Immune Profile of Blood, Tissue and Peritoneal Fluid: A Comparative Study in High Grade Serous Epithelial Ovarian Cancer Patients at Interval Debulking Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Enrollment
2.2. Preparation of Tumor-Associated Lymphocytes from HGSOC Patients
2.3. Single Cells Preparation of Tissue Specimens from HGSOC Patients
2.4. Surface Staining of Peripheral Lymphocytes, Tumor-Associated Lymphocytes, and Tumor-Infiltrating Lymphocytes for Flow Cytometry
2.5. Soluble Cytokine and Ligand Levels in Peritoneal Fluid and Serum
2.6. Analysis of the Soluble Level of MICA, MICB, in Peritoneal Fluid and Serum
2.7. Statistical Analysis
3. Results
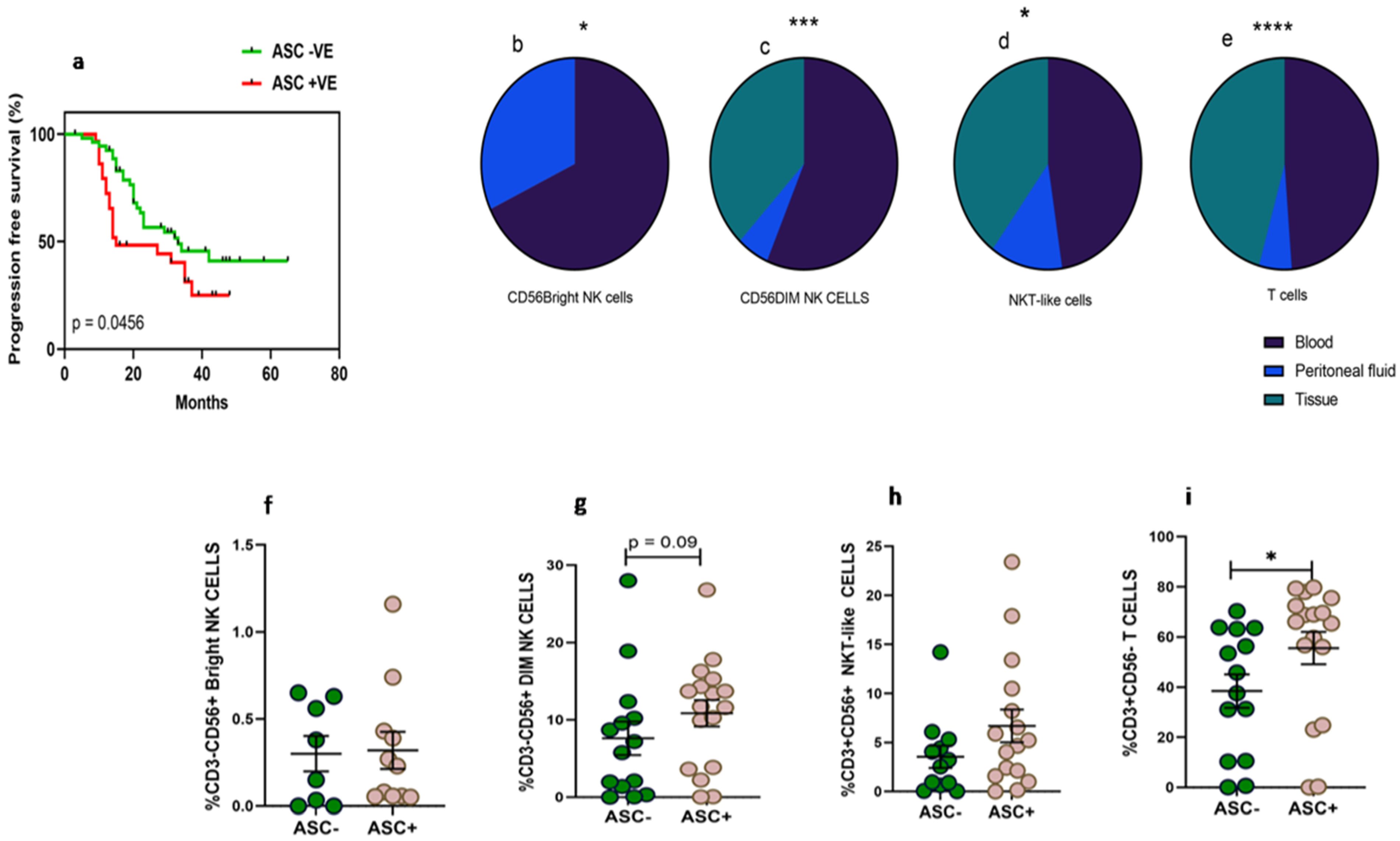
3.1. Detailed Patient Characteristics and Association of Fluid Cytology with Progression-Free Survival
3.2. Higher Frequency of NKT-Like and T Cells in Peritoneal Fluid Than Tumor Specimens of HGSOC
3.3. Reduced Activating Receptors Expression on CD56BrightNK Cells in Peritoneal Fluid
3.4. Improved Expression of Activating NKp46 on CD56DimNK Cells in Peritoneal Fluid
3.5. Receptor Expression Profile of NKT-Like and T Cells
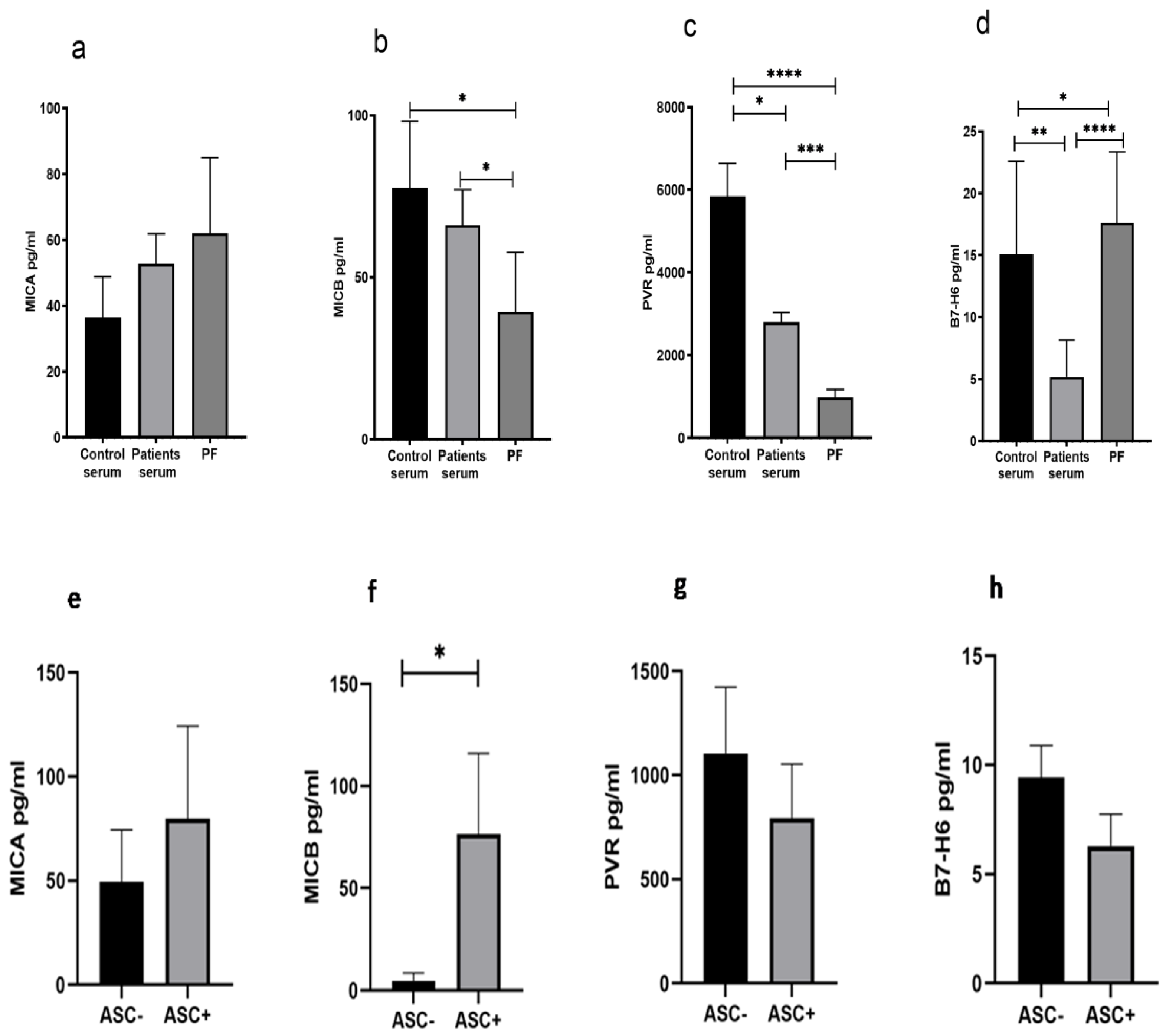
3.6. Reduced Soluble MICB and PVR in Peritoneal Fluid of HGSOC Patients
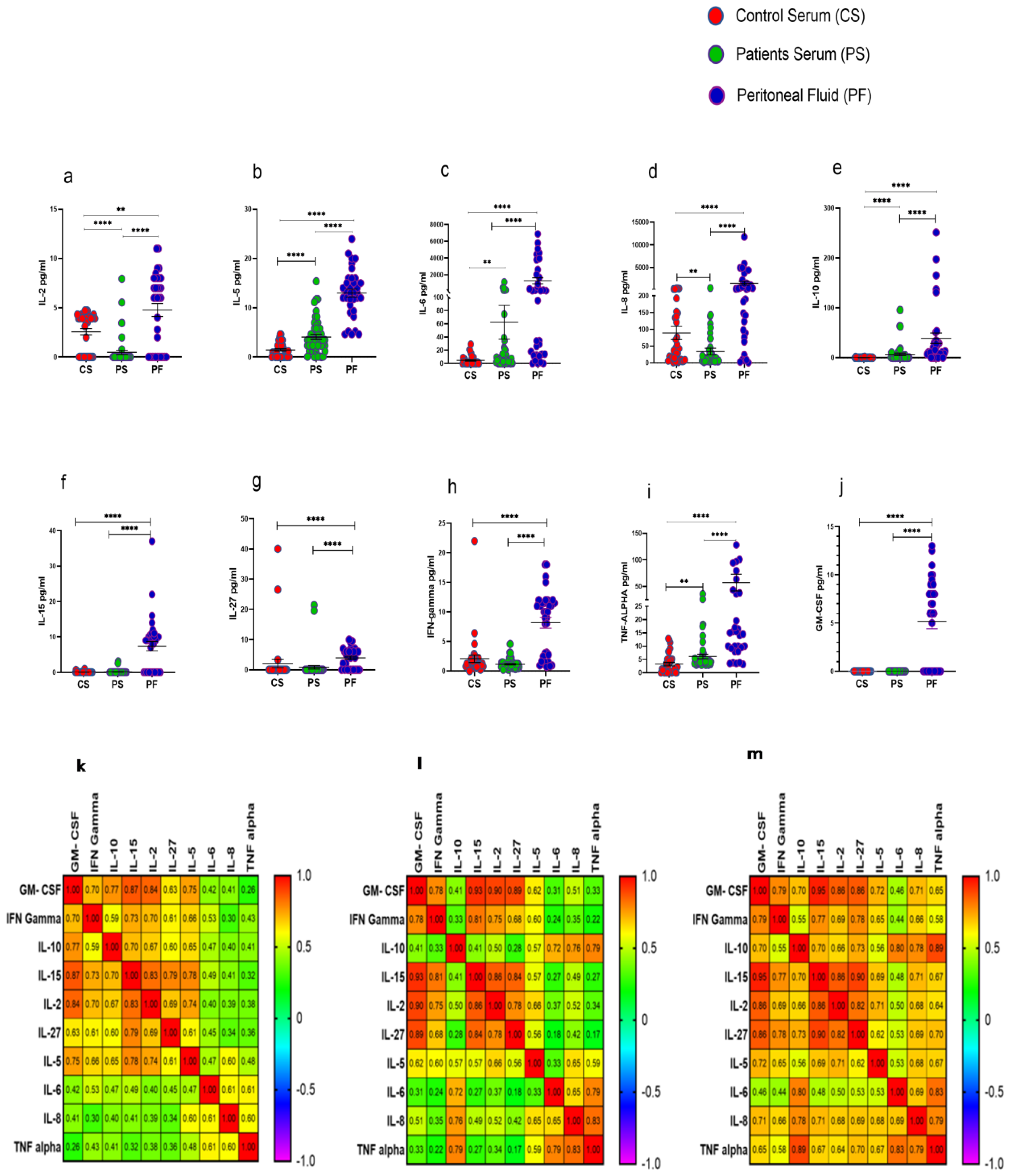
3.7. Elevated Levels of Cytokines in Peritoneal Fluid of HGSOC Patients
3.8. Differential Immune Profile of Chemo Response Score 1 (CRS1) and Chemo Response Score 2/3 (CRS2/3) Group of Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [PubMed]
- Kossaï, M.; Leary, A.; Scoazec, J.Y.; Genestie, C. Ovarian Cancer: A Heterogeneous Disease. Pathobiology 2017, 85, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Hamanishi, J.; Mandai, M.; Konishi, I. Immune checkpoint inhibition in ovarian cancer. Int. Immunol. 2016, 28, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Cole, C.B.; Annunziata, C.M. Driving Immune Responses in the Ovarian Tumor Microenvironment. Front. Oncol. 2021, 10, 604084. [Google Scholar] [CrossRef]
