SARS-CoV-2 IgG Antibody Levels in Women with IBD Vaccinated during Pregnancy
Abstract
1. Introduction
2. Materials and Methods
- Pregnant women with IBD group: patients with an established diagnosis of IBD, followed up on and treated during pregnancy in dedicated IBD–Pregnancy joint clinics conducted at two medical centers in Israel (Shaare Zedek and Rabin medical center) between March and December 2021. As part of regular clinical care, consenting patients were evaluated in a prospective follow-up that included at least one visit per trimester, for clinical assessment and blood analysis. In consenting patients under biologic therapies, cord blood was also collected.
- Pregnant women without IBD—control group: consecutive parturient women, admitted for delivery at two tertiary medical centers in Israel (Shaare Zedek and Hadassah medical center) between January and August 2021. Eligible women were recruited on admittance to the delivery room or operating room in elective cesarean delivery cases to a prospective study aimed to assess maternal and neonatal SARS-CoV-2 immunoglobulin G (IgG) antibody levels.
- Non-pregnant women with IBD—control group: IBD female patients in reproductive age, included in a prospective study conducted at Rabin medical center, between December 2020 and May 2021, to assess response to the BNT162b2 mRNA COVID-19 vaccine in IBD patients and healthy controls. Non-pregnant women with IBD were selected according to age, IBD type, and anti-TNF therapy to compare with pregnant women with IBD.
2.1. Study Procedures and Data Collection
- Pregnant women with IBD group: patients were evaluated at each pregnancy trimester for regular clinical assessment and follow-up. Laboratory tests were performed at each pregnancy trimester visit, including COVID-19 serology (SARS-CoV-2 IgG quantitative testing, anti-S and SARS-CoV-2 nucleocapsid (N) IgG). In consenting patients, an umbilical cord blood sample was obtained within 30 min of delivery. During regular follow-up visits in the joint IBD-pregnancy clinics, data regarding baseline demographics and IBD characteristics were collected, including age, BMI, parity, smoking status, comorbidities, IBD type and phenotype, IBD-related surgery, IBD-related medications during pregnancy, date and gestational age at the first and second vaccine doses and gestational age at birth. The time interval between the second vaccine dose and blood sample obtainment for COVID-19 serology was calculated.
- Pregnant women without IBD—control group: upon admittance to the delivery room or operating room in cases of elective cesarean delivery, maternal blood samples were obtained for COVID-19 serology (SARS-CoV-2 IgG quantitative testing, anti-S and SARS-CoV-2 nucleocapsid (N)-IgG). An umbilical cord blood sample was obtained within 30 min of delivery. After enrolment, demographic and clinical data were collected, including maternal age, BMI, parity, date and gestational age at the first and second vaccine doses and gestational age at birth. The time interval between the second vaccine dose and delivery was calculated.
- Non-pregnant women with IBD—control group: at enrollment, patients were assessed for baseline demographics and IBD characteristics. Blood samples for COVID-19 serology were obtained 21–35 days after the second vaccine dose (SARS-CoV-2 IgG quantitative testing, anti-S and SARS-CoV-2 nucleocapsid (N) IgG). The time interval between the second vaccine dose and blood sample obtainment for COVID-19 serology was calculated.
2.2. Laboratory Methods
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Vaccine Administration and Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Authors Statement
References
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. Can. Med. Assoc. J. 2021, 193, E540–E548. [Google Scholar] [CrossRef] [PubMed]
- Antoun, L.; El Taweel, N.; Ahmed, I.; Patni, S.; Honest, H. Maternal COVID-19 infection, clinical characteristics, pregnancy, and neonatal outcome: A prospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100107. [Google Scholar] [CrossRef] [PubMed]
- CDC. COVID-19 Vaccines While Pregnant or Breastfeeding. Cent. Dis. Control 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html (accessed on 9 September 2022).
