Measles Virus Neutralizing Antibody Response and Durability Two Years after One or Two Doses of Measles–Mumps–Rubella Vaccine among Young Seronegative Healthcare Workers
Abstract
1. Introduction
2. Materials and Methods
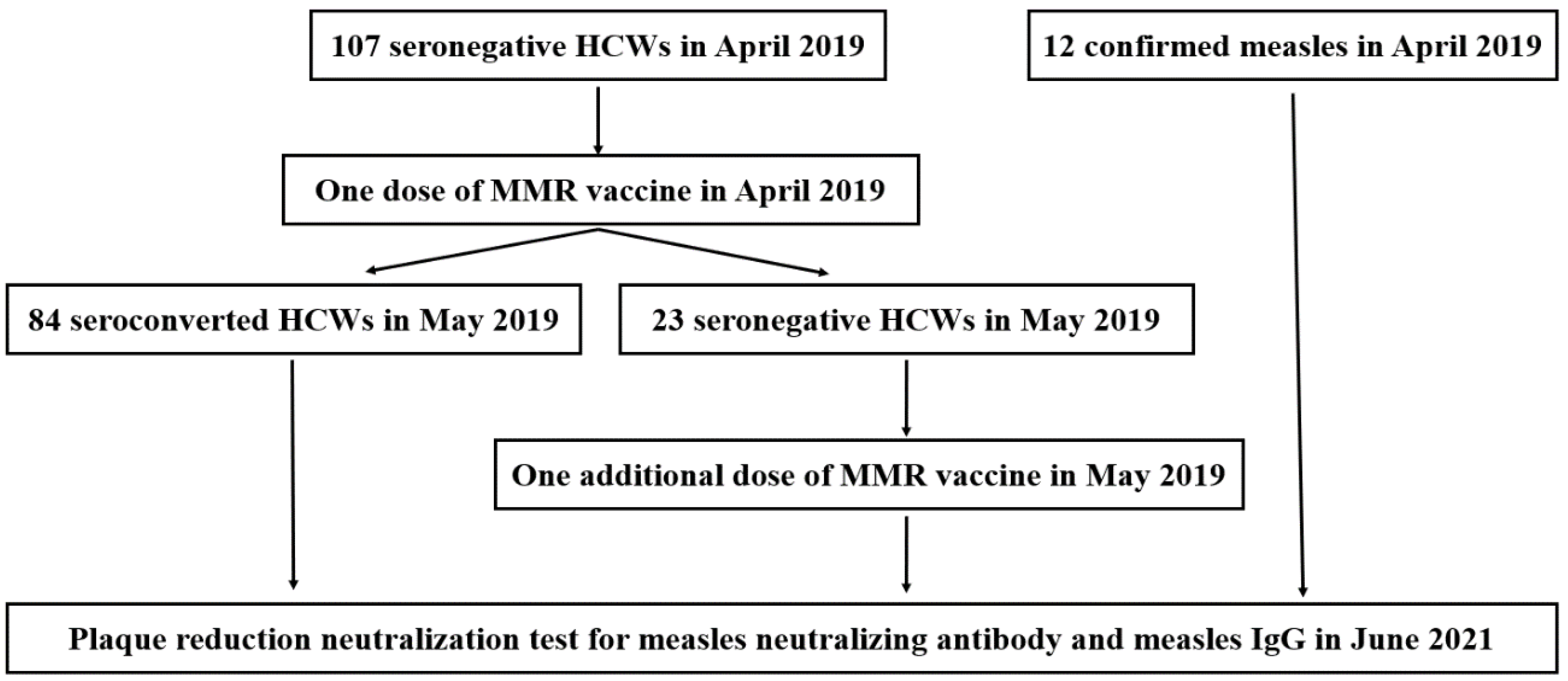
2.1. Study Population and Clinical Data Collection
2.2. Plaque Reduction Neutralization Test
2.3. Chemiluminescent Immunoassay for Measles-Specific IgGs Assay
2.4. Statistical Analysis
3. Results
3.1. Enrollment and Baseline Characteristics
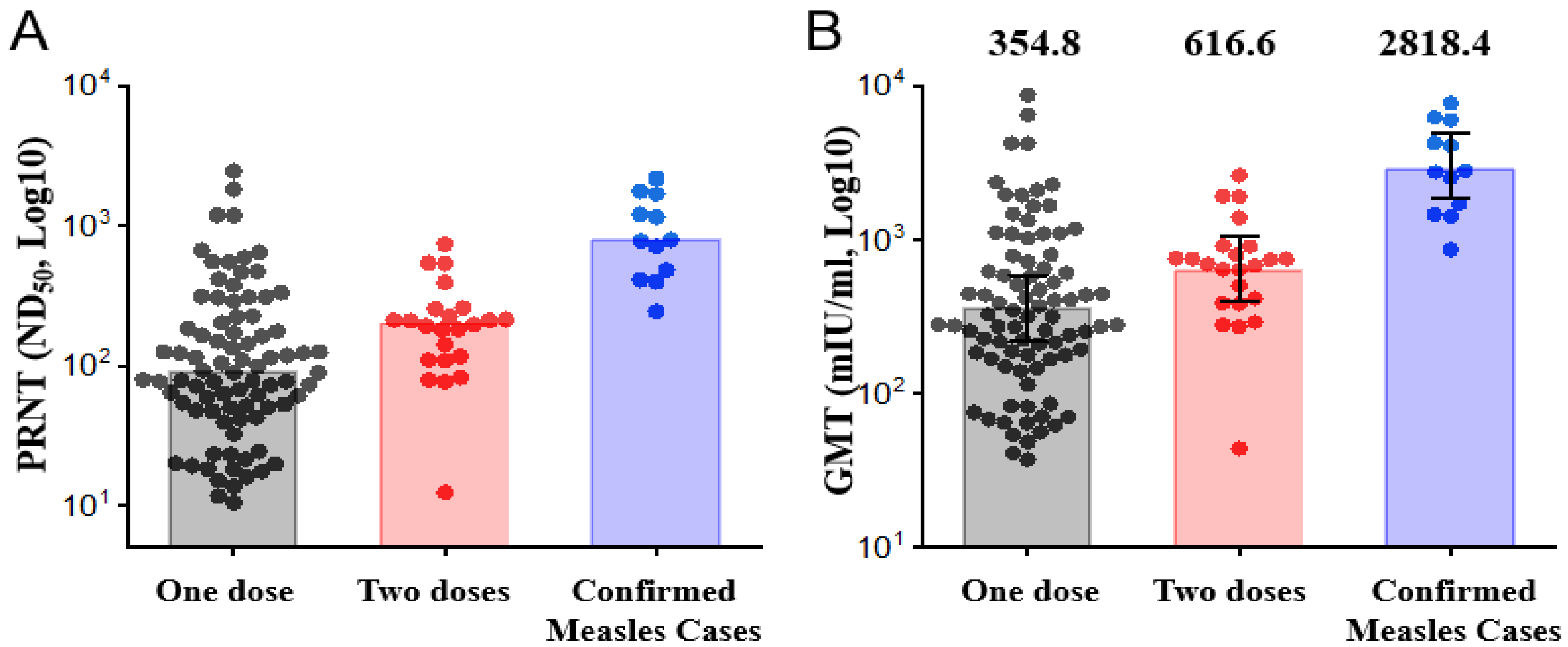
3.2. Measles Neutralizing Antibody Response 2 Years after One or Two Doses of MMR Booster
3.3. Measles-Specific IgGs Response 2 Years after One or Two Doses of the MMR Vaccine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hübschen, J.M.; Gouandjika-Vasilache, I.; Dina, J. Measles. Lancet 2022, 399, 678–690. [Google Scholar] [CrossRef]
- O’Connor, P.; Jankovic, D.; Muscat, M.; Ben-Mamou, M.; Reef, S.; Papania, M.; Singh, S.; Kaloumenos, T.; Butler, R.; Datta, S. Measles and Rubella Elimination in the WHO Region for Europe: Progress and Challenges. Clin. Microbiol. Infect. 2017, 23, 504–510. [Google Scholar] [CrossRef]
- Patel, M.K.; Orenstein, W.A. Classification of Global Measles Cases in 2013-17 as Due to Policy or Vaccination Failure: A Retrospective Review of Global Surveillance Data. Lancet Glob. Health 2019, 7, e313–e320. [Google Scholar] [CrossRef]
- Kang, H.J.; Han, Y.W.; Kim, S.J.; Kim, Y.-J.; Kim, A.-R.; Kim, J.A.; Jung, H.-D.; Eom, H.E.; Park, O.; Kim, S.S. An Increasing, Potentially Measles-Susceptible Population over Time after Vaccination in Korea. Vaccine 2017, 35, 4126–4132. [Google Scholar] [CrossRef]
- Kontio, M.; Jokinen, S.; Paunio, M.; Peltola, H.; Davidkin, I. Waning Antibody Levels and Avidity: Implications for MMR Vaccine-Induced Protection. J. Infect. Dis. 2012, 206, 1542–1548. [Google Scholar] [CrossRef]
- Basu, S.; Giri, P.; Adisesh, A.; McNAUGHT, R. Healthcare Workers and Measles-Mumps-Rubella (MMR) Status: How Worried Should We Be about Further Outbreaks? Epidemiol. Infect. 2014, 142, 1688–1694. [Google Scholar] [CrossRef]
- Freund, R.; Krivine, A.; Prévost, V.; Cantin, D.; Aslangul, E.; Avril, M.-F.; Claessens, Y.-E.; Rozenberg, F.; Casetta, A.; Baixench, M.-T.; et al. Measles Immunity and Measles Vaccine Acceptance among Healthcare Workers in Paris, France. J. Hosp. Infect. 2013, 84, 38–43. [Google Scholar] [CrossRef]
- Jung, J.; Kim, S.-K.; Kwak, S.H.; Hong, M.J.; Kim, S.-H. Seroprevalence of Measles in Healthcare Workers in South Korea. Infect. Chemother. 2019, 51, 58. [Google Scholar] [CrossRef]