- Pautu, J.L.; Kumar, L. Intratumoral T cells and survival in epithelial ovarian cancer. Natl. Med. J. India 2003, 16, 150–151. [Google Scholar]
- Wefers, C.; Boer, T.D.-D.; Yigit, R.; Zusterzeel, P.L.M.; van Altena, A.M.; Massuger, L.F.A.G.; De Vries, I.J.M. Survival of ovarian cancer patients is independent of the presence of DC and T cell subsets in ascites. Front. Immunol. 2019, 9, 3156. [Google Scholar] [CrossRef]
- Lutz, P.; Jeffery, H.C.; Jones, N.; Birtwistle, J.; Kramer, B.; Nattermann, J.; Spengler, U.; Strassburg, C.P.; Adams, D.H.; Oo, Y.H. NK cells in ascites from liver disease patients display a particular phenotype and take part in antibacterial immune response. Front. Immunol. 2019, 10, 1838. [Google Scholar] [CrossRef]
- Cózar, B.; Greppi, M.; Carpentier, S.; Narni-mancinelli, E. Europe PMC Funders Group Tumor-infiltrating natural killer cells. Cancer Discov. 2021, 11, 34–44. [Google Scholar] [CrossRef]
- Tonetti, C.; de Souza-Araújo, C.; Yoshida, A.; da Silva, R.; Alves, P.; Mazzola, T.; Derchain, S.; Fernandes, L.; Guimarães, F. Ovarian cancer-associated ascites have high proportions of cytokine-responsive CD56bright NK cells. Cells 2021, 10, 1702. [Google Scholar] [CrossRef]
- Vazquez, J.; Chavarria, M.; Lopez, G.E.; Felder, M.A.; Kapur, A.; Chavez, A.R.; Karst, N.; Barroilhet, L.; Patankar, M.S.; Stanic, A.K. Identification of unique clusters of T, dendritic, and innate lymphoid cells in the peritoneal fluid of ovarian cancer patients. Am. J. Reprod. Immunol. 2020, 84, e13284. [Google Scholar] [CrossRef]
- Di Modica, M.; Sfondrini, L.; Regondi, V.; Varchetta, S.; Oliviero, B.; Mariani, G.; Bianchi, G.V.; Generali, D.; Balsari, A.; Triulzi, T.; et al. Taxanes enhance trastuzumab-mediated ADCC on tumor cells through NKG2D-mediated NK cell recognition. Oncotarget 2015, 7, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Kopp, L.; Ray, A.; Denman, C.J.; Senyukov, V.S.; Somanchi, S.S.; Zhu, S.; Lee, D. Decitabine has a biphasic effect on natural killer cell viability, phenotype, and function under proliferative conditions. Mol. Immunol. 