Morphology of Lymphoid Tissue in the Lungs of Guinea Pigs Infected with Mycobacterium bovis against the Background of Vaccine Immunity and the Action of Betulin and Its Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Pharmacophore Models and Molecular Docking
2.2. In Vivo Anti-Tuberculosis Activity Study
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Daniel, T.M. The history of tuberculosis. Respir. Med. 2006, 100, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Finci, I.; Albertini, A.; Merker, M.; Andres, S.; Bablishvili, N.; Barilar, I.; Cáceres, T.; Crudu, V.; Gotuzzo, E.; Hapeela, N.; et al. Investigating resistance in clinical Mycobacterium tuberculosis complex isolates with genomic and phenotypic antimicrobial susceptibility testing: A multicentre observational study. Lancet Microbe 2022, 3, e672–e682. [Google Scholar] [PubMed]
- Bajaj, A.O.; Saraswat, S.; Knuuttila, J.E.A.; Freeke, J.; Stielow, J.B.; Barker, A.P. Accurate Identification of Closely Related Mycobacterium tuberculosis Complex Species by High Resolution Tandem Mass Spectrometry. Front. Cell. Infect. Microbiol. 2021, 22, 656880. [Google Scholar] [CrossRef] [PubMed]
- Borham, M.; Oreiby, A.; El-Gedawy, A.; Hegazy, Y.; Khalifa, H.O.; Al-Gaabary, M.; Matsumoto, T. Review on Bovine Tuberculosis: An Emerging Disease Associated with Multidrug-Resistant Mycobacterium Species. Pathogens 2022, 21, 715. [Google Scholar]
- Gagneux, S. Ecology and evolution of Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018, 16, 202–213. [Google Scholar] [CrossRef]
- Li, J.; Zhan, L.; Qin, C. The double-sided effects of Mycobacterium Bovis bacillus Calmette-Guérin vaccine. NPJ Vaccines 2021, 25, 14. [Google Scholar]
- Dunlap, M.D.; Prince, O.A.; Rangel-Moreno, J.; Thomas, K.A.; Scordo, J.M.; Torrelles, J.B.; Cox, J.; Steyn, A.J.C.; Zúñiga, J.; Kaushal, D.; et al. Formation of Lung Inducible Bronchus Associated Lymphoid Tissue Is Regulated by Mycobacterium tuberculosis Expressed Determinants. Front. Immunol. 2020, 30, 1325. [Google Scholar] [CrossRef]
