Molecular Lipopolysaccharide Di-Vaccine Protects from Shiga-Toxin Producing Epidemic Strains of Escherichia coli O157:H7 and O104:H4
Abstract
1. Introduction
2. Results
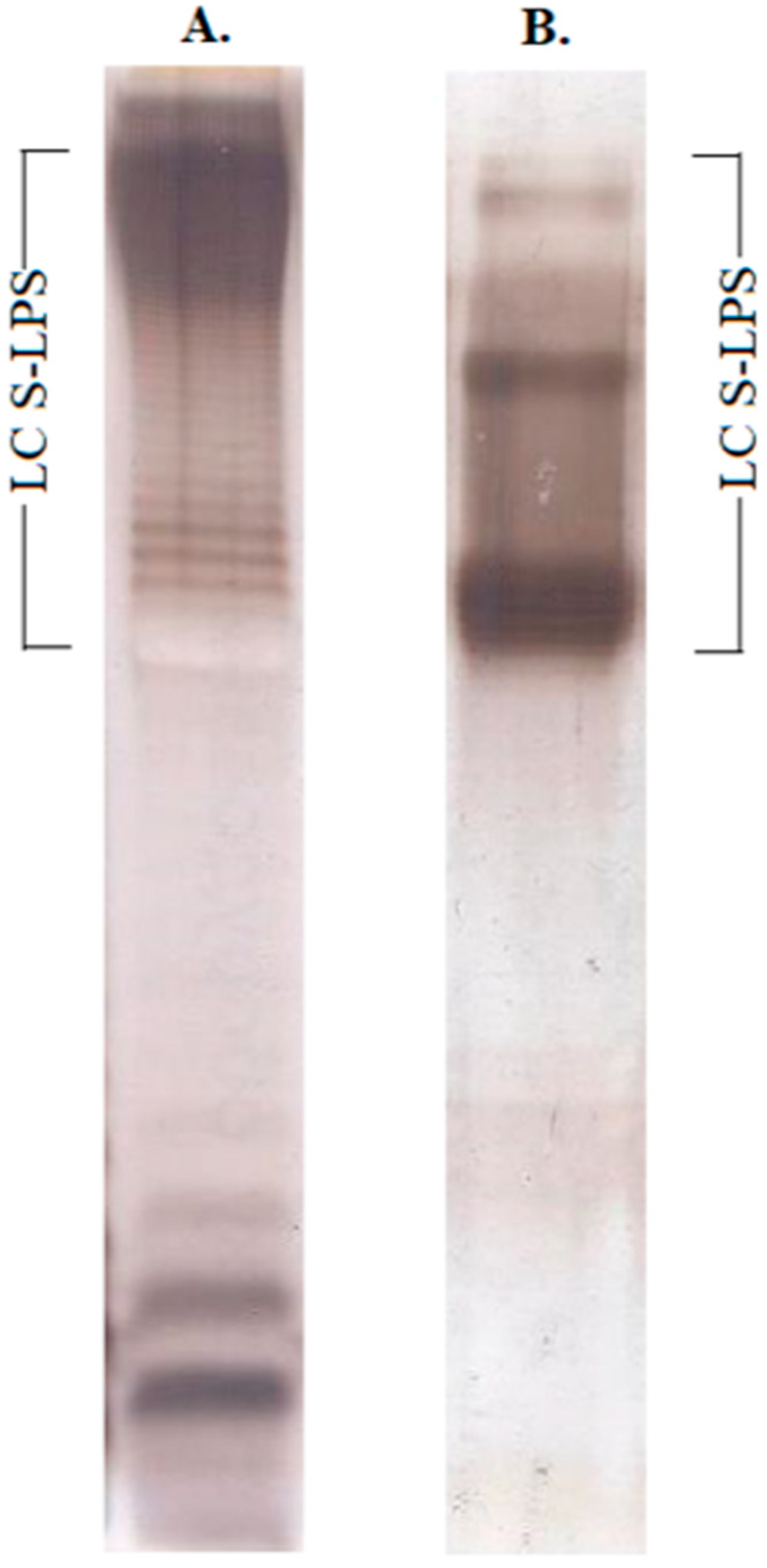
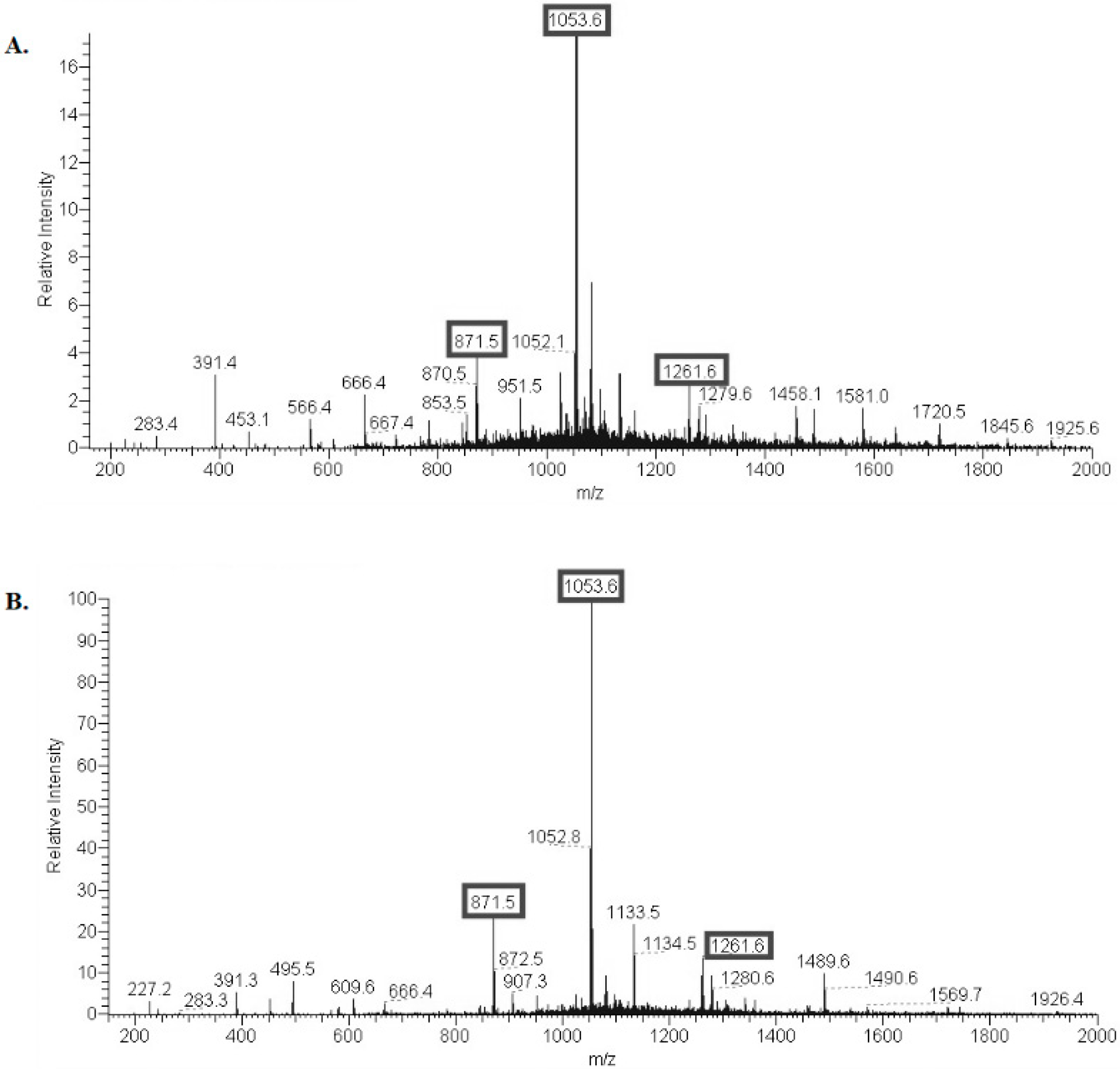
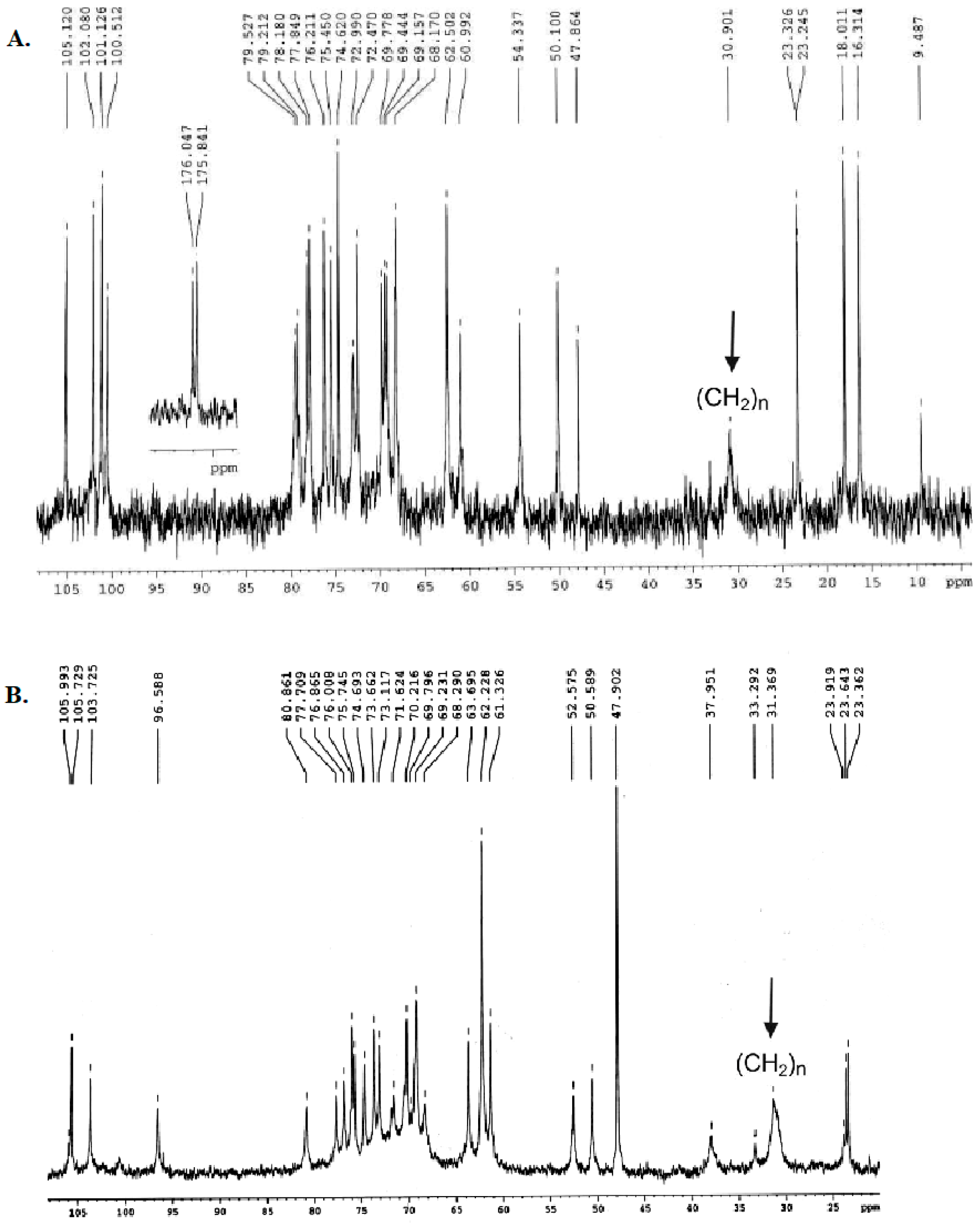
2.1. Structural Characterization of Ac3-S-LPS from STEC Strains
2.2. Immunobiological Study of Modified LPS
2.3. Immunization Provides Effective Protection of Mice against Intraperitoneal STEC Infection
2.4. Protective and Immunogenic Properties of the STEC Di-Vaccine
2.5. Non-Lethal Intragastric Colonization Study in Mice Immunized with E. coli (O157 + O104) Ac3-S-LPS Di-Vaccine and Its Individual Components
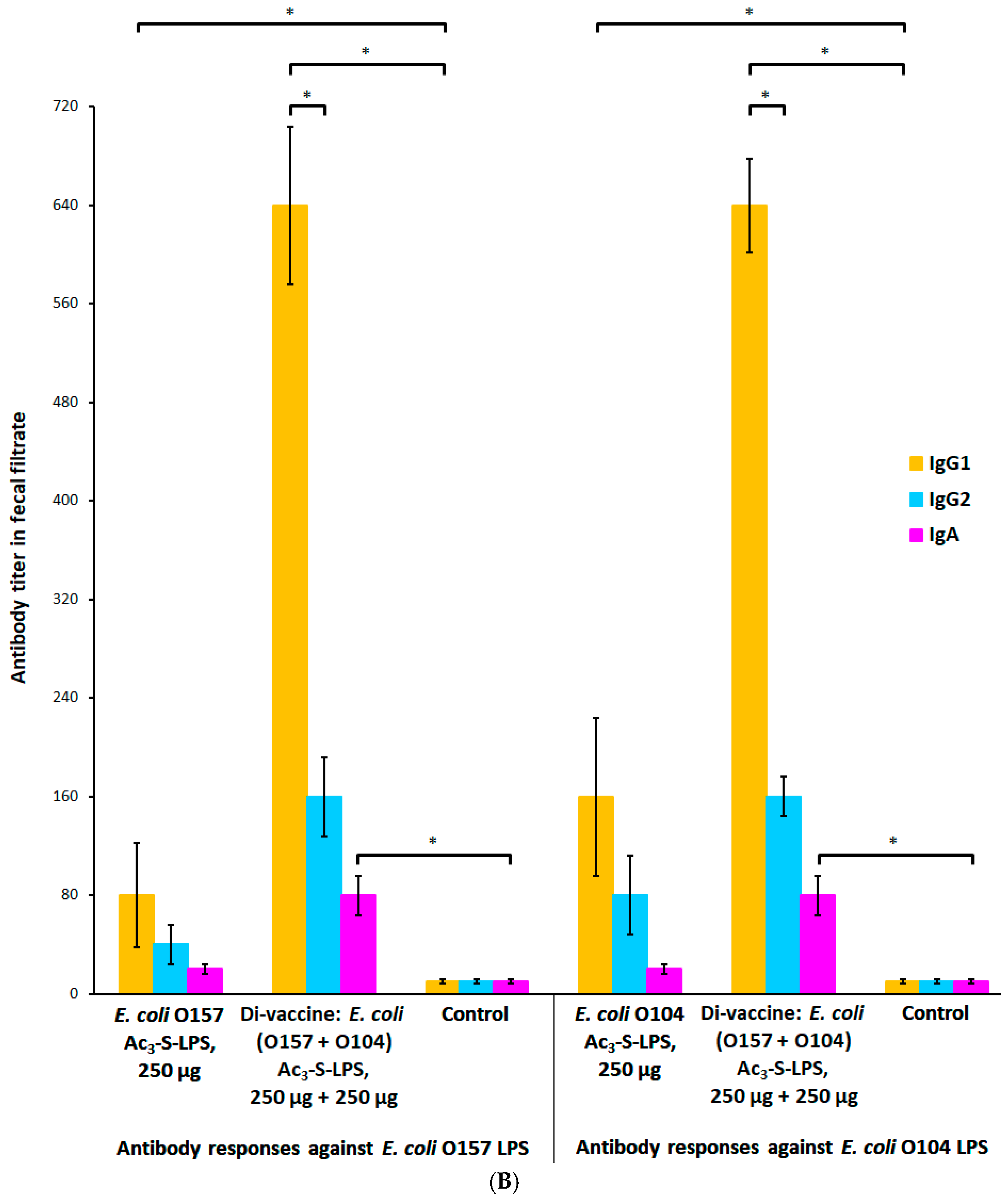
2.6. Dose-Dependent Modification of the Systemic and Mucosal Immune Response with Ac3-S-LPS STEC Antigens
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Preparation of S-LPS
4.3. Partial Deacylation of S-LPS
4.4. Analyses
4.5. Safety Characteristics of Ac3-S-LPS
4.6. Mice
4.7. Immunization
4.8. Serology
4.9. Intraperitoneal Challenge Model
4.10. Non-Lethal Intragastric Colonization Study
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aleksic, S.; Karch, H.; Bockemuhl, J. A biotyping scheme for Shiga-like (Vero) toxin-producing Escherichia coli 0157 and a list of serological cross-reactions between 0157 and other Gram-negative bacteria. Int. J. Med. Microbiol. 1992, 276, 221–230. [Google Scholar] [CrossRef]
- Chart, H.; Law, D.; Rowe, B.; Acheson, D.W.K. Patients with haemolytic uraemic syndrome caused by Escherichia coli 0157: Absence of antibodies to Vero cytotoxin 1 (VT1) or VT2. J. Clin. Pathol. 1993, 46, 1053–1054. [Google Scholar] [CrossRef] [PubMed]