2013, 54, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Giuntoli, R.L.; Webb, T.J.; Zoso, A.; Rogers, O.; Diaz-Montes, T.P.; Bristow, R.E.; Mathias, O. Ovarian Cancer-associated Ascites Demonstrates altered immune environment-2009. Anticancer Res. 2009, 29, 2875–2884. [Google Scholar]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Rådestad, E.F.S.; Klynning, C.; Stikvoort, A.; Mogensen, O.; Nava, S.; Magalhaes, I.; Uhlin, M. Immune profiling and identification of prognostic immune-related risk factors in human ovarian cancer. OncoImmunology 2018, 8, e1535730. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, W.; Hu, B.; Wang, P.; Lv, X.; Chen, S.; Shao, Z. Prognostic Significance of Tumor-Infiltrating Natural Killer Cells in Solid Tumors: A Systematic Review and Meta-Analysis. Front. Immunol. 2020, 11, 1242. [Google Scholar] [CrossRef]
- Sivori, S.; Marcenaro, E. Different Features of Tumor-Associated NK Cells in Patients with Low-Grade or High-Grade Peritoneal Carcinomatosis. Front. Immunol. 2019, 10, 1963. [Google Scholar]
- Desbois, M.; Udyavar, A.R.; Ryner, L.; Kozlowski, C.; Guan, Y.; Dürrbaum, M.; Lu, S.; Fortin, J.-P.; Koeppen, H.; Ziai, J.; et al. Integrated digital pathology and transcriptome analysis identifies molecular mediators of T-cell exclusion in ovarian cancer. Nat. Commun. 2020, 11, 5583. [Google Scholar] [CrossRef]
- Poli, A.; Michel, T.; Thérésine, M.; Andrès, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef]
- Poznanski, S.M.; Ashkar, A.A. Shining light on the significance of NK cell CD56 brightness. Cell Mol. Immunol. 2018, 15, 1071–1073. [Google Scholar] [CrossRef]
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F.; et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019, 177, 1701–1713.e16. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Correa, B.; Gayoso, I.; Bergua, J.M.; Casado, J.G.; Morgado, S.; Solana, R.; Tarazona, R. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol. Cell Biol. 2011, 90, 109–115. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, L.F.; Smyth, M.J.; Martinet, L. DNAM-1 control of natural killer cells functions through nectin and nectin-like proteins. Immunol. Cell Biol. 2013, 92, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Liontos, M.; Andrikopoulou, A.; Koutsoukos, K.; Markellos, C.; Skafida, E.; Fiste, O.; Kaparelou, M.; Thomakos, N.; Haidopoulos, D.; Rodolakis, A.; et al. Neutrophil-to-lymphocyte ratio and chemotherapy response score as prognostic markers in ovarian cancer patients treated with neoadjuvant chemotherapy. J. Ovarian Res. 2021, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.; Essers, P.B.M.; de Jong, M.C.; de Roest, R.H.; Sanduleanu, S.; Verhagen, C.V.M.; Hamming-Vrieze, O.; Hoebers, F.; Lambin, P.; Bartelink, H.; et al. Biological Determinants of Chemo-Radiotherapy Response in HPV-Negative Head and Neck Cancer: A Multicentric External Validation. Front Oncol. 2020, 9, 1470. [Google Scholar] [CrossRef]
- Garg, S.; Shekhawat, U.; Vohra, R.; Gupta, R. Pretreatment Immune Status, Predicts Response to Definite Chemo Radiotherapy in Advanced Stages of Cervical Cancer Patients. J. Obs. Gynecol. India 2022, 72, 319–325. [Google Scholar] [CrossRef]
- Ingram, Z.; Madan, S.; Merchant, J.; Carter, Z.; Gordon, Z.; Carey, G.; Webb, T. Targeting natural killer T cells in solid malignancies. Cells 2021, 10, 1329. [Google Scholar] [CrossRef]
- Gilfillan, S.; Chan, C.J.; Cella, M.; Haynes, N.M.; Rapaport, A.S.; Boles, K.S.; Andrews, D.; Smyth, M.; Colonna, M. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J. Exp. Med. 2008, 205, 2965–2973. [Google Scholar] [CrossRef]
- Konduri, V.; Oyewole-Said, D.; Vazquez-Perez, J.; Weldon, S.A.; Halpert, M.M.; Levitt, J.M.; Decker, W.K. CD8+CD161+ T-Cells: Cytotoxic Memory Cells with High Therapeutic Potential. Front. Immunol. 2021, 11, 613204. [Google Scholar] [CrossRef]
- González-Foruria, I.; Santulli, P.; Chouzenoux, S.; Carmona, F.; Batteux, F.; Chapron, C. Soluble ligands for the NKG2D receptor are released during endometriosis and correlate with disease severity. PLoS ONE 2015, 10, e0119961. [Google Scholar] [CrossRef]