- Moyron-Quiroz, J.E.; Rangel-Moreno, J.; Kusser, K.; Hartson, L.; Sprague, F.; Goodrich, S.; Woodland, D.L.; Lund, F.E.; Randall, T.D. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 2004, 10, 927–934. [Google Scholar]
- Pabst, R.; Gehrke, I. Is the bronchus-associated lymphoid tissue (BALT) an integral structure of the lung in normal mammals, including humans? Am. J. Respir. Cell Mol. Biol. 1990, 3, 131–135. [Google Scholar] [CrossRef]
- Bienenstock, J.; Befus, D. Gut- and bronchus-associated lymphoid tissue. Am. J. Anat. 1984, 170, 437–445. [Google Scholar] [CrossRef]
- Randall, T.D. Bronchus-associated lymphoid tissue (BALT) structure and function. Adv. Immunol. 2010, 107, 187–241. [Google Scholar]
- Tschernig, T.; Pabst, R. Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology 2000, 68, 1–8. [Google Scholar] [CrossRef]
- Doz, E.; Lombard, R.; Carreras, F.; Buzoni-Gatel, D.; Winter, N. Mycobacteria-infected dendritic cells attract neutrophils that produce IL-10 and specifically shut down Th17 CD4 T cells through their IL-10 receptor. J. Immunol. 2013, 191, 3818–3826. [Google Scholar] [CrossRef]
- Lai, R.; Jeyanathan, M.; Shaler, C.R.; Damjanovic, D.; Khera, A.; Horvath, C.; Ashkar, A.A.; Xing, Z. Restoration of innate immune activation accelerates Th1-cell priming and protection following pulmonary mycobacterial infection. Eur. J. Immunol. 2014, 44, 1375–1386. [Google Scholar] [CrossRef]
- Demangel, C.; Bertolino, P.; Britton, W.J. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur. J. Immunol. 2002, 32, 994–1002. [Google Scholar] [CrossRef]
- Kang, D.D.; Lin, Y.; Moreno, J.R.; Randall, T.D.; Khader, S.A. Profiling early lung immune responses in the mouse model of tuberculosis. PLoS ONE 2011, 6, e16161. [Google Scholar] [CrossRef]
- Blomgran, R.; Desvignes, L.; Briken, V.; Ernst, J.D. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host Microbe 2012, 11, 81–90. [Google Scholar]
