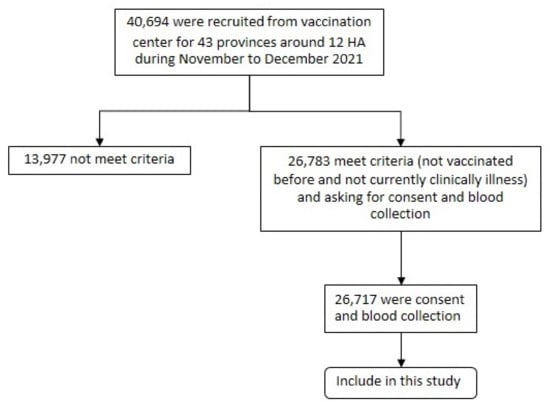
Figure 1.
The sampling strategy: in total, 40,694 were enrolled based on voluntary participation at vaccination center. Only 26,783 met the criteria and were asked to donate a blood sample.
2.3. Blood Collection and Processing
Blood samples (3–5 mL) were separated into serum samples with cold chains, and aliquots were placed into two microtubes: one for SARs-CoV-2 antibody testing and another for back up. All the specimens were kept at −20 °C until laboratory testing was performed.
2.4. Laboratory Testing
2.4.1. SARS-CoV-2 Antibody Testing by In-House Immunoassay (ELISA)
In-house immunoassay testing was conducted at the laboratory of Medical Life Sciences Institute. The ELISA microplate (Thermo Scientific, Roskilde, Denmark) was coated over-night at 4 °C with 0.02 ug of SARS-CoV-2 spike RBD proteins per well and further washed with 400 µL of wash buffer (PBST; 0.02% in phosphate-buffered saline) 3 times. Then, the ELISA microplate was blocked with 300 µL/well of blocking buffer (5% skim milk in PBST) at ambient temperature for 1 h; then, 50 µL/well of the test and control serum (1:200 dilution) was added into the microplate, and incubated at ambient temperatures for 1 h. After 3-time washing, 50 µL of 1:10,000 diluted polyclonal rabbit anti-human IgG conjugated HRP (Dako, cat no. P0214) was added. The microplate was incubated for 1 h at ambient temperatures and washed 5 times. After that, 50 µL of TMB peroxidase substrate (SeraCare, Milford, MA, USA) was added and further incubated in the dark at ambient temperatures for 30 min. The reaction was stopped by adding 50 µL of 1 M H2SO4, and the optical density (OD) was read at 450/570 nm with an ELISA microplate reader (TECAN, Männedorf, Switzerland). Finally, the OD ratio was calculated by dividing the OD of the sample by the OD of the negative control.
The performance of the in-house ELISA was evaluated by testing with 173 confirmed positive samples and 228 confirmed negative samples, and compared to the commercial assay (Quant IgG II, Abbott Ireland, Sligo, Ireland). Using a cut-off value of 1.5, which is determined as the mean values of OD ratio derived from confirmed negative samples plus two standard deviations, demonstrated a sensitivity and specificity of 100% and 95.61%, respectively. The positive samples were further confirmed by the commercial quantitative test.
2.4.2. Quantitative for SARS-CoV-2
Individual serum was quantified for SARS-CoV-2 antibodies relative to S1 SARS-CoV-2 subunit spike proteins by a commercial assay (Quant IgGII, Abbott Ireland, Sligo, Ireland), which is a chemiluminescent microparticle immunoassay (CMIA), run on automatic analyzer with the ARCHITECT I System (Abbott, Abbott Park, IL, USA). The reportable range of this kit was 6.8–80,000 Abbott Arbitrary Unit (AU/mL). The correlation level of the antibody when compared with WHO’s International Standard (NIBSC code 20-136) can be converted into a binding antibody unit (BAU/mL) by multiplying 0.142 at the 0.999 correlation level. The sample, which has over 50 AU/mL, is considered positive for SARS-CoV-2 antibodies.
2.5. Data Collection and Data Analysis
Individual data were collected in case record form (CRF); we collected the participants’ characteristics data, such as gender, age, occupation, subject’s residence area (public health area (PH) 1 to 12), body mass index, underlying disease, the number of people the subject lived with, and close exposure to COVID-19 cases.