- Cleary, T.G.; Mathewson, J.J.; Faris, E.; Pickering, L.K. Shiga-like cytotoxin production by enteropathogenic Escherichia coli serogroups. Infect. Immun. 1985, 47, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.W.; Remis, R.S.; Helgerson, S.D.; McGee, H.B.; Wells, J.G.; Davis, B.R.; Hebert, R.J.; Olcott, E.S.; Johnson, L.M.; Hargrett, N.T.; et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 1983, 308, 681–685. [Google Scholar] [CrossRef]
- Johnson, W.M.; Lior, H.; Bezanson, G.S. Cytotoxic Escherichia coli 0157:H7 associated with hemorrhagic colitis in Canada. Lancet 1983, 1, 76. [Google Scholar] [CrossRef]
- MacDonald, K.L.; O’Leary, M.J.; Cohen, M.L.; Norris, P.; Wells, J.G.; Noll, E.; Kobayashi, J.M.; Blake, P.A. E. coli 0157:H7: An emerging gastrointestinal pathogen. JAMA 1988, 259, 3567–3570. [Google Scholar] [CrossRef]
- Tarr, P.I. Escherichia coli 0157:H7: Clinical, diagnostic, and epidemiological aspects of human infection. Clin. Infect. Dis. 1995, 20, 1–8. [Google Scholar] [CrossRef]
- Griffin, P.M.; Tauxe, R.V. The epidemiology of infections caused by E. coli 0157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 1991, 13, 60–98. [Google Scholar] [CrossRef]
- Bitzan, M.; Ludwig, K.; Klemt, M.; Konig, H.; Buren, J.; Muller-Wiefel, D.E. The role of Escherichia coli 0 157 infections in the classical (enteropathic) haemolytic uremic syndrome: Results of a central European, multicentre study. Epidemiol. Infect. 1993, 110, 183–196. [Google Scholar] [CrossRef]
- Michino, H.; Araki, K.; Minami, S.; Takaya, S.; Sakai, N.; Miyazaki, M.; Ono, A.; Yanagawaet, H. Massive outbreak of Escherichia coli 0157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am. J. Epidemiol. 1999, 150, 797–803. [Google Scholar] [CrossRef]