- American Colleague of Obstetrics and Gynecology. Vaccinating Pregnant and Lactating Patients Against Summary of Key Information and Recommendations. ACOG Web 2021. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjr7JHEh4r7AhXo63MBHSDVAakQFnoECBEQAQ&url=http%3A%2F%2Fwww.aofog.net%2Fpdf%2FVaccinating%2520Pregnant%2520and%2520Lactating%2520Patients%2520Against%2520COVID-19%2520_%2520ACOG.pdf&usg=AOvVaw2zL-Wxm3-JGRAgltnferwO (accessed on 9 September 2022).
- Poliquin, V. SOGC Statement on COVID-19 Vaccination in Pregnancy. Ott. Soc. Obstet. Gynaecol. Can. 2021. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjJy9KXiIr7AhXSluYKHcQSASMQFnoECAsQAQ&url=https%3A%2F%2Fsogc.org%2Fcommon%2FUploaded%2520files%2FLatest%2520News%2FSOGC_Statement_COVID-19_Vaccination_in_Pregnancy.pdf&usg=AOvVaw1LcvtZ0ZrRfoATR9C0jTAy (accessed on 9 September 2022).
- Stafford, I.A.; Parchem, J.G.; Sibai, B.M. The COVID-19 vaccine in pregnancy: Risks benefits and recommendations. Am. J. Obstet. Gynecol. 2021, 224, 484–495. [Google Scholar] [CrossRef]
- Giles, M.; Gunatilaka, A.; Palmer, K.; Sharma, K.; Roach, V. Alignment of national COVID-19 vaccine recommendations for pregnant and lactating women. Bull. World Health Organ. 2021, 99, 739–746. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Seow, C.H.; Maxwell, C.; Huang, V.; Leung, Y.; Jones, J.; Leontiadis, G.I.; Tse, F.; Mahadevan, U.; van der Woude, C.J.; et al. The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterology 2016, 150, 734–757. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohn’s Colitis 2021, 16, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Van Der Woude, C.; Ardizzone, S.; Bengtson, M.; Fiorino, G.; Fraser, G.; Katsanos, K.; Kolacek, S.; Juillerat, P.; Mulders, A.; Pedersen, N.; et al. The Second European Evidenced-Based Consensus on Reproduction and Pregnancy in Inflammatory Bowel Disease. J. Crohn’s Colitis 2015, 9, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, U.; Robinson, C.; Bernasko, N.; Boland, B.; Chambers, C.D.; Dubinsky, M.; Friedman, S.; Kane, S.; Manthey, J.; Sauberan, J.; et al. Inflammatory Bowel Disease in Pregnancy Clinical Care Pathway: A Report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology 2019, 156, 1508–1524. [Google Scholar] [CrossRef]