3. Results
3.1. Participant Data
From November to December 2021, 40,694 people were asked to participate in the project based on voluntary participation, and they met the basic criterion of not being vaccinated up until that point and not being clinically ill. Participants numbering 26,783 were enrolled; however, those providing consent and who were willing to donate blood samples resulted in a final number of 26,717 participants. The median age of participants was 31 years (IQR: 25–50); 51.9% were female; 53.2% were within the normal body mass index; most (79.4%) did not have underlying diseases; 64.7% had a non-salary base occupation; 55.6% stayed with 3–5 persons in the same house; and only 1.7% came in close contact with COVID-19 cases (the data on the participant’s characteristics are shown in Table 1). The number of participants in each public health area ranged from 1084 to 4084, as shown in Table 2.

Table 1.
Participant characteristics.

Table 2.
Percentage of seropositive participants by the geography of Thailand.
3.2. Serological Test Result
A total of 26,717 blood samples were interpreted as serological test results, as shown in the flowchart in Figure 2; 367 clinical specimens tested positive for anti-SARS-CoV-2 antibodies, which indicates a 1.4% seroprevalence in this investigation (Table 2). The distribution of seropositive results and the data on participant characteristics are shown in Table 2 and Table 3, respectively. Public health area 1 in the northern region and public health area 3 in the central region indicate the lowest seropositive cases at 0.4% (95% CI: 0.15 to 0.95), while public health area 12 in the southern region hits the highest seropositive peak at 5.8% (95% CI: 4.48 to 7.29).
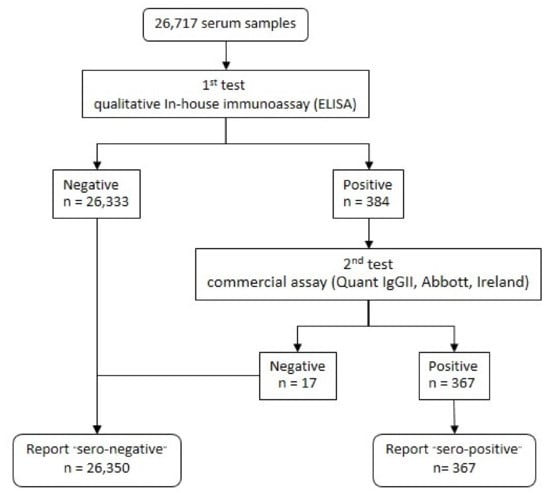
Figure 2.
A flowchart of the interpreted sample results of the 1st and 2nd SARS-CoV-2 antibody tests.

Table 3.
SARS-CoV-2 IgG serological test result.
3.3. Seroprevalence by Geographic Data
This survey study employed samples from Thai people who visited vaccination points and those that voluntarily agreed to participate in the cross-sectional study of epidemiological analyses, as shown in Figure 3. The 12 public health areas are categorized by geographic region: northern, northeastern, central, and southern regions. To begin with, in the northern region, clinical specimens were collected from four provinces from a total of eight provinces in public health area 1, including Chaing Rai, Lampang, Phayao, and Lamphun; in addition, the results show a 0.4% seropositive result (6/1416), whereas Phetchabun and Pitsanulok from public health area 2 have four-times-higher seropositive results at 1.6% (24/1431). The central region consists of public health area 3, 4, 5, and 6. The lowest seropositive results belong to public health area 3 at 0.4% (6/1366), with collected specimens from Nakhon sawan and Uthai Thani. In contrast, public health areas 4, 5, and 6 demonstrated results of 2.5% (34/1382), 2.7% (29/1084), and 2.8% (70/2517), respectively. Public health areas 7, 8, 9, and 10 are located in the northeastern region; and their seropositive rates are 1.1% (40/3726), 0.9% (27/3137), 0.9% (35/4084), and 0.5% (17/3702), respectively. Furthermore, the southern zone comprises public health areas 11 and 12. Public health area 11 shows 0.8% in terms of seropositivity results. Distinctly, public health area 12 displays the greatest seroprevalence rate at 5.8%.