- Cadena, A.M.; Fortune, S.M.; Flynn, J.L. Heterogeneity in tuberculosis. Nat. Rev. Immunol. 2017, 17, 691–702. [Google Scholar]
- Ulrichs, T.; Kosmiadi, G.A.; Trusov, V.; Jörg, S.; Pradl, L.; Titukhina, M.; Mishenko, V.; Gushina, N.; Kaufmann, S.H. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J. Pathol. 2004, 204, 217–228. [Google Scholar] [CrossRef]
- Ganchua, S.K.C.; Cadena, A.M.; Maiello, P.; Gideon, H.P.; Myers, A.J.; Junecko, B.F.; Klein, E.C.; Lin, P.L.; Mattila, J.T.; Flynn, J.L. Lymph nodes are sites of prolonged bacterial persistence during Mycobacterium tuberculosis infection in macaques. PLoS Pathog. 2018, 14, e1007337. [Google Scholar]
- Slight, S.R.; Rangel-Moreno, J.; Gopal, R.; Lin, Y.; Fallert Junecko, B.A.; Mehra, S.; Selman, M.; Becerril-Villanueva, E.; Baquera-Heredia, J.; Pavon, L.; et al. CXCR5+ T helper cells mediate protective immunity against tuberculosis. J. Clin. Investig. 2013, 123, 712–726. [Google Scholar] [PubMed]
- Bachvarova, M.; Stefanova, T.; Nikolaeva, S.; Chouchkova, M. Tuberculin sensitivity and morphological immune response in guinea pigs after application of minimal sensitizing dose of BCG vaccine, substrain Sofia SL222. Int. Immunopharmacol. 2009, 9, 1010–1015. [Google Scholar] [PubMed]
- Shoen, C.M.; DeStefano, M.S.; Hager, C.C.; Tham, K.T.; Braunstein, M.; Allen, A.D.; Gates, H.O.; Cynamon, M.H.; Kernodle, D.S. A Modified Bacillus Calmette-Guérin (BCG) Vaccine with Reduced Activity of Antioxidants and Glutamine Synthetase Exhibits Enhanced Protection of Mice despite Diminished in Vivo Persistence. Vaccines 2013, 1, 34–57. [Google Scholar] [PubMed]
- Koshkin, I.N.; Vlasenko, V.S.; Kulakov, I.V. The Effect of Experimental BCG Antigen–Betulin-Derived Conjugates on the Guinea Pig Immunological Response. Russ. J. Bioorg. Chem. 2021, 47, 837–844. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- BIOVIA; Dassault Systèmes. BIOVIA Discovery Studio, 2020; Dassault Systèmes: San Diego, CA, USA, 2020. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Barthel, A.; Stark, S.; Csuk, R. Oxidative transformations of betulinol. Tetrahedron 2008, 64, 9225–9229. [Google Scholar]