- Chart, H.; Rowe, B.; van der Kar, N.; Monnens, L.A. Serological identification of Escherichia coli 0157 as a cause of haemolytic uraemic syndrome in Netherlands. Lancet 1991, 337, 437. [Google Scholar] [CrossRef]
- Carter, A.O.; Borczyk, A.A.; Carlson, J.A.; Harvey, B.; Hockin, J.C.; Karmali, M.A.; Krishnan, C.; Korn, D.A.; Lior, H. A severe outbreak of Escherichia coli 0157:H7- associated hemorrhagic colitis in a nursing home. N. Engl. J. Med. 1987, 317, 1496–1500. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.L.; Diaz, M.; Grinstein, S.; Devoto, S.; Mendilaharzu, F.; Murray, B.E.; Ashkenazi, S.; Rubeglio, E.; Woloj, M.; Vasquez, M.; et al. Hemolytic uremic syndrome and diarrhea in Argentine children: The role of Shiga-like toxins. J. Infect. Dis. 1989, 160, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Seriwatana, J.; Brown, J.E.; Echeverria, P.; Taylor, D.N.; Suthienkul, O.; Newland, J. DNA probes to identify Shiga-like toxin I and II producing enteric bacterial pathogens isolated from patients with diarrhea in Thailand. J. Clin. Microbiol. 1988, 26, 1614–1615. [Google Scholar] [CrossRef]
- Smith, H.R.; Rowe, B.; Gross, R.J.; Fry, N.K.; Scotland, S.M. Hemorrhagic colitis and vero-cytotoxin-producing Escherichia coli in England and Wales. Lancet 1987, 329, 1062–1065. [Google Scholar] [CrossRef]
- Tarr, P.I.; Gordon, C.A.; Chandler, W.L. Shiga-toxin-producing Escherichia coli and hemolytic uremic syndrome. Lancet 2005, 365, 1073–1086. [Google Scholar]
- Watanabe, H.; Wada, A.; Inagak, Y.; Tamura, K. Outbreaks of enterohaemorragic Escherichia coli O157:H7 infection by two different genotype strains in Japan. Lancet 1996, 348, 831–832. [Google Scholar] [CrossRef]
- Launders, N.; Locking, M.E.; Hanson, M.; Willshaw, G.; Charlett, A.; Salmon, R.; Cowden, J.; Harker, K.S.; Adak, G.K. A large Great Britain-wide outbreak of STEC O157 phage type 8 linked to handling of raw leeks and potatoes. Epidemiol. Infect. 2016, 144, 171–181. [Google Scholar] [CrossRef]