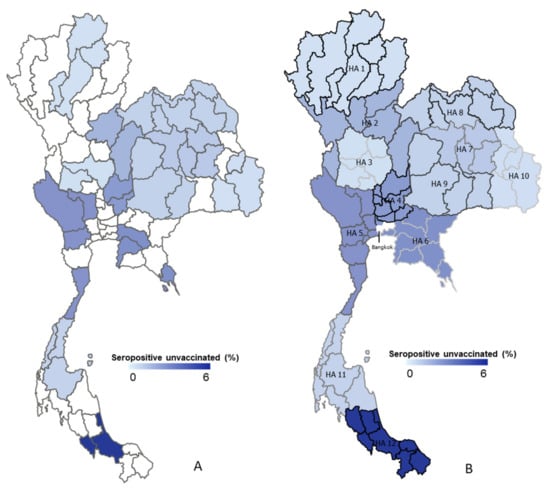
Figure 3.
Mapping 13 public health areas and seropositive distributions in Thailand. (A) The random province sites around Thailand that show each HA (see Appendix A Table A1). (B) The density of seropositivity in each area of public health, which ranges from 0% to 6% seropositivity, is demonstrated by a choropleth map. HA, health area.
4. Discussion
This cross-sectional survey aimed to study the seroprevalence of IgG SARS-CoV-2 antibodies in order to estimate the burden of infections during the late-fourth-waves of the pandemic in Thailand (November–December 2021). This period of time shows the decreasing number of confirmed COVID-19 cases (approximately 5000 cases/day) compared to the highest infection of the fourth wave in August 2021 (approximately 20,000 cases/day) [3]. In this study, serum samples were collected from unvaccinated donors who did not have historical reports of SARS-CoV-2 infections: 26,717 persons. These participants should be illustrative of the naïve immunity that Thai subjects have; however, seropositive results in these groups are characterized as asymptomatic COVID-19 patients. We found a seroprevalence of 1.4% in the studies. This prevalence increases from the beginning of the fourth wave (April 2021) with 0.2% seropositive results [11,16].
There were only minor differences in seropositivity between sexes, age groups, and BMI categories. However, seroprevalence in women was slightly higher than men. The seroprevalence in age groups ranged from 1.2 to 1.6%. The lowest rate of seropositivity was observed in the 18–27 age group and the highest rate was in the 28–37 age group. These findings can be partially explained by sociological data from a national survey and are related to the highest number of COVID-19 patients in the same age group during the fourth wave (April–May 2021) [17]. The highest seropositivity prevalence was observed in persons with BMI > 30 kg/m2. This result suggested that BMI may be related to SARS-CoV-2 infections; moreover, there are supporting data from UK that BMI is related to SAR-CoV-2 seropositive results and COVID-19-related death [18].
In Thailand, the ministry of public health established 13 health areas for public health management. This governing structure is becoming more prominent during responses to the pandemic. The health regions with a high number of tourism activity or immigrant workers are susceptible to COVID-19 outbreaks, and reflected in the better responses observed in disease control. Health regions with exposure to border areas include health regions 1, 2, 4, and 5 in the north of Thailand, which are located next to Myanmar; health region 6, located next to Cambodia; and health region 12, located next to Malaysia. These health regions are the primary areas responding to the initial clusters of COVID-19. At the end of 2020, immigrant worker outbreaks were detected in health region 5, where immigrant workers from Myanmar were displaced from their countries. As a result of immigrant workers coming from Cambodia in mid-2021, the SARS-CoV-2 delta variant was introduced in Thailand. Afterward, the delta variants spread from Bangkok across the country during the latter half of 2021. On the other hand, the SARS-CoV-2 beta variant circulated concomitantly in health region 12, which is extended from Malaysia. With mixed SARS-CoV-2 variants, the seropositivity rate in various health regions supported the epidemiology, while the immunoassay used in this study could not discriminate the effects of various strains due to the non-specificity of the anti-S against various SARS-CoV-2 variants of concern (VOCs). The detailed neutralization-based assay may be able to describe more details with respect to the increasing viral infections and immune escapes [19,20].