- Botelho-Nevers, E.; Gautret, P.; Biellik, R.; Brouqui, P. Nosocomial Transmission of Measles: An Updated Review. Vaccine 2012, 30, 3996–4001. [Google Scholar] [CrossRef]
- Sydnor, E.; Perl, T.M. Healthcare Providers as Sources of Vaccine-Preventable Diseases. Vaccine 2014, 32, 4814–4822. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Botelho-Nevers, E.; Brantsæter, A.B.; Carlsson, R.-M.; Heininger, U.; Hübschen, J.M.; Josefsdottir, K.S.; Kassianos, G.; Kyncl, J.; Ledda, C.; et al. Vaccination of Healthcare Personnel in Europe: Update to Current Policies. Vaccine 2019, 37, 7576–7584. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Park, H.-Y.; Kim, S.-H. A Third Dose of Measles Vaccine Is Needed in Young Korean Health Care Workers. Vaccine 2018, 36, 3888–3889. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.; Park, D.W.; Kim, K.N.; Kim, M.J.; Kim, S.-H.; Kim, J.Y.; Park, S.E.; Park, S.Y.; Eun, B.W.; Lee, M.S.; et al. Report of the Korean Society of Infectious Diseases Roundtable Discussion on Responses to the Measles Outbreaks in Korea in 2019. Infect. Chemother. 2021, 53, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention (CDC). Immunization of Health-Care Personnel: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2011, 60, 1–45. [Google Scholar]
- Fiebelkorn, A.P.; Coleman, L.A.; Belongia, E.A.; Freeman, S.K.; York, D.; Bi, D.; Kulkarni, A.; Audet, S.; Mercader, S.; McGrew, M.; et al. Measles Virus Neutralizing Antibody Response, Cell-Mediated Immunity, and Immunoglobulin G Antibody Avidity before and after Receipt of a Third Dose of Measles, Mumps, and Rubella Vaccine in Young Adults. J. Infect. Dis. 2016, 213, 1115–1123. [Google Scholar] [CrossRef]
- Han, S.B.; Park, S.H.; Yi, Y.; Ji, S.K.; Jang, S.H.; Park, M.H.; Lee, J.E.; Jeong, H.S.; Shin, S. Measles Seroprevalence among Healthcare Workers in South Korea during the Post-Elimination Period. Hum. Vaccin. Immunother. 2021, 17, 2517–2521. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Stefanizzi, P.; De Nitto, S.; Larocca, A.M.V.; Germinario, C.; Tafuri, S. Long-Term Immunogenicity of Measles Vaccine: An Italian Retrospective Cohort Study. J. Infect. Dis. 2020, 221, 721–728. [Google Scholar] [CrossRef]
- Kim, C.-J.; Bae, J.-Y.; Jun, K.-I.; Chung, H.-S.; Kim, A.; Kim, J.; Son, H.-J.; Lee, M.; Choi, H.-J. Risk of Absence of Measles Antibody in Healthcare Personnel and Efficacy of Booster Vaccination. Vaccines 2021, 9, 501. [Google Scholar] [CrossRef]