- Flekhter, O.B.; Karachurina, L.T.; Poroĭkov, V.V.; Nigmatullina, L.P.; Baltina, L.A.; Zarudiĭ, F.S.; Davydova, V.A.; Spirikhin, L.V.; Baĭkova, I.P.; Galin, F.Z.; et al. Synthesis of the lupane group triterpenoids and there hepatoprotective activity. Russ. J. Bioorg. Chem. 2000, 26, 192–200. [Google Scholar]
- Flekhter, O.B.; Boreko, E.I.; Nigmatullina, L.R.; Pavlova, N.I.; Nikolaeva, S.N.; Savinova, O.V.; Eremin, V.F.; Baltina, L.A.; Galin, F.Z.; Tolstikov, G.A. Synthesis and antiviral activity of hydrazides and substituted benzalhydrazides of betulinic acid and its derivatives. Russ. J. Bioorg. Chem. 2003, 29, 296–302. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Bajusz, D.; Rácz, A.; Héberger, K. Why is Tanimoto index an appropriate choice for fingerprint-based similarity calculations? J. Cheminform. 2015, 7, 20. [Google Scholar]
- Backman, T.W.; Cao, Y.; Girke, T. ChemMine tools: An online service for analyzing and clustering small molecules. Nucleic Acids Res. 2011, 39, W486–W491. [Google Scholar] [CrossRef]
- Ortega, J.; Roy, A.; Díaz-Castillo, A.; de Juan, L.; Romero, B.; Sáez-Llorente, J.L.; Domínguez, L.; Regal, P.; Infantes-Lorenzo, J.A.; Álvarez, J.; et al. Effect of the topical administration of corticosteroids and tuberculin pre-sensitisation on the diagnosis of tuberculosis in goats. BMC Vet. Res. 2022, 18, 58. [Google Scholar]
- Huang, T.M.; Kuo, K.C.; Wang, Y.H.; Wang, C.Y.; Lai, C.C.; Wang, H.C.; Chen, L.; Yu, C.J.; On the behalf of Taiwan Clinical Trial Consortium for Respiratory Diseases (TCORE). Risk of active tuberculosis among COPD patients treated with fixed combinations of long-acting beta2 agonists and inhaled corticosteroids. BMC Infect. Dis. 2020, 20, 706. [Google Scholar]
- Vozoris, N.T.; Seemangal, J.; Batt, J. Prevalence, screening and treatment of latent tuberculosis among oral corticosteroid recipients. Eur. Respir. J. 2014, 44, 1373–1375. [Google Scholar] [CrossRef]