- Onishchenko, G.G.; Dyatlov, I.A.; Svetoch, E.A.; Volozhantsev, N.V.; Bannov, V.A.; Kartsev, N.N.; Borzenkov, V.N.; Fursova, N.K.; Shemyakin, I.G.; Bogun, A.G.; et al. Molecular-genetic characterization of shiga-toxin producing Escherichia coli isolated during a food-borne outbreak in St. Petersburg in 2013. Vestn. Ross. Akad. Med. Nauk 2015, 1, 70–81. (In Russian) [Google Scholar] [CrossRef]
- Karmali, M.A.; Petric, M.; Lim, C. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J. Infect. Dis. 1985, 151, 775–782. [Google Scholar] [CrossRef]
- Karmali, M.A.; Steele, B.T.; Petric, M.; Lim, C. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet 1983, 1, 619–620. [Google Scholar] [CrossRef]
- Pavia, A.T.; Nichols, C.R.; Green, D.P.; Tauxe, R.V.; Mottice, S.; Greene, K.D.; Wells, J.G.; Siegler, R.L.; Brewer, E.D.; Hannon, D.; et al. Hemolytic-uremic syndrome during an outbreak of Escherichia coli 0157:H7 infections in institutions for mentally retarded persons: Clinical and epidemiologic observations. J. Pediatr. 1990, 116, 544–551. [Google Scholar] [CrossRef]
- Caprioli, A.; Morabito, S.; Brugère, H.; Oswald, E. Enterohaemorrhagic Escherichia coli: Emerging issues on virulence and modes of transmission. Vet. Res. 2005, 36, 289–311. [Google Scholar] [CrossRef] [PubMed]
- Karmali, M.A. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 1989, 2, 15–38. [Google Scholar] [CrossRef]
- Muniesa, M.; Hammerl, J.A.; Hertwi, S.; Appel, B.; Brüssow, H. Shiga toxin-producing Escherichia coli O104:H4: A new challenge for microbiology. Appl. Environ. Microbiol. 2012, 12, 40654073. [Google Scholar] [CrossRef]