- Rubin, R. Pregnant People’s Paradox—Excluded from Vaccine Trials Despite Having a Higher Risk of COVID-19 Complications. JAMA 2021, 325, 1027–1028. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Biron-Shental, T.; Makov-Assif, M.; Key, C.; Kohane, I.S.; Hernán, M.A.; Lipsitch, M.; Hernandez-Diaz, S.; Reis, B.Y.; et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat. Med. 2021, 27, 1693–1695. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.-R.Y.; McMahan, K.; Yu, J.; Tostanoski, L.H.; Aguayo, R.; Ansel, J.; Chandrashekar, A.; Patel, S.; Bondzie, E.A.; Sellers, D.; et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA 2021, 325, 2370. [Google Scholar] [CrossRef]
- Beharier, O.; Mayo, R.P.; Raz, T.; Sacks, K.N.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Investig. 2021, 131, e150319. [Google Scholar] [CrossRef]
- Doherty, J.; Fennessy, S.; Stack, R.; Morain, N.O.; Cullen, G.; Ryan, E.J.; De Gascun, C.; Doherty, G.A. Review Article: Vaccination for patients with inflammatory bowel disease during the COVID-19 pandemic. Aliment. Pharmacol. Ther. 2021, 54, 1110–1123. [Google Scholar] [CrossRef]
- Rahier, J.F.; Magro, F.; Abreu, C.; Armuzzi, A.; Ben-Horin, S.; Chowers, Y.; Cottone, M.; de Ridder, L.; Doherty, G.; Ehehalt, R.; et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J. Crohn’s Colitis 2014, 8, 443–468. [Google Scholar] [CrossRef]
- Fiorino, G.; Peyrin-Biroulet, L.; Naccarato, P.; Szabò, H.; Sociale, O.R.; Vetrano, S.; Fries, W.; Montanelli, A.; Repici, A.; Malesci, A.; et al. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: A prospective study. Inflamm. Bowel Dis. 2012, 18, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Melmed, G.Y.; Agarwal, N.; Frenck, R.W.; Ippoliti, A.F.; Ibanez, P.; A Papadakis, K.; Simpson, P.; Barolet-Garcia, C.; Ward, J.; Targan, S.R.; et al. Immunosuppression Impairs Response to Pneumococcal Polysaccharide Vaccination in Patients with Inflammatory Bowel Disease. Am. J. Gastroenterol. 2010, 105, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Kim, H.-S.; Ye, B.D.; Lee, K.-M.; Kim, Y.S.; Rhee, S.Y.; Kim, H.-J.; Yang, S.-K.; Moon, W.; Koo, J.