However, Bangkok, which inhibits public health area 13, was not included in this serosurveillance study because a number of unvaccinated people were in the minor age group. In addition, Bangkok had a 98.5% vaccination coverage and a significant number of SARS-CoV-2-infected cases. As a result, estimating seroprevalences in the Bangkok region was insufficient, and vaccination may have resulted in a larger true prevalence of seropositivity.
5. Conclusions
After the fourth wave of the COVID-19 pandemic in Thailand (November–December 2021), our study estimated that seroprevalence is around 58.7% for unvaccinated Thai subjects (illustrated for asymptomatic cases), confirmatory cases by RT-PCR (illustrated for infected cases), and vaccination (illustrated for immunized cases), which shows that Thai subjects had seroprevalence rates that are lower than those of some developed countries; for example, 83% seroprevalence is observed in the United States of America and 89% is observed in Germany [20,21]. The advantage of our study is that it is the largest study of the seroprevalence of anti-SARS-CoV-2 IgG antibodies in Thailand. According to the WHO population-based age-stratified seroepidemiological investigation protocol, the major strengths of our study include size and coverage. Confirmed COVID-19 patients comprise 2.6% of the Thai population; meanwhile, asymptomatic patients that are not included in the detection system comprise around 1.4%.
Author Contributions
Conceptualization, S.M. and S.S. (Supakit Sirilak); methodology, S.M. and P.D.; software, N.T.; validation, S.M., W.P. and P.D.; formal analysis, S.M., W.P. and N.T.; investigation, K.J. (Kritchai Juntaped), T.C., J.S., J.C., R.W., N.C., P.C., K.M., S.T., K.S., N.S., K.J. (Kodcharad Jongpitisub), P.P., L.P. and S.S. (Sakulrat Soonthorncharttrawat); resources, P.D. and N.P.; data curation, W.P. and N.T.; writing—original draft preparation, W.P., P.P., N.T., L.P. and S.A.; writing—review and editing, S.M. and N.W.; visualization, N.T. and W.P.; supervision, S.M., A.R. and B.U.; project administration, W.P. and K.L.; funding acquisition, S.S. (Sakulrat Soonthorncharttrawat), C.T. and P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Medical Science, Ministry of Public Health, Thailand. The funding sources had no role in the study’s design; in the writing of the report; or in the decision to submit the paper for publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Department of Medical Sciences, Ministry of Public Health, Thailand, on 18 October 2021 (ethical number DMSC EC 18/2564) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
We thank all collaborators from the 14 Regional Medical Sciences Centers around Thailand—Regional Medical Sciences 1 Chiang Mai Center, Regional Medical Sciences 1-1 Chiang Rai Center, Regional Medical Sciences 2 Pisanulok Center, Regional Medical Sciences 3 Nakornsawan Center, Regional Medical Sciences 4 Saraburi Center, Regional Medical Sciences 5 Samut Songkhram Center, Regional Medical Sciences 6 Chonburi Center, Regional Medical Sciences 7 Khonkhen Center, Regional Medical Sciences 8 Udonthani Center, Regional Medical Sciences 9 Nakornratchasrima Center, Regional Medical Sciences 10 Ubonratchathani Center, Regional Medical Sciences 11 Surathani Center, Regional Medical Sciences 12 Songkla Center, and Regional Medical Sciences 12-1 Trang Center—for administrative support, subject enrolment, the obtainment of informed consent, specimens collection, transportation, and the other technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Random provinces in Thailand.
Table A1.
Random provinces in Thailand.