- Frank, C.; Cramer, J.P.; Askar, M.; Faber, M.; Heiden, M.; Bernard, H.; Fruth, A.; Prager, R.; Spode, A.; Wadl, M.; et al. Epidemic profile of shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N. Engl. J. Med. 2011, 365, 1771–1780. [Google Scholar] [CrossRef]
- Safdar, N.; Said, A.; Gangnon, R.E.; Maki, D.G. Risk of hemolytic uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 enteritis: A meta-analysis. J. Am. Med. Assoc. 2002, 288, 996–1001. [Google Scholar] [CrossRef]
- Wong, C.S.; Mooney, J.C.; Brandt, J.R.; Staples, A.O.; Jelacic, S.; Boster, D.R.; Watkins, S.L.; Tarr, P.I. Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: A multivariable analysis. Clin. Infect. Dis. 2012, 55, 33–41. [Google Scholar] [CrossRef]
- Amani, J.; Salmanian, A.H.; Rafati, S.; Mousavi, S.L. Immunogenic properties of chimeric protein from espA, eae and tir genes of Escherichia coli O157:H7. Vaccine 2010, 28, 6923–6929. [Google Scholar] [CrossRef]
- Cai, K.; Gao, X.; Li, T.; Wang, Q.; Hou, X.; Tu, W.; Xiao, L.; Tian, M.; Liu, Y.; Wang, H. Enhanced immunogenicity of a novel Stx2Am-StxlB fusion protein in a mice model of enterohemorrhagic Escherichia coli O157:H7 infection. Vaccine 2011, 29, 946–952. [Google Scholar] [CrossRef]
- Lin, R.; Zhu, B.; Zhang, Y.; Bai, Y.; Zhi, F.; Long, B.; Li, Y.; Wu, Y.; Wu, X.; Fan, H. Intranasal immunization with novel EspA-Tir-M fusion protein induces protective immunity against enterohemorrhagic Escherichia coli O157:H7 challenge in mice. Microb. Pathog. 2017, 105, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Konadu, E.; Robbins, J.B.; Shiloach, J.; Bryla, D.A.; Szu, S.C. Preparation, characterization, and immunological properties in mice of Escherichia coli O157 O-specific polysaccharide-protein conjugate vaccines. Infect. Immun. 