- Singh, V.; Dziwornu, G.A.; Mabhula, A.; Chibale, K. Rv0684/fusA1, an Essential Gene, Is the Target of Fusidic Acid and Its Derivatives in Mycobacterium tuberculosis. ACS Infect. Dis. 2021, 7, 2437–2444. [Google Scholar] [CrossRef]
- Tükenmez, H.; Edström, I.; Kalsum, S.; Braian, C.; Ummanni, R.; Fick, S.B.; Sundin, C.; Lerm, M.; Elofsson, M.; Larsson, C. Corticosteroids protect infected cells against mycobacterial killing in vitro. Biochem. Biophys. Res. Commun. 2019, 511, 117–121. [Google Scholar] [CrossRef]
- Yang, X.; Dubnau, E.; Smith, I.; Sampson, N.S. Rv1106c from Mycobacterium tuberculosis is a 3beta-hydroxysteroid dehydrogenase. Biochemistry 2007, 46, 9058–9067. [Google Scholar] [CrossRef] [PubMed]
- Marrink, S.J.; Corradi, V.; Souza, P.C.T.; Ingólfsson, H.I.; Tieleman, D.P.; Sansom, M.S.P. Computational Modeling of Realistic Cell Membranes. Chem. Rev. 2019, 119, 6184–6226. [Google Scholar] [CrossRef] [PubMed]
- Ordway, D.; Henao-Tamayo, M.; Shanley, C.; Smith, E.E.; Palanisamy, G.; Wang, B.; Basaraba, R.J.; Orme, I.M. Influence of Mycobacterium bovis BCG vaccination on cellular immune response of guinea pigs challenged with Mycobacterium tuberculosis. Clin. Vaccine Immunol. 2008, 15, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Taylor, J.; Troudt, J.; Keyser, A.; Arnett, K.; Izzo, L.; Rholl, D.; Izzo, A. Kinetics of the immune response profile in guinea pigs after vaccination with Mycobacterium bovis BCG and infection with Mycobacterium tuberculosis. Infect. Immun. 2009, 77, 4837–4846. [Google Scholar]
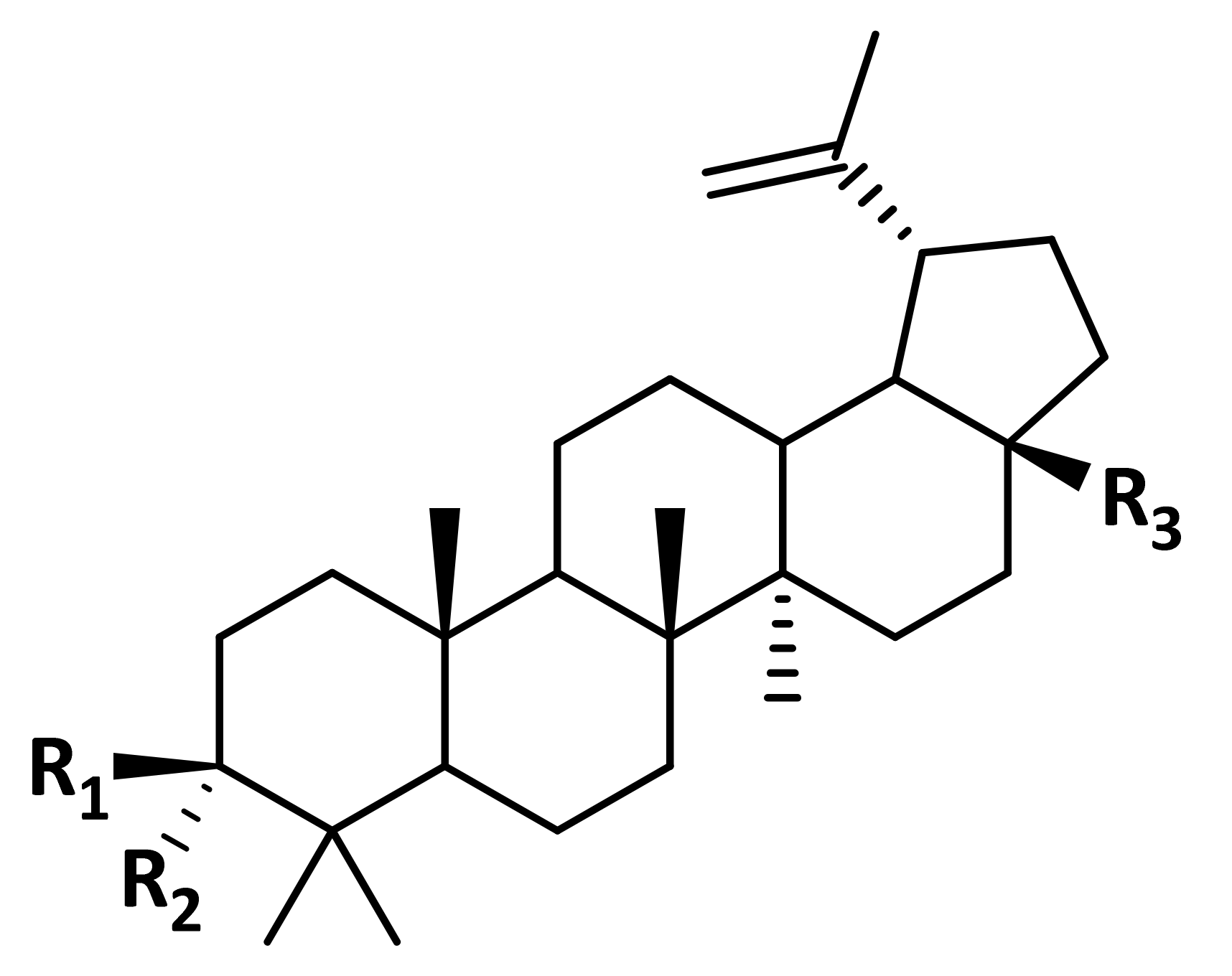

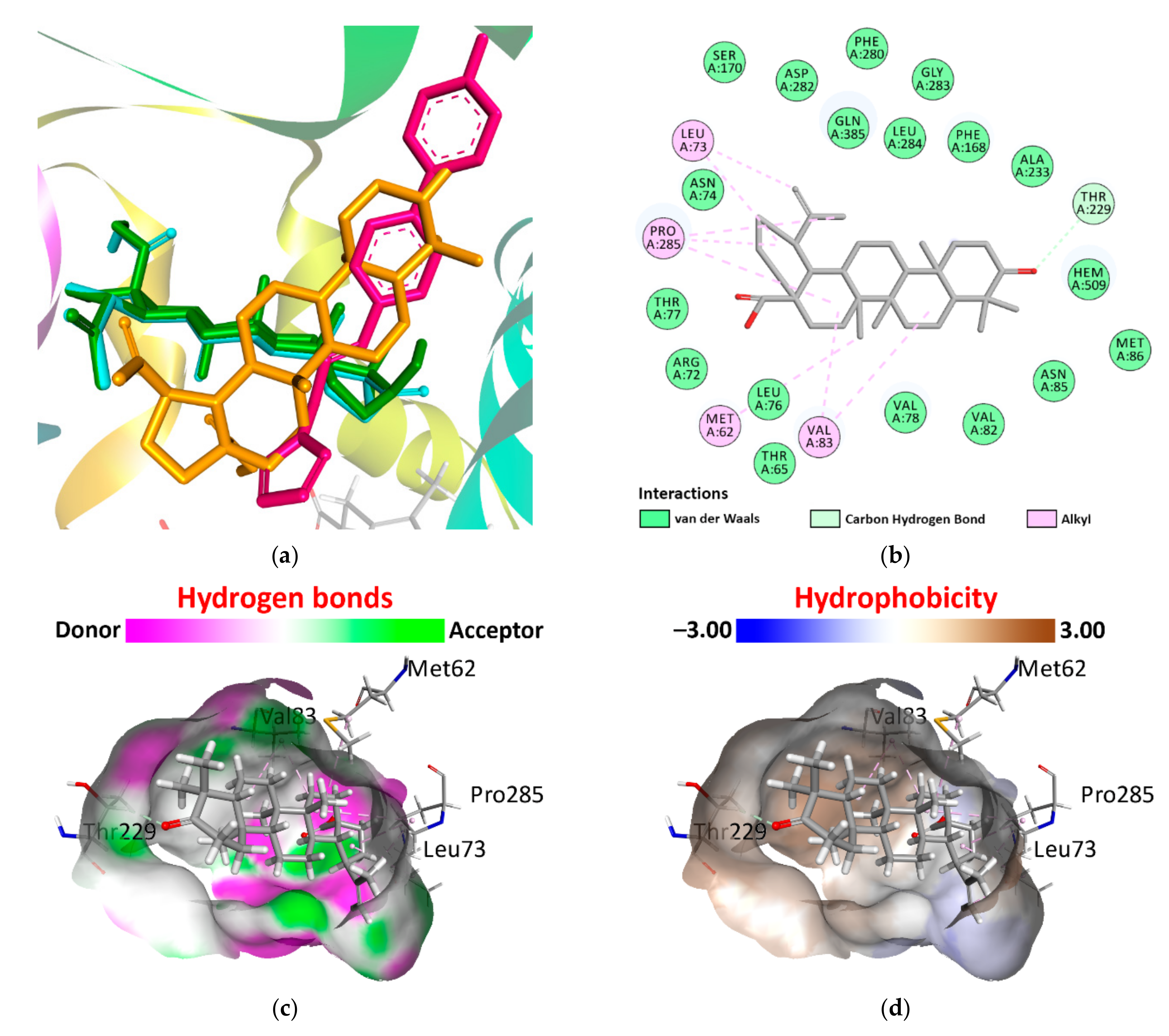


| Drug Name | MCS Tanimoto Coefficient 1 | ||
|---|---|---|---|
| Betulin | Betulonic Acid | Betulinic Acid | |
| Betamethasone | 0.4286 | 0.4186 | 0.4186 |
| Budesonide | 0.4000 | 0.3617 | 0.3913 |
| Cortisone acetate | 0.4524 | 0.4762 | 0.4419 |
| Deflazacort | 0.3913 | 0.3540 | 0.3830 |