-S.; et al. Patients with Crohn’s disease on anti-tumor necrosis factor therapy are at significant risk of inadequate response to the 23-valent pneumococcal polysaccharide vaccine. J. Crohn’s Colitis 2014, 8, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.K.; Kuenzig, M.E.; Windsor, J.W.; Ghia, J.-E.; Griffiths, A.M.; Panaccione, R.; Seow, C.H.; I Benchimol, E.; Bernstein, C.N.; Bitton, A.; et al. Crohn’s and Colitis Canada’s 2021 Impact of COVID-19 and Inflammatory Bowel Disease in Canada: COVID-19 Vaccines—Biology, Current Evidence and Recommendations. J. Can. Assoc. Gastroenterol. 2021, 4, S54–S60. [Google Scholar] [CrossRef] [PubMed]
- Zion, R.L.; Focht, G.; Lujan, R.; Mendelovici, A.; Friss, C.; Greenfeld, S.; Kariv, R.; Ben-Tov, A.; Matz, E.; Nevo, D.; et al. DOP26 COVID-19 vaccine effectiveness in Inflammatory Bowel Disease patients on tumor-necrosis factor inhibitors: Real world data from a mass-vaccination campaign. J. Crohn’s Colitis 2022, 16, i078–i079. [Google Scholar] [CrossRef]
- Edelman-Klapper, H.; Zittan, E.; Shitrit, A.B.-G.; Rabinowitz, K.M.; Goren, I.; Avni-Biron, I.; Ollech, J.E.; Lichtenstein, L.; Banai-Eran, H.; Yanai, H.; et al. Lower Serologic Response to COVID-19 mRNA Vaccine in Patients with Inflammatory Bowel Diseases Treated with Anti-TNFα. Gastroenterology 2021, 162, 454–467. [Google Scholar] [CrossRef]
- Chanchlani, N.; Lin, S.; Chee, D.; Hamilton, B.; Nice, R.; Arkir, Z.; Bewshea, C.; Cipriano, B.; Derikx, L.A.A.P.; Dunlop, A.; et al. Adalimumab and Infliximab Impair SARS-CoV-2 Antibody Responses: Results from a Therapeutic Drug Monitoring Study in 11 422 Biologic-Treated Patients. J. Crohn’s Colitis 2021, 16, 389–397. [Google Scholar] [CrossRef]
- A Kennedy, N.; Lin, S.; Goodhand, J.R.; Chanchlani, N.; Hamilton, B.; Bewshea, C.; Nice, R.; Chee, D.; Cummings, J.F.; Fraser, A.; et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021, 70, 1884–1893. [Google Scholar] [CrossRef]
- A Kennedy, N.; Goodhand, J.R.; Bewshea, C.; Nice, R.; Chee, D.; Lin, S.; Chanchlani, N.; Butterworth, J.; Cooney, R.; Croft, N.M.; et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut 2021, 70, 865–875. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Notarte, K.I.; Guerrero-Arguero, I.; Velasco, J.V.; Ver, A.T.; de Oliveira, M.H.S.; Catahay, J.A.; Khan, S.R.; Pastrana, A.; Juszczyk, G.; Torrelles, J.B.; et al. Characterization of the significant decline in humoral immune response six months post-SARS-CoV-2 mRNA vaccination: A systematic review. J. Med. Virol. 2022, 94, 2939–2961. [Google Scholar] [CrossRef] [PubMed]