1994, 62, 5048–5054. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.B.; Chu, C.; Schneerson, R. Hypothesis for vaccine development: Protective immunity to enteric diseases caused by nontyphoidal Salmonellae and Shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin. Infect. Dis. 1992, 15, 346–361. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, A.D.; Holmes, R.K. Shiga and Shiga-like toxins. Microbiol. Rev. 1987, 51, 206–220. [Google Scholar] [CrossRef]
- Siegler, R.; Oakes, R. Hemolytic uremic syndrome; pathogenesis, treatment, and outcome. Curr. Opin. Pediatr. 2005, 17, 200–204. [Google Scholar] [CrossRef]
- Arenas, J. The role of bacterial lipopolysaccharides as immune modulator in vaccine and drug development. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 221–235. [Google Scholar] [CrossRef]
- Barrett, T.J.; Potter, M.E.; Wachsmuth, I.K. Bacterial endotoxin both enhances and inhibits the toxicity of Shiga-like toxin II in rabbits and mice. Infect. Immun. 1989, 57, 3434–3437. [Google Scholar] [CrossRef]
- Karpman, D.; Connell, H.; Svensson, M.; Scheutz, F.; Alm, P.; Svanborg, C. The role of lipopolysaccharide and Shiga-like toxin in a mouse model of Escherichia coli O157:H7 infection. J. Infect. Dis. 1997, 175, 611–620. [Google Scholar] [CrossRef]
- Ikeda, M.; Ito, S.; Honda, M. Hemolytic uremic syndrome induced by lipopolysaccharide and Shiga-like toxin. Pediatr. Nephrol. 2004, 19, 485–489. [Google Scholar] [CrossRef]
- Keepers, T.R.; Psotka, M.A.; Gross, L.K.; Obrig, T.G. A Murine Model of HUS: Shiga Toxin with Lipopolysaccharide Mimics the Renal Damage and Physiologic Response of Human Disease. J. Am. Soc. Nephrol. 2006, 17, 3404–3414. [Google Scholar] [CrossRef]