| Dexamethasone | 0.4286 | 0.4186 | 0.4186 |
| Fludrocortisone | 0.4750 | 0.5000 | 0.4634 |
| Flunisolide | 0.4000 | 0.3617 | 0.3913 |
| Fluprednisolone | 0.4390 | 0.3953 | 0.4286 |
| Fusidic acid | 0.4082 | 0.3725 | 0.4000 |
| Hydrocortisone | 0.4873 | 0.5128 | 0.4750 |
| Meprednisone | 0.4390 | 0.4286 | 0.4286 |
| Methylprednisolone | 0.4390 | 0.4286 | 0.4286 |
| Prednisolone | 0.4500 | 0.4048 | 0.4390 |
| Prednisone | 0.4500 | 0.4048 | 0.4390 |
| Triamcinolone | 0.4286 | 0.3864 | 0.4186 |
| Trilostane | 0.4737 | 0.4615 | 0.4615 |
| Protein | PDB ID | Ligand | Binding Energy (kcal/mol) | pKi |
|---|---|---|---|---|
| Carbohydrate-recognition domain of the C-type lectin mincle | 4KZW | FLC | −4.20 | 3.06 |
| Betulin | −5.97 | 4.35 | ||
| Betulonic acid | −6.02 | 4.39 | ||
| Betulinic acid | −5.81 | 4.23 | ||
| Mtb adenosine kinase | 4UBE | 2FA | −9.92 | 7.23 |
| Betulin | −6.84 | 4.98 | ||
| Betulonic acid | −7.01 | 5.11 | ||
| Betulinic acid | −6.92 | 5.04 | ||
| Mtb CYP121 | 6TET | N5Z | −9.80 | 7.14 |
| Betulin | −9.79 | 7.13 | ||
| Betulonic acid | −11.02 | 8.03 | ||
| Betulinic acid | −9.52 | 6.94 | ||
| UDP-N-acetylmuramic Acid L-alanine ligase (MurC) | 7BVB | UD1 | −7.94 | 5.78 |
| Betulin | −7.45 | 5.43 | ||
| Betulonic acid | −7.66 | 5.58 | ||
| Betulinic acid | −7.53 | 5.49 | ||
| Mtb tryptophanyl-tRNA synthetase | 7ENS | ATP | −8.92 | 6.50 |
| 5BX | −7.95 | 5.79 | ||
| Betulin | −5.39 | 3.93 | ||
| Betulonic acid | −6.24 | 4.55 | ||
| Betulinic acid | −7.12 | 5.19 |
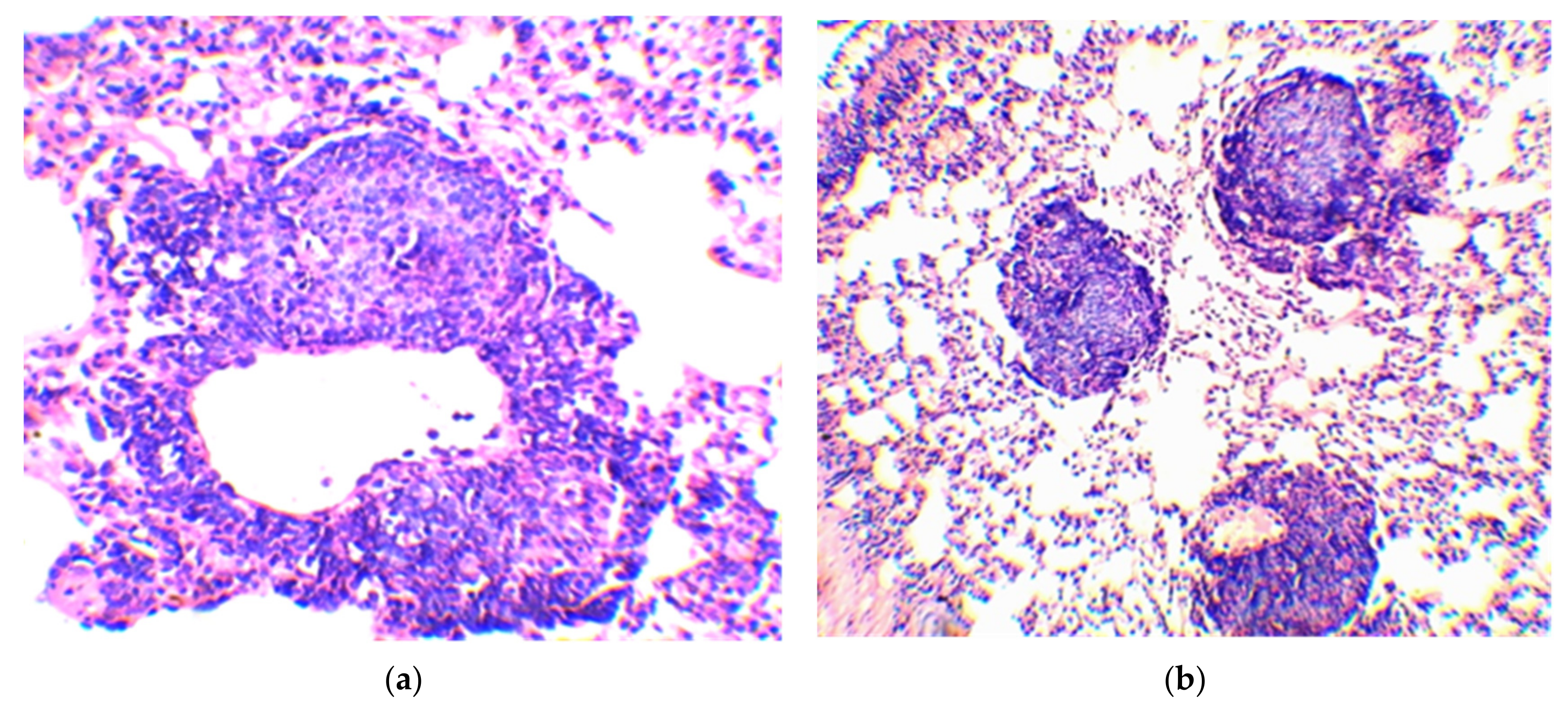
| Group No. | BCG Vaccine | Compound | Mean Follicle Area (µm2) | Mean Follicle Diameter (µm) | Follicle Size Distribution by Diameter (µm) | Number of Follicles of the Same Size (%) |
|---|---|---|---|---|---|---|
| 1 | − | − | 34,103.0 ± 2766.5 | 203.0 ± 8.8 | 70–100 | 3.3 |
| 110–160 | 23.3 | |||||
| 170–220 | 40.0 | |||||
| 230–270 | 26.7 | |||||
| 280–300 | 6.7 | |||||
| 2 | + | Betulinic acid | 37,478.8 ± 4532.1 | 216.3 ± 14.0 | 70–100 | 6.7 |