- Schlecker, E.; Fiegler, N.; Arnold, A.; Altevogt, P.; Rose-John, S.; Moldenhauer, G.; Sucker, A.; Paschen, A.; von Strandmann, E.P.; Textor, S.; et al. Metalloprotease-mediated tumor cell shedding of B7-H6, the ligand of the natural killer cell-activating receptor NKp30. Cancer Res. 2014, 74, 3429–3440. [Google Scholar] [CrossRef]
- Pesce, S.; Tabellini, G.; Cantoni, C.; Patrizi, O.; Coltrini, D.; Rampinelli, F.; Matta, J.; Vivier, E.; Moretta, A.; Parolini, S.; et al. B7-H6-mediated downregulation of NKp30 in NK cells contributes to ovarian carcinoma immune escape. Oncoimmunology 2015, 4, e1001224. [Google Scholar] [CrossRef]
- Mantovani, G.; Macciò, A.; Versace, R.; Pisano, M.; Lai, P.; Esu, S.; Ghiani, M.; Dessi, D.; Turnu, E.; Santona, M.C.; et al. Tumor-associated lymphocytes (TAL) are competent to produce higher levels of cytokines in neoplastic pleural and peritoneal effusions than those found in sera and are able to release into culture higher levels of IL-2 and IL-6 than those released by PBMC. Klin. Wochenschr. 1995, 73, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Thongchot, S.; Vidoni, C.; Ferraresi, A.; Loilome, W.; Khuntikeo, N.; Sangkhamanon, S.; Titapun, A.; Isidoro, C.; Namwat, N. Cancer associated fibroblast derived IL6 determines unfavorable prognosis in cholangiocarcinoma by affecting autophagy-associated chemoresponse. Cancers 2021, 13, 2134. [Google Scholar] [CrossRef] [PubMed]
- Ham, I.-H.; Oh, H.J.; Jin, H.; Bae, C.A.; Jeon, S.-M.; Choi, K.S.; Son, S.-Y.; Han, S.-U.; Brekken, R.A.; Lee, D.; et al. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol. Cancer 2019, 18, 68. [Google Scholar] [CrossRef] [PubMed]




| Patients Enrolled | Patients Characteristics | Healthy Controls |
|---|---|---|
| n = 33 | n = 34 | |
| Median age (range) in years | 56.5 (32–76) | 51 (35–67) |
| Chemotherapy at the time of enrollment | 3 cycles (Paclitaxel+Carboplatin) | Not applicable |
| Stage | Not applicable | |
| II | 4 (12.1%) | |
| III | 21 (63.4%) | |