- Song, K.; Lee, J.M.; Lee, E.J.; Lee, B.R.; Choi, J.Y.; Yun, J.; Lee, S.N.; Jang, M.Y.; Kim, H.W.; Kim, H.-S.; et al. Control of a Nosocomial Measles Outbreak among Previously Vaccinated Adults in a Population with High Vaccine Coverage: Korea, 2019. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 455–466. [Google Scholar] [CrossRef]
- Cohen, B.J.; Audet, S.; Andrews, N.; Beeler, J. Plaque Reduction Neutralization Test for Measles Antibodies: Description of a Standardised Laboratory Method for Use in Immunogenicity Studies of Aerosol Vaccination. Vaccine 2007, 26, 59–66. [Google Scholar] [CrossRef]
- Cohen, B.J.; Parry, R.P.; Doblas, D.; Samuel, D.; Warrener, L.; Andrews, N.; Brown, D. Measles Immunity Testing: Comparison of Two Measles IgG ELISAs with Plaque Reduction Neutralisation Assay. J. Virol. Methods 2006, 131, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Haralambieva, I.H.; Ovsyannikova, I.G.; O’Byrne, M.; Pankratz, V.S.; Jacobson, R.M.; Poland, G.A. A Large Observational Study to Concurrently Assess Persistence of Measles Specific B-Cell and T-Cell Immunity in Individuals Following Two Doses of MMR Vaccine. Vaccine 2011, 29, 4485–4491. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.T.; Markowitz, L.E.; Albrecht, P.; Stewart, J.A.; Mofenson, L.M.; Preblud, S.R.; Orenstein, W.A. Measles Antibody: Reevaluation of Protective Titers. J. Infect. Dis. 1990, 162, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Samb, B.; Aaby, P.; Whittle, H.C.; Coll Seck, A.M.; Rahman, S.; Bennett, J.; Markowitz, L.; Simondon, F. Serologic Status and Measles Attack Rates among Vaccinated and Unvaccinated Children in Rural Senegal. Pediatr. Infect. Dis. J. 1995, 14, 203–208. [Google Scholar] [CrossRef][Green Version]
- LeBaron, C.W.; Beeler, J.; Sullivan, B.J.; Forghani, B.; Bi, D.; Beck, C.; Audet, S.; Gargiullo, P. Persistence of Measles Antibodies after 2 Doses of Measles Vaccine in a Postelimination Environment. Arch. Pediatr. Adolesc. Med. 2007, 161, 294. [Google Scholar] [CrossRef]
- Davidkin, I.; Jokinen, S.; Broman, M.; Leinikki, P.; Peltola, H. Persistence of Measles, Mumps, and Rubella Antibodies in an MMR-vaccinated Cohort: A 20-year Follow-up. J. Infect. Dis. 2008, 197, 950–956. [Google Scholar] [CrossRef]
- Davidkin, I.; Valle, M. Vaccine-Induced Measles Virus Antibodies after Two Doses of Combined Measles, Mumps and Rubella Vaccine: A 12-Year Follow-up in Two Cohorts. Vaccine 1998, 16, 2052–2057. [Google Scholar] [CrossRef]