| 110–160 | 16.7 | |||||
| 170–220 | 33.3 | |||||
| 230–270 | 25.7 | |||||
| 280–300 | 16.7 | |||||
| 3 | + | Betulin | 19,080.7 ± 1905.0 1 | 147.0 ± 9.0 2 | 70–100 | 10.0 |
| 110–160 | 53.5 | |||||
| 170–220 | 36.7 | |||||
| 4 | + | Betulonic acid | 11,458.4 ± 1240.7 2 | 115.7 ± 6.5 2 | 70–100 | 43.3 |
| 110–160 | 46.7 | |||||
| 170–190 | 10.0 | |||||
| 5 | + | − | 20,742.3 ± 2386.4 1 | 155.0 ± 9.1 2 | 70–100 | 13.3 |
| 110–160 | 50.0 | |||||
| 170–220 | 23.4 | |||||
| 230–250 | 13.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshkin, I.N.; Vlasenko, V.S.; Pleshakova, V.I.; Alkhimova, L.E.; Elyshev, A.V.; Kulakov, I.V. Morphology of Lymphoid Tissue in the Lungs of Guinea Pigs Infected with Mycobacterium bovis against the Background of Vaccine Immunity and the Action of Betulin and Its Derivatives. Vaccines 2022, 10, 2084. https://doi.org/10.3390/vaccines10122084
Koshkin IN, Vlasenko VS, Pleshakova VI, Alkhimova LE, Elyshev AV, Kulakov IV. Morphology of Lymphoid Tissue in the Lungs of Guinea Pigs Infected with Mycobacterium bovis against the Background of Vaccine Immunity and the Action of Betulin and Its Derivatives. Vaccines. 2022; 10(12):2084. https://doi.org/10.3390/vaccines10122084
Chicago/Turabian StyleKoshkin, Ivan N., Vasily S. Vlasenko, Valentina I. Pleshakova, Larisa E. Alkhimova, Andrey V. Elyshev, and Ivan V. Kulakov. 2022. "Morphology of Lymphoid Tissue in the Lungs of Guinea Pigs Infected with Mycobacterium bovis against the Background of Vaccine Immunity and the Action of Betulin and Its Derivatives" Vaccines 10, no. 12: 2084. https://doi.org/10.3390/vaccines10122084
APA StyleKoshkin, I. N., Vlasenko, V. S., Pleshakova, V. I., Alkhimova, L. E., Elyshev, A. V., & Kulakov, I. V. (2022). Morphology of Lymphoid Tissue in the Lungs of Guinea Pigs Infected with Mycobacterium bovis against the Background of Vaccine Immunity and the Action of Betulin and Its Derivatives. Vaccines, 10(12), 2084. https://doi.org/10.3390/vaccines10122084