- Bitzan, M.; Moebius, E.; Ludwig, K.; Müller-Wiefel, D.E.; Heesemann, J.; Karch, H. High incidence of serum antibodies to Escherichia coli O157 lipopolysaccharide in children with hemolytic-uremic syndrome. J. Pediatr. 1991, 119, 380–385. [Google Scholar] [CrossRef]
- Luzzi, I.; Tozzi, A.E.; Rizzoni, G.; Niccolini, A.; Benedetti, I.; Minelli, F.; Caprioli, A. Detection of serum antibodies to the lipopolysaccharide of Escherichia coli O103 in patients with hemolytic-uremic syndrome. J. Infect. Dis. 1995, 171, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Frey, E.; Mackenzie, A.M.; Finlay, B.B. Human response to Escherichia coli O157:H7 infection: Antibodies to secreted virulence factors. Infect. Immun. 2000, 68, 5090–5095. [Google Scholar] [CrossRef] [PubMed]
- Ledov, V.A.; Golovina, M.E.; Markina, A.A.; Knirel, Y.A.; L’vov, V.L.; Kovalchuk, A.L.; Aparin, P.G. Highly homogenous tri-acylated S-LPS acts as a novel clinically applicable vaccine against Shigella flexneri 2a infection. Vaccine 2019, 37, 1062–1072. [Google Scholar] [CrossRef]
- Westphal, O. Bacterial lipopolysaccharide-extraction with phenol water and further application of procedure. Met. Carbohydr. Chem. 1965, 1, 83–91. [Google Scholar]
- Perry, M.B.; MacLean, L.; Griffith, D.W. Structure of the O-chain polysaccharide of the phenol-phase soluble lipopolysaccharide of Escherichia coli O:157:H7. Biochem. Cell Biol. 1986, 64, 21–28. [Google Scholar] [CrossRef]
- Gamian, A.; Romanowska, E.; Ulrich, J.; Defaye, J. The structure of the sialic acid-containing Escherichia coli O104 O-specific polysaccharide and its linkage to the core region in lipopolysaccharide. Carbohydr. Res. 1992, 236, 195–208. [Google Scholar] [CrossRef]
- WHO. Requirements for Vi-Polysaccharide Typhoid Vaccine; Technical Report Series No.840 Annex 1; WHO: Geneva, Switzerland, 1994.