- Carryn, S.; Feyssaguet, M.; Povey, M.; Di Paolo, E. Long-Term Immunogenicity of Measles, Mumps and Rubella-Containing Vaccines in Healthy Young Children: A 10-Year Follow-Up. Vaccine 2019, 37, 5323–5331. [Google Scholar] [CrossRef]
- Seok, H.; Españo, E.; Kim, J.; Jeon, J.H.; Choi, W.S.; Kim, Y.-K.; Kim, J.-K.; Park, D.W. Immunogenicity after Outbreak Response Immunization Activities among Young Healthcare Workers with Secondary Vaccine Failure during the Measles Epidemic in Korea, 2019. BMC Infect. Dis. 2022, 22, 530. [Google Scholar] [CrossRef]
- Kaaijk, P.; Nicolaie, M.A.; van Rooijen, D.; van Houten, M.A.; van der Klis, F.R.; Buisman, A.-M.; van Binnendijk, R.S. Dynamics of the Antibody Response after a Third Dose of Measles-Mumps-Rubella Vaccine Indicate a Slower Decline Compared with a Second Dose. Open Forum Infect. Dis. 2020, 7, ofaa505. [Google Scholar] [CrossRef]
- Kaaijk, P.; Wijmenga-Monsuur, A.J.; Ten Hulscher, H.I.; Kerkhof, J.; Smits, G.; Nicolaie, M.A.; van Houten, M.A.; van Binnendijk, R.S. Antibody Levels at 3-Years Follow-up of a Third Dose of Measles-Mumps-Rubella Vaccine in Young Adults. Vaccines 2022, 10, 132. [Google Scholar] [CrossRef]
- Kennedy, R.B.; Ovsyannikova, I.G.; Thomas, A.; Larrabee, B.R.; Rubin, S.; Poland, G.A. Differential Durability of Immune Responses to Measles and Mumps Following MMR Vaccination. Vaccine 2019, 37, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Ruckdeschel, J.C.; Graziano, K.D.; Mardiney, M.R., Jr. Additional Evidence That the Cell-Associated Immune System Is the Primary Host Defense against Measles (Rubeola). Cell. Immunol. 1975, 17, 11–18. [Google Scholar] [CrossRef]
- Griffin, D.E. Measles Vaccine. Viral Immunol. 2018, 31, 86–95. [Google Scholar] [CrossRef]
- Park, J.W.; Yu, S.N.; Park, E.; Lee, Y.; Park, S.M.; Jeon, M.H. Modified Measles in an Anti-Measles Immunoglobulin G-Negative Healthcare Worker Who Had Received Two Doses of Measles-Containing Vaccine. Infect. Chemother. 2019, 51, 305. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.B.; Rota, J.S.; Hickman, C.J.; Sowers, S.B.; Mercader, S.; Rota, P.A.; Bellini, W.J.; Huang, A.J.; Doll, M.K.; Zucker, J.R.; et al. Outbreak of Measles among Persons with Prior Evidence of Immunity, New York City, 2011. Clin. Infect. Dis. 2014, 58, 1205–1210. [Google Scholar] [CrossRef]
- Choe, Y.J.; Park, Y.-J.; Kim, J.W.; Eom, H.E.; Park, O.; Oh, M.-D.; Lee, J.-K. An Outbreak of Measles in a University in Korea, 2014. J. Korean Med. Sci. 2017, 32, 1876. [Google Scholar] [CrossRef]
- Hales, C.M.; Johnson, E.; Helgenberger, L.; Papania, M.J.; Larzelere, M.; Gopalani, S.V.; Lebo, E.; Wallace, G.; Moturi, E.; Hickman, C.J.; et al. Measles Outbreak Associated with Low Vaccine Effectiveness among Adults in Pohnpei State, Federated States of Micronesia, 2014. Open Forum Infect. Dis. 2016, 3, ofw064. [Google Scholar] [CrossRef]
- Haviari, S.; Bénet, T.; Saadatian-Elahi, M.; André, P.; Loulergue, P.; Vanhems, P. Vaccination of Healthcare Workers: A Review. Hum. Vaccin. Immunother. 2015, 11, 2522–2537. [Google Scholar] [CrossRef]
- Chang, H.-H.; Kim, S.-W.; Kwon, K.T.; Kim, H.I.; Kim, M.J.; Ryu, S.Y.; Kim, H.A.; Hur, J.; Kwon, H.H.; Hong, H.-L. Preliminary Report of Seroprevalence of Anti-Measles Immunoglobulin G among Healthcare Workers of 6 Teaching Hospitals of Daegu, Korea in 2019. Infect. Chemother. 2019, 51, 54. [Google Scholar] [CrossRef]
- Sá Machado, R.; Perez Duque, M.; Almeida, S.; Cruz, I.; Sottomayor, A.; Almeida, I.; Oliveira, J.R.; Antunes, D. Measles Outbreak in a Tertiary Level Hospital, Porto, Portugal, 2018: Challenges in the Post-Elimination Era. Eurosurveillance 2018, 23, 18-00224. [Google Scholar] [CrossRef] [PubMed]
- Gohil, S.K.; Okubo, S.; Klish, S.; Dickey, L.; Huang, S.S.; Zahn, M. Healthcare Workers and Post-Elimination Era Measles: Lessons on Acquisition and Exposure Prevention. Clin. Infect. Dis. 2016, 62, 166–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coppeta, L.; Balbi, O.; Baldi, S.; Pietroiusti, A.; Magrini, A. Pre-Vaccination IgG Screening for Mumps Is the Most Cost-Effectiveness Immunization Strategy among Health Care Workers. Hum. Vaccin. Immunother. 2019, 15, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Jeong, O.; Yang, H. Screening and Vaccination against Measles and Varicella among Health Care Workers: A Cost-Effectiveness Analysis. Asia Pac. J. Public Health 2021, 33, 508–515. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Mascipinto, S.; Stefanizzi, P.; De Nitto, S.; Germinario, C.; Tafuri, S. Long-Term Immunogenicity after Measles Vaccine vs. Wild Infection: An Italian Retrospective Cohort Study. Hum. Vaccin. Immunother. 2021, 17, 2078–2084. [Google Scholar] [CrossRef]
- Anichini, G.; Gandolfo, C.; Fabrizi, S.; Miceli, G.B.; Terrosi, C.; Gori Savellini, G.; Prathyumnan, S.; Orsi, D.; Battista, G.; Cusi, M.G. Seroprevalence to Measles Virus after Vaccination or Natural Infection in an Adult Population, in Italy. Vaccines 2020, 8, 66. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, M.S.; Kim, S.R.; Kwak, Y.G. A Nationwide Survey on the Hospital Vaccination Policies in Korea. J. Korean Med. Sci. 2020, 35, e76. [Google Scholar] [CrossRef]