- Laing, E.D.; Weiss, C.D.; Samuels, E.C.; Coggins, S.A.; Wang, W.; Wang, R.; Vassell, R.; Sterling, S.L.; Tso, M.S.; Conner, T.; et al. Durability of Antibody Response and Frequency of SARS-CoV-2 Infection 6 Months after COVID-19 Vaccination in Healthcare Workers. Emerg. Infect. Dis. 2022, 28, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, B.; Richards, N.E.; Workman, L.J.; Patel, J.; Muehling, L.M.; Canderan, G.; Murphy, D.D.; Brovero, S.G.; Ailsworth, S.M.; Eschenbacher, W.H.; et al. Trajectory of IgG to SARS-CoV-2 After Vaccination with BNT162b2 or mRNA-1273 in an Employee Cohort and Comparison with Natural Infection. Front. Immunol. 2022, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.; James, D.; Singh, A.K.; Dutta, U.; Sebastian, S.; Sharma, V. Effectiveness and Durability of COVID-19 Vaccination in 9447 Patients With IBD: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 1456–1479. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, K.M.; Navon, M.; Edelman-Klapper, H.; Zittan, E.; Shitrit, A.B.-G.; Goren, I.; Avni-Biron, I.; E Ollech, J.; Lichtenstein, L.; Banai-Eran, H.; et al. P313 Within, 6 months from COVID-19 BNT162b2 vaccine patients with Inflammatory Bowel Diseases treated with Anti-TNFα have significantly lower serologic responses. J. Crohn’s Colitis 2022, 16, i337–i338. [Google Scholar] [CrossRef]
- Kugelman, N.; Nahshon, C.; Shaked-Mishan, P.; Cohen, N.; Sher, M.L.; Gruber, M.; Marom, I.; Zolotarevsky, A.; Lavie, O.; Damti, A.; et al. Maternal and Neonatal SARS-CoV-2 Immunoglobulin G Antibody Levels at Delivery After Receipt of the BNT162b2 Messenger RNA COVID-19 Vaccine During the Second Trimester of Pregnancy. JAMA Pediatr. 2022, 176, 290. [Google Scholar] [CrossRef] [PubMed]
- Nir, O.; Schwartz, A.; Toussia-Cohen, S.; Leibovitch, L.; Strauss, T.; Asraf, K.; Doolman, R.; Sharabi, S.; Cohen, C.; Lustig, Y.; et al. Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy. Am. J. Obstet. Gynecol. MFM 2022, 4, 100492. [Google Scholar] [CrossRef]