| IV | 8 (24.2%) | |
| Grade | Not applicable | |
| High | 33 (100%) | |
| Low | 0 | |
| Unknown | 0 | |
| Co-morbidity | ||
| Yes | 18 (54.5%) | Arbitrary Healthy No active chronic infection Diabetes, Hypertension, etc. |
| No | 10 (30.3%) | |
| Unknown | 5 (15.1%) | |
| Fluid cytology | Not applicable | |
| Positive | 18 (54.5%) | |
| Negative | 15 (45.4%) | |
| Unknown | 0 | |
| Lymph node metastasis | Not applicable | |
| Positive | 16 (48.4%) | |
| Negative | 10 (30.3%) | |
| Unknown | 7 (21.1%) | |
| Chemo response score (CRS) | Not applicable | |
| CRS 1 | 14 (42.4%) | |
| CRS 2 | 16 (48.4%) | |
| CRS 3 | 3 (0.09%) | |
| CA-125 Median (Range) | Not applicable | |
| Pre-treatment | 665.5 (125.4–27,428) units/mL | Not applicable |
| Post-treatment | 37.94 (6.9–679.5) units/mL | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.; Ranmale, S.; Tongaonkar, H.; Mania-Pramanik, J. Immune Profile of Blood, Tissue and Peritoneal Fluid: A Comparative Study in High Grade Serous Epithelial Ovarian Cancer Patients at Interval Debulking Surgery. Vaccines 2022, 10, 2121. https://doi.org/10.3390/vaccines10122121
Kumar P, Ranmale S, Tongaonkar H, Mania-Pramanik J. Immune Profile of Blood, Tissue and Peritoneal Fluid: A Comparative Study in High Grade Serous Epithelial Ovarian Cancer Patients at Interval Debulking Surgery. Vaccines. 2022; 10(12):2121. https://doi.org/10.3390/vaccines10122121
Chicago/Turabian StyleKumar, Pavan, Samruddhi Ranmale, Hemant Tongaonkar, and Jayanti Mania-Pramanik. 2022. "Immune Profile of Blood, Tissue and Peritoneal Fluid: A Comparative Study in High Grade Serous Epithelial Ovarian Cancer Patients at Interval Debulking Surgery" Vaccines 10, no. 12: 2121. https://doi.org/10.3390/vaccines10122121
APA StyleKumar, P., Ranmale, S., Tongaonkar, H., & Mania-Pramanik, J. (2022). Immune Profile of Blood, Tissue and Peritoneal Fluid: A Comparative Study in High Grade Serous Epithelial Ovarian Cancer Patients at Interval Debulking Surgery. Vaccines, 10(12), 2121. https://doi.org/10.3390/vaccines10122121