- Cui, A.; Wang, H.; Zhu, Z.; Mao, N.; Song, J.; Zhang, Y.; Xu, W. Measles Vaccine-Associated Rash Illness in China: An Emerging Issue in the Process of Measles Elimination. J. Clin. Microbiol. 2020, 58, e01472-20. [Google Scholar] [CrossRef]
- Marin, M.; Fiebelkorn, A.P.; Bi, D.; Coleman, L.A.; Routh, J.; Curns, A.T.; McLean, H.Q. Adverse Events among Young Adults Following a Third Dose of Measles-Mumps-Rubella Vaccine. Clin. Infect. Dis. 2021, 73, e1546–e1553. [Google Scholar] [CrossRef]
- Kang, J.H. Review of Measles in Korea: Quarantine and Elimination. Infect. Chemother. 2020, 52, 113–122. [Google Scholar] [CrossRef]
- Wiedermann, U.; Garner-Spitzer, E.; Wagner, A. Primary vaccine failure to routine vaccines: Why and what to do? Hum. Vaccin. Immunother. 2016, 12, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Poland, G. The Association between HLA Class I Alleles and Measles Vaccine-Induced Antibody Response: Evidence of a Significant Association. Vaccine 1998, 16, 1869–1871. [Google Scholar] [CrossRef]
- Bellanti, J.A.; Sanga, R.L.; Klutinis, B.; Brandt, B.; Artenstein, M.S. Antibody responses in serum and nasal secretions of children immunized with inactivated and attenuated measles-virus vaccines. N. Engl. J. Med. 1969, 280, 628–633. [Google Scholar] [CrossRef] [PubMed]
- de Ory, F.; Minguito, T.; Balfagón, P.; Sanz, J.C. Comparison of Chemiluminescent Immunoassay and ELISA for Measles IgG and IgM. APMIS 2015, 123, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Dorigo-Zetsma, J.W.; Leverstein-van Hall, M.A.; Vreeswijk, J.; de Vries, J.J.C.; Vossen, A.C.T.M.; ten Hulscher, H.I.; Kerkhof, J.; Smits, G.P.; Ruijs, W.L.M.; Koopmans, M.P.G.; et al. Immune Status of Health Care Workers to Measles Virus: Evaluation of Protective Titers in Four Measles IgG EIAs. J. Clin. Virol. 2015, 69, 214–218. [Google Scholar] [CrossRef]
| Variables | One Dose (n = 84) | Two Doses (n = 23) | Confirmed Case (n = 12) |
|---|---|---|---|
| Age, median years | 27 (24–31) | 24 (23–26) | 25 (24–27) |
| Male | 22 (26.2) | 19 (82.6) | 3 (25.0) |
| Documented measles vaccination history before study enrollment | |||
| Unknown | 29 (34.5) | 9 (39.1) | 1 (8.3) |
| 1 dose received | 47 (56.0) | 14 (60.9) | 3 (25.0) |
| 2 doses received | 8 (9.5) | 0 (0) | 8 (66.7) |
| Time from the last MMR vaccine to antibody sampling, years | 18 (18–19) | 18 (17–19) | 18 (18–19) |
| Age at the time of first MMR vaccine, months | 60 (32–78) | NA | 32 (12–73) |
| Age at the time of second MMR vaccine, years | 7 (6–9) | NA | 7 (5–9) |