| Public Health Areas | Random Provinces | Population |
|---|---|---|
| North | ||
| Public health area 1 | 5,876,353 | |
| Chiang Mai, Chiang Rai, Lampang, Phayao, Phrae, Nan, Lamphun, Mae Hong Son | Chiang Rai, Lampang, Lamphun, Phayao | |
| Public health area 2 | 3,538,314 | |
| Phetchabun, Phitsanulok, Tak, Uttaradit, Sukhothai | Phetchabun, Phitsanulok | |
| Central | ||
| Public health area 3 | 2,935,081 | |
| Nakhon Sawan, Uthai Thani, Kamphaeng Phet, Phichit, Chai Nat | Nakhon Sawan, Uthai Thani | |
| Public health area 4 | 5,401,564 | |
| Ang Thong, Lop Buri, Sing Buri, Saraburi, Nonthaburi, Nakhon Nayok, Phra Nakhon Si Ayutthaya, Pathum Thani | Ang Thong, Lop Buri, Sing Buri, Saraburi | |
| Public health area 5 | 5,331,768 | |
| Kanchanaburi, Nakhon Pathom, Prachuap Khiri Khan, Ratchaburi, Suphan Buri, Samut Songkhram, Samut Sakhon, Phetchaburi | Kanchanaburi, Nakhon Pathom, Prachuap Khiri Khan, Ratchaburi, Suphan Buri, Samut Songkhram | |
| Public health area 6 | 6,199,296 | |
| Chon Buri, Chachoengsao, Trat, Sa Kaeo, Prachin Buri, Samut Prakan, Chanthaburi, Rayong | Chon Buri, Chachoengsao, Trat | |
| North-East | ||
| Public health area 7 | 5,024,006 | |
| Khon Kaen, Kalasin, Maha Sarakham, Roi Et | Khon Kaen, Kalasin, Maha Sarakham, Roi Et | |
| Public health area 8 | 5,519,803 | |
| Bueng Kan, Nong Bua Lam Phu, Nong Khai, Loei, Nakhon Phanom, Sakon Nakhon, Udon Thani | Bueng Kan, Nong Bua Lam Phu, Nong Khai, Loei, Nakhon Phanom, Sakon Nakhon, Udon Thani | |
| Public health area 9 | 6,717,536 | |
| Buri Ram, Chaiyaphum, Nakhon Ratchasima, Surin | Buri Ram, Chaiyaphum, Nakhon Ratchasima | |
| Public health area 10 | 4,586,883 | |
| Si Sa Ket, Ubon Ratchathani, Amnat Charoen, Mukdahan | Si Sa Ket, Ubon Ratchathani, Amnat Charoen | |
| South | ||
| Public health area 11 | 4,482,497 | |
| Chumphon, Ranong, Surat Thani, Nakhon Si Thammarat, Phuket, Krabi | Chumphon, Ranong, Surat Thani | |
| Public health area 12 | 4,985,404 | |
| Songkhla, Satun, Trang, Phatthalung, Pattani, Yala, Narathiwat | Songkhla, Satun | |
| Public health area 13 (Bangkok) | - | 5,588,222 |
| Grand Total | 66,186,727 |
References
- Dousari, A.S.; Moghadam, M.T.; Satarzadeh, N. COVID-19 (Coronavirus Disease 2019): A New Coronavirus Disease. Infect. Drug Resist. 2020, 13, 2819–2828. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): A Global Pandemic and Treatment Strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization WHO Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/ (accessed on 5 July 2022).
- Uansri, S.; Tuangratananon, T.; Phaiyarom, M.; Rajatanavin, N.; Suphanchaimat, R.; Jaruwanno, W. Predicted Impact of the Lockdown Measure in Response to Coronavirus Disease 2019 (COVID-19) in Greater Bangkok, Thailand, 2021. Int. J. Environ. Res. Public Health 2021, 18, 12816. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, A.; Ardebili, A. Real-Time RT-PCR in COVID-19 Detection: Issues Affecting the Results. Expert Rev. Mol. Diagn. 2020, 20, 453–454. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Coronavirus Disease 2019 (COVID-19): Situation Report, 26. Available online: https://apps.who.int/iris/handle/10665/331443 (accessed on 5 July 2022).
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.Y.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020, 323, 1406–1407. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal Dynamics in Viral Shedding and Transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Laboratory Testing for Coronavirus Disease (COVID-19) in Suspected Human Cases: Interim Guidance, 19 March 2020. Available online: https://apps.who.int/iris/handle/10665/331501 (accessed on 17 September 2022).