- Atyeo, C.G.; Shook, L.L.; Brigida, S.; De Guzman, R.M.; Demidkin, S.; Muir, C.; Akinwunmi, B.; Baez, A.M.; Sheehan, M.L.; McSweeney, E.; et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. Nat. Commun. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Soysal, A.; Bilazer, C.; Gönüllü, E.; Barın, E.; Çivilibal, M. Cord blood antibody following maternal SARS-CoV-2 inactive vaccine (CoronaVac) administration during the pregnancy. Hum. Vaccines Immunother. 2021, 17, 1–3. [Google Scholar] [CrossRef]
- De Rose, D.U.; Salvatori, G.; Dotta, A.; Auriti, C. SARS-CoV-2 Vaccines during Pregnancy and Breastfeeding: A Systematic Review of Maternal and Neonatal Outcomes. Viruses 2022, 14, 539. [Google Scholar] [CrossRef]
- Liu, S.; Zhong, J.; Zhang, D. Transplacental Transfer of Maternal Antibody against SARS-CoV-2 and Its Influencing Factors: A Review. Vaccines 2022, 10, 1083. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, K.M.; Navon, M.; Edelman-Klapper, H.; Zittan, E.; Shitrit, A.B.-G.; Goren, I.; Avni-Biron, I.; Ollech, J.E.; Lichtenstein, L.; Banai-Eran, H.; et al. Anti-TNFα Treatment Impairs Long-Term Immune Responses to COVID-19 mRNA Vaccine in Patients with Inflammatory Bowel Diseases. Vaccines 2022, 10, 1186. [Google Scholar] [CrossRef] [PubMed]
| Pregnant with IBD | Pregnant without IBD | Non-Pregnant, IBD | p Value | |
|---|---|---|---|---|
| Characteristic | N = 36 | N = 61 | N = 42 | |
| Mean age, years (SD) | 32.1 (5.5) | 29.4 (5.5) | 31.5 (6.5) | 0.073 |
| Mean weight, kg (SD) | 70.8 (15.1) | 64.8 (13.1) | 61.3 (11.6) | 0.012 |
| Mean BMI, kg/m2 (SD) | 26.9 (4.9) | 24.2 (4.7) | 23.6 (4.6) | 0.004 |
| Parity (SD) | 2.3 (2.1) | 2.2 (1.9) | 0.9 (1.5) | <0.001 |
| Smoking: | ||||
| Never | 32 (88.9%) | 59 (98.3%) | 37 (88.1%) | 0.085 |
| Past/present | 4 (11.1%) | 1 (1.7%) | 5 (11.9%) | |
| IBD type: | ||||
| Crohn’s disease | 22 (61.1%) | - | 29 (69.0%) | 0.484 |
| Ulcerative colitis | 14 (38.9%) | - | 13 (31.0%) | |
| Mean CRP (SD) | 1.1 (1.8) | - | 0.8 (1.5) | 0.006 |
| Anti-TNF: | ||||
| No | 25 (69.4%) | - | 29 (69.0%) | 1.000 |
| Yes | 11 (30.6%) | - | 13 (31.0%) | |
| Gestational age at delivery, mean (SD) | 38.8 (1.9) | 39.1 (2.6) | 0.570 | |
| Mode of delivery: | ||||
| Vaginal | 21 (61.8%) | 55 (91.7%) | - | <0.001 |
| Cesarean | 13 (38.2%) | 5 (8.3%) | - | |
| Birthweight, mean (SD) | 3135 (527.8) | 3235 (527.2) | - | 0.390 |
| Mean gestational age at 1st vaccine dose administration (weeks) | 19.7 (8.8) | 20.1 (6.7) | 0.786 | |
| Mean gestational age at 2nd vaccine dose administration (weeks) | 22.0 (8.2) | 23.2 (6.8) | 0.464 | |
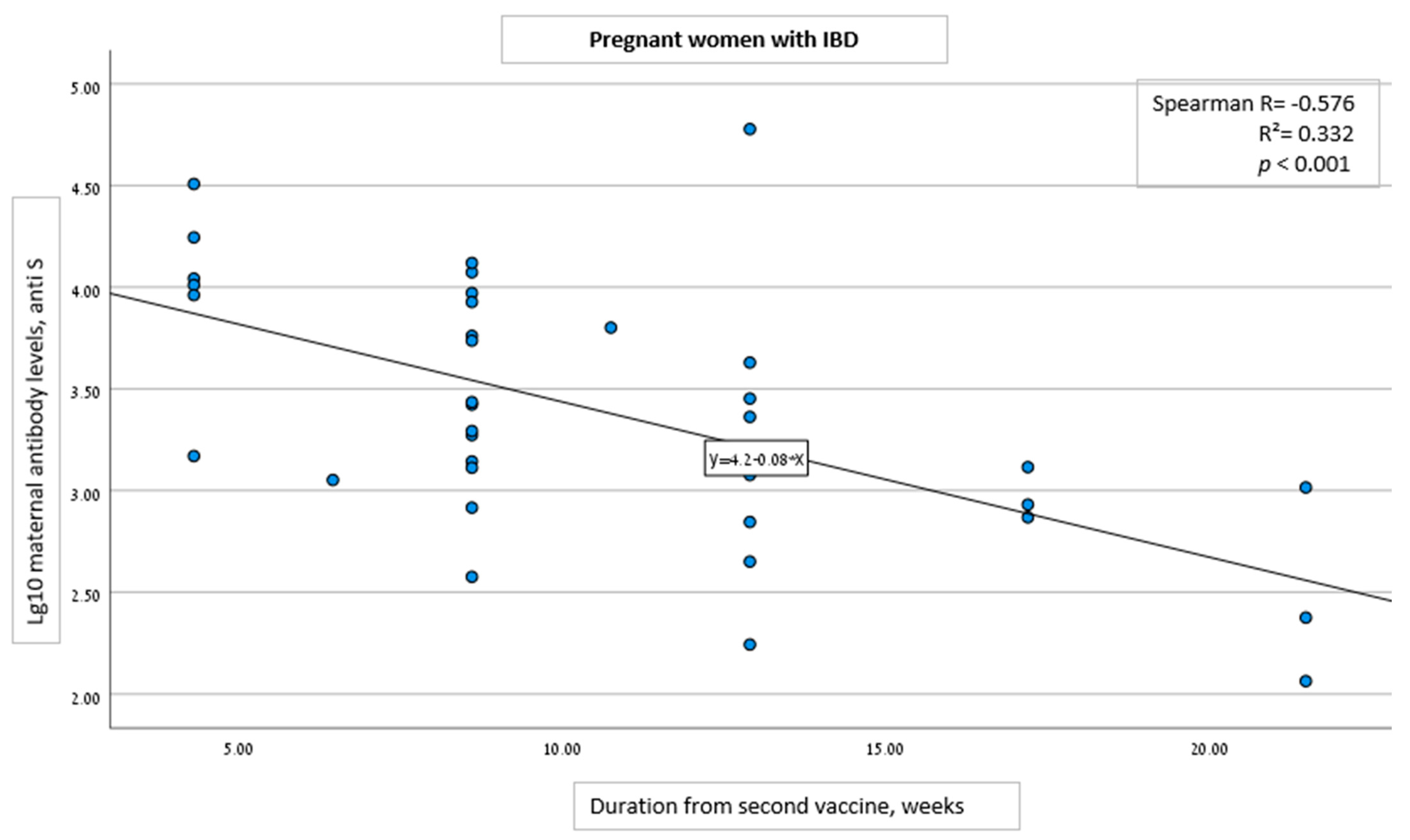
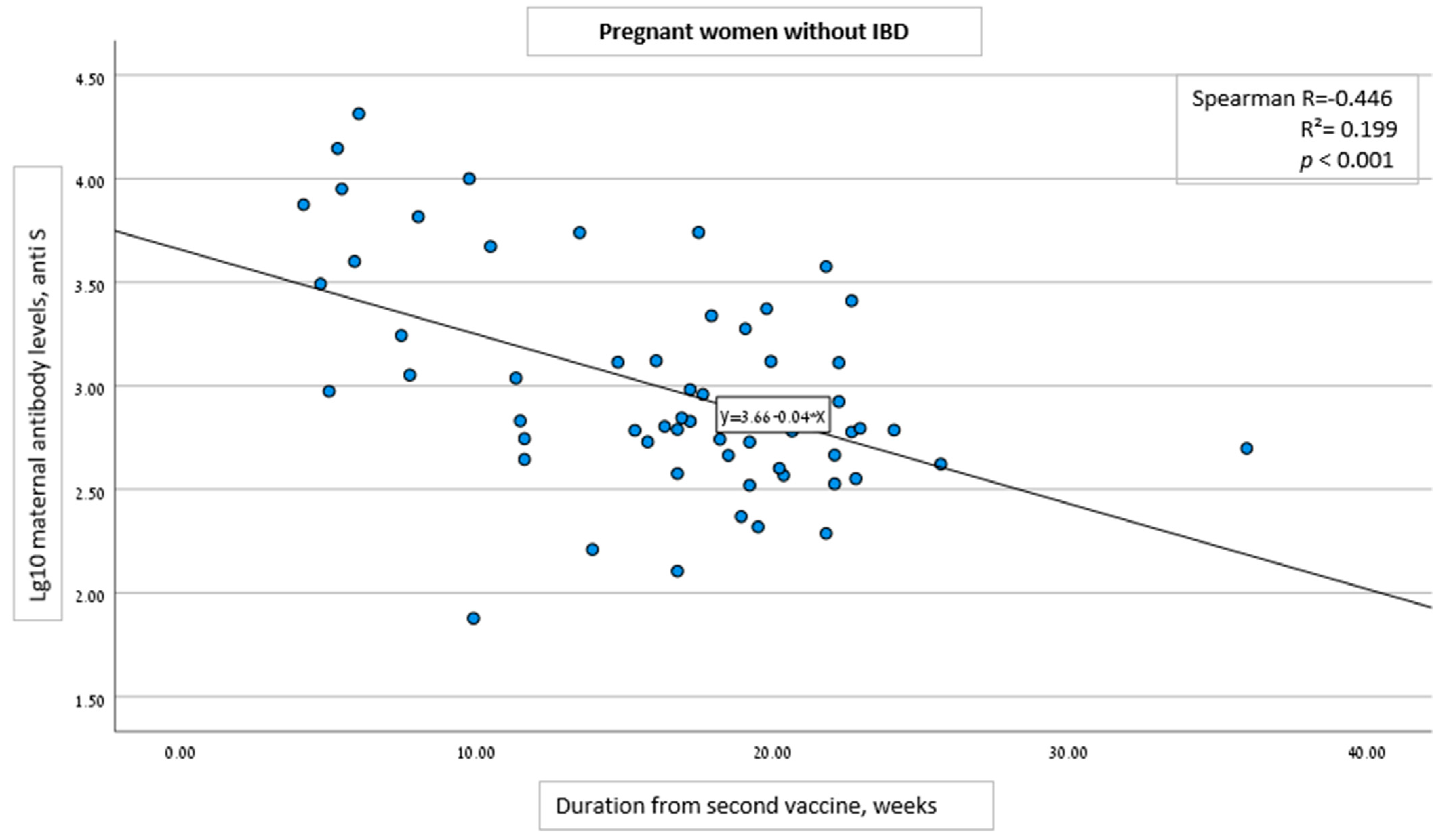
| Time duration from 2nd vaccine to serology assessment, weeks, (SD) | 10.6 (4.9) | 16.4 (6.3) | 4.3 (0.99) | <0.001 |
| Pregnant with IBD (N = 36) | Non-Pregnant with IBD (N = 42) | |
|---|---|---|
| Mean age, years (SD) | 32.1 (5.5) | 31.5 (6.5) |
| Mean BMI, kg/m2 (SD) | 26.9 (4.9) | 23.6 (4.6) |
| Smoking status: | ||
| Present/past | 4 (11.1%) | 5 (11.9%) |
| No | 32 (88.9%) | 37 (88.1%) |
| Comorbidities | 7 (19.4%) | 12 (28.6%) |
| IBD-related surgery | 8 (22.9%) | - |
| IBD phenotype: | ||
| CD | 22 (61.1%) | 29 (69%) |
| UC | 14 (38.9%) | 13 (31%) |
| Medical therapy: | ||
| Infliximab | 5 (13.9%) | 5 (11.9%) |
| Adalimumab | 6 (16.7%) | 9 (21.4%) |
| Vedolizumab | 4 (11.1%) | 8 (19.0%) |
| Ustekinumab | 2 (5.6%) | 4 (9.5%) |
| 5-ASA | 15 (41.7%) | 12 (28.6%) |
| Corticosteroids | 2 (5.6%) | 7 (16.7%) |
| 6mp/azathioprine | 8 (22.2%) | 5 (11.9%) |
| JAK inhibitor | 0 (0%) | 4 (9.5%) |
| No medical therapy | 4 (11.1%) | 3 (7.1%) |
| Pregnant with IBD | Pregnant without IBD | Non-Pregnant with IBD | p Value | |
|---|---|---|---|---|
| SARS-CoV-2 IgG antibody levels, GMC anti-S (95% CI) | N = 36 | N = 61 | N = 42 | |
| Total | 2442 (1489–4004) | 977 (716–1332) | 7416 (4966–11,073) | <0.001 |
| Anti-TNF | ||||
| No | 2457 (1419–4253) n = 25 | - | 8920 (5812–13,690) n = 29 | |
| Yes | 2408 (718–8071) n = 11 | - | 4911 (1915–12,595) n = 13 | |
| IBD type | ||||
| Crohn’s disease | 1900 (1061–3403) n = 22 | - | 7601 (4679–12,346) n = 29 | |
| Ulcerative colitis | 3620 (1403–9337) n = 14 | - | 7018 (3081–15,985) n = 13 | |
| Variable | Change in Antibody Level% (95% CI) | p Value |
|---|---|---|
| Pregnant women with vs. without IBD | ||
| Per 1-week increase from 2nd vaccine dose to serology analysis | −0.124 (−0.162 to −0.086) | <0.001 |
| Group | ||
| Pregnant IBD | 0.090 | 0.365 |
| Pregnant without IBD | reference | |