| Time from the first to the second MMR vaccine, months | 15 (4–39) | NA | 30 (23–61) |
| Time from the first MMR vaccine to antibody sampling, years | 19 (18–22) | NA | 21 (19–23) |
| Time from the second MMR vaccine to antibody sampling, years | 18 (18–18) | NA | 18 (17–18) |
| Variables | Booster Dose | Confirmed Case (n = 12) | p Value a | p Value b | ||
|---|---|---|---|---|---|---|
| One Dose (n = 84) | Two Doses (n = 23) | Total (n = 107) | ||||
| PRNT, ND50 | 88.6 (47.0–196.8) | 195.2 (108.8–253.3) | 113.6 (54.3–223.5) | 779.8 (426.3–1558.9) | 0.007 | <0.001 |
| Low (8–120) | 51 (60.7) | 7 (30.4) | 58 (54.2) | 0 (0) | 0.010 | <0.001 |
| Medium (121–900) | 29 (34.5) | 16 (69.6) | 45 (42.1) | 7 (58.3) | 0.003 | 0.31 |
| High (>900) | 4 (4.8) | 0 (0) | 4 (3.7) | 5 (41.7) | 0.58 | <0.001 |
| GMT (mIU/mL), mean (95% CI) | 354.8 (273.8–459.8) | 616.6 (438.6–866.9) | 398.1 (319.5–496.0) | 2818.4 (1917.3–4143.1) | 0.009 | <0.001 |
| Variables | Booster Dose | Confirmed Case (n = 12) | p Value a | p Value b | ||
|---|---|---|---|---|---|---|
| One Dose (n = 84) | Two Doses (n = 23) | Total (n = 107) | ||||
| Measles IgG positive (CLIA) | 72 (85.7) | 14 (60.9) | 88 (82.2) | 12 (100) | 0.008 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, B.; Kim, H.W.; Kim, H.-S.; Park, J.Y.; Seo, H.; Kim, Y.K. Measles Virus Neutralizing Antibody Response and Durability Two Years after One or Two Doses of Measles–Mumps–Rubella Vaccine among Young Seronegative Healthcare Workers. Vaccines 2022, 10, 1812. https://doi.org/10.3390/vaccines10111812
Jang B, Kim HW, Kim H-S, Park JY, Seo H, Kim YK. Measles Virus Neutralizing Antibody Response and Durability Two Years after One or Two Doses of Measles–Mumps–Rubella Vaccine among Young Seronegative Healthcare Workers. Vaccines. 2022; 10(11):1812. https://doi.org/10.3390/vaccines10111812
Chicago/Turabian StyleJang, Byungki, Han Wool Kim, Han-Sung Kim, Ji Young Park, Hyeonji Seo, and Yong Kyun Kim. 2022. "Measles Virus Neutralizing Antibody Response and Durability Two Years after One or Two Doses of Measles–Mumps–Rubella Vaccine among Young Seronegative Healthcare Workers" Vaccines 10, no. 11: 1812. https://doi.org/10.3390/vaccines10111812
APA StyleJang, B., Kim, H. W., Kim, H.-S., Park, J. Y., Seo, H., & Kim, Y. K. (2022). Measles Virus Neutralizing Antibody Response and Durability Two Years after One or Two Doses of Measles–Mumps–Rubella Vaccine among Young Seronegative Healthcare Workers. Vaccines, 10(11), 1812. https://doi.org/10.3390/vaccines10111812