- Takalay, S.; Ngo-Giang-Huong, N.; Kongnim, W.; Mongkolwat, P.; Phoseng, P.; Wangsaeng, N.; Hongjaisee, S.; Butr-Indr, B.; Tragoolpua, K.; Jourdain, G.; et al. Prevalences of SARS-CoV-2 RNA and Anti-SARS-CoV-2 among at-Risk Populations in Chiang Mai and Lamphun Provinces, Thailand, during November 2020–January 2021. PLoS ONE 2022, 17, e0263127. [Google Scholar] [CrossRef] [PubMed]
- Kittikraisak, W.; Piyaraj, P.; Vachiraphan, A.; Wongrapee, T.; Punjasamanvong, S.; Hunsawong, T.; Sinthuwattanawibool, C.; Leepiyasakulchai, C.; Yoocharoen, P.; Azziz-Baumgartner, E.; et al. Sero-Surveillance for SARS-CoV-2 Infection among Healthcare Providers in Four Hospitals in Thailand One Year after the First Community Outbreak. PLoS ONE 2021, 16, e0254563. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Opsomer, J.D.; Stone, M.; Benoit, T.; Ferg, R.A.; Stramer, S.L.; Busch, M.P. Updated US Infection- and Vaccine-Induced SARS-CoV-2 Seroprevalence Estimates Based on Blood Donations, July 2020–December 2021. JAMA 2022, 328, 298–301. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Immunity Task Force. Latest Canadian Blood Services Data Reflect Uptick in Infections Prior to Omicron Surge—COVID-19 Immunity Task Force. Available online: https://www.covid19immunitytaskforce.ca/latest-canadian-blood-services-data-reflect-uptick-in-infections-prior-to-omicron-surge/ (accessed on 18 September 2022).
- Scotland, P.H. Enhanced Surveillance of COVID-19 in Scotland—Population-Based Seroprevalence Surveillance 19 January 2022. Public Health 2022, 190, 132–134. [Google Scholar] [CrossRef]
- Cable, R.; Coleman, C.; Glatt, T.; Grebe, E.; Mhlanga, L.; Nyoni, C.; Pieterson, N.; Swanevelder, R.; Swarts, A.; Sykes, W.; et al. Estimates of Prevalence of Anti-SARS-CoV-2 Antibodies among Blood Donors in Eight Provinces of South Africa in November 2021; Research Square: Durham, NC, USA, 2022. [Google Scholar] [CrossRef]
- Nopsopon, T.; Pongpirul, K.; Chotirosniramit, K.; Hiransuthikul, N. COVID-19 Seroprevalence among Hospital Staff and Preprocedural Patients in Thai Community Hospitals: A Cross-Sectional Study. BMJ Open 2021, 11, e046676. [Google Scholar] [CrossRef] [PubMed]
- Kunno, J.; Supawattanabodee, B.; Sumanasrethakul, C.; Wiriyasivaj, B.; Kuratong, S.; Kaewchandee, C. Comparison of Different Waves during the COVID-19 Pandemic: Retrospective Descriptive Study in Thailand. Adv. Prev. Med. 2021, 2021, 5807056. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Ho, F.K.; Gill, J.M.; Ghouri, N.; Gray, S.R.; Celis-Morales, C.A.; Katikireddi, S.V.; Berry, C.; Pell, J.P.; McMurray, J.J.; et al. BMI and Future Risk for COVID-19 Infection and Death across Sex, Age and Ethnicity: Preliminary Findings from UK Biobank. Diabetes Metab. Syndr. 2020, 14, 1149–1151. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Plante, K.S.; Plante, J.A.; Xie, X.; Zhang, X.; Ku, Z.; An, Z.; Scharton, D.; Schindewolf, C.; et al. The N501Y Spike Substitution Enhances SARS-CoV-2 Infection and Transmission. Nature 2021, 602, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P.A. SARS-CoV-2 Reinfections: Overview of Efficacy and Duration of Natural and Hybrid Immunity. Environ. Res. 2022, 209, 112911. [Google Scholar] [CrossRef] [PubMed]
- Gornyk, D.; Harries, M.; Glöckner, S.; Strengert, M.; Kerrinnes, T.; Heise, J.K.; Maaß, H.; Ortmann, J.; Kessel, B.; Kemmling, Y.; et al. SARS-CoV-2 Seroprevalence in Germany: A Population-Based Sequential Study in Seven Regions. Dtsch. Ärzteblatt Int. 2021, 118, 824. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).