| Pregnancy week at 2nd vaccine | −0.121 | 0.262 |
| IBD pregnant vs. IBD non-pregnant | ||
| Per 1-week increase from 2nd vaccine dose to serology analysis | −0.172 (−0.233 to −0.110) | <0.001 |
| Group | ||
| IBD pregnant | −0.021 | 0.876 |
| IBD non-pregnant | reference | |
| Variable | Change in antibody level% (95% CI) | p value |
| Pregnant women with vs. without IBD | ||
| Per 1-week increase from 2nd vaccine dose to serology analysis | −0.124 (−0.162 to −0.086) | <0.001 |
| Group | ||
| Pregnant IBD | 0.090 | 0.365 |
| Pregnant without IBD | reference | |
| Pregnancy week at 2nd vaccine | −0.121 | 0.262 |
| IBD pregnant vs. IBD non-pregnant | ||
| Per 1-week increase from 2nd vaccine dose to serology analysis | −0.172 (−0.233 to −0.110) | <0.001 |
| Group | ||
| IBD pregnant | −0.021 | 0.876 |
| IBD non-pregnant | reference | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avni Biron, I.; Maayan, Y.; Mishael, T.; Hadar, E.; Neeman, M.; Plitman Mayo, R.; Sela, H.Y.; Yagel, S.; Goldenberg, R.; Ben Ya’acov, A.; et al. SARS-CoV-2 IgG Antibody Levels in Women with IBD Vaccinated during Pregnancy. Vaccines 2022, 10, 1833. https://doi.org/10.3390/vaccines10111833
Avni Biron I, Maayan Y, Mishael T, Hadar E, Neeman M, Plitman Mayo R, Sela HY, Yagel S, Goldenberg R, Ben Ya’acov A, et al. SARS-CoV-2 IgG Antibody Levels in Women with IBD Vaccinated during Pregnancy. Vaccines. 2022; 10(11):1833. https://doi.org/10.3390/vaccines10111833
Chicago/Turabian StyleAvni Biron, Irit, Yair Maayan, Tali Mishael, Eran Hadar, Michal Neeman, Romina Plitman Mayo, Hen Y. Sela, Simcha Yagel, Rosalind Goldenberg, Ami Ben Ya’acov, and et al. 2022. "SARS-CoV-2 IgG Antibody Levels in Women with IBD Vaccinated during Pregnancy" Vaccines 10, no. 11: 1833. https://doi.org/10.3390/vaccines10111833
APA StyleAvni Biron, I., Maayan, Y., Mishael, T., Hadar, E., Neeman, M., Plitman Mayo, R., Sela, H. Y., Yagel, S., Goldenberg, R., Ben Ya’acov, A., Grisaru Granovsky, S., Ollech, J. E., Edelman-Klapper, H., Rabinowitz, K. M., Pauker, M. H., Yanai, H., Goren, S., Cohen, D., Dotan, I., & Bar-Gil Shitrit, A. (2022). SARS-CoV-2 IgG Antibody Levels in Women with IBD Vaccinated during Pregnancy. Vaccines, 10(11), 1833. https://doi.org/10.3390/vaccines10111833