- Rojas-Lopez, M.; Monterio, R.; Pizza, M.; Desvaux, M.; Rosini, R. Intestinal pathogenic Escherichia coli: Insights for vaccine development. Front. Microb. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Lu, X.; Skurnik, D.; Pozzi, C.; Roux, D.; Cywes-Bentley, C.; Ritchie, J.M.; Munera, D.; Gening, M.L.; Tsvetkov, Y.E.; Nifantiev, N.E.; et al. A Poly-N-Acetylglucosamine − Shiga toxin broad-spectrum conjugate vaccine for Shiga toxin-producing Escherichia coli. mBio 2014, 5, e00974. [Google Scholar] [CrossRef]
- Gening, M.L.; Pier, G.B.; Nifantiev, N.E. Broadly protective semi-synthetic glycoconjugate vaccine against pathogens capable of producing poly-(1→6)-N-acetyl-D-glucosamine exopolysaccharide. Drug Discov. Today Technol. 2020, 35–36, 13–21. [Google Scholar] [CrossRef]
- Maira-Litrán, T.; Bentancor, L.V.; Bozkurt-Guzel, C.; O’Malley, J.M.; Cywes-Bentley, C.; Pier, G.B. Synthesis and Evaluation of a Conjugate Vaccine Composed of Staphylococcus aureus Poly-N-Acetyl-Glucosamine and Clumping Factor A. PLoS ONE 2012, 7, e43813. [Google Scholar] [CrossRef] [PubMed]
- Konadu, E.; Donohue-Rolfe, A.; Calderwood, S.B.; Pozsgay, V.; Shiloach, J.; Robbins, J.B.; Szu, S.C. Syntheses and immunologic properties of Escherichia coli O157 O-specific polysaccharide and Shiga toxin 1 B subunit conjugates in mice. Infect. Immun. 1999, 67, 6191–6193. [Google Scholar] [CrossRef] [PubMed]
- Rokhsartalab-Azar, S.; Shapouri, R.; Rahnema, M.; Najafzadeh, F.; Kianmehr, A. Synthesis, characterization and immunological properties of Escherichia coli 0157:H7 lipopolysaccharide-diphtheria toxoid conjugate vaccine. Iran. J. Microbiol. 2015, 7, 150–155. [Google Scholar] [PubMed]
- Konadu, E.Y.; Parke, J.C., Jr.; Tran, H.T.; Bryla, D.A.; Robbins, J.B.; Szu, S.C. Investigational vaccine for Escherichia coli O157: Phase 1 study of O157 O-specific polysaccharide Pseudomonas aeruginosa recombinant exoprotein A conjugates in adults. J. Infect. Dis. 1998, 177, 383–387. [Google Scholar] [CrossRef]
- Conlan, J.W.; Cox, A.D.; KuoLee, R.; Webb, A.; Perry, M.B. Parenteral immunization with a glycoconjugate vaccine containing the O157 antigen of Escherichia coli O157:H7 elicits a systemic humoral immune response in mice, but fails to prevent colonization by the pathogen. Can. J. Microbiol. 1999, 45, 279–286. [Google Scholar] [CrossRef]
- Conlan, J.W.; KuoLee, R.; Webb, A.; Cox, A.D.; Perry, M.B. Oral immunization of mice with a glycoconjugate vaccine containing the O157 antigen of Escherichia coli O157:H7 admixed with cholera toxin fails to elicit protection against subsequent colonization by the pathogen. Can. J. Microbiol. 2000, 46, 283–290. [Google Scholar] [CrossRef]
- Ademokoya, A.A.; Adebolu, T.T.; Ogundare, A.O. Evaluation of the Lipopolysaccharide of Escherichia coli O157:H7 for prophylactic ability against diarrhea caused by homologous organism in Wistar albino rats. Glob. Adv. Res. J. Med. Sci. 2015, 4, 117–120. [Google Scholar]
- Robbins, J.B.; Schneerson, R.; Szu, S.C. Perspective: Hypothesis: Serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J. Infect. Dis. 1995, 171, 1387–1398. [Google Scholar] [CrossRef]
- Paton, A.W.; Voss, E.; Manning, P.A.; Paton, J.C. Antibodies to lipopolysaccharide block adherence of Shiga toxin-producing Escherichia coli to human intestinal epithelial (Henle 407) cells. Microb. Pathog. 1998, 24, 57–63. [Google Scholar] [CrossRef]
- Peterson, A.A.; McGroarty, E.J. High-molecular-weight components in lipopolysaccharides of Salmonella typhimurium, Salmonella minnesota, and Escherichia coli. J. Bacteriol. 1985, 162, 738–745. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Spirin, A.S. Spectrophotometric determination of total nucleic acids. Biokhimiia 1958, 23, 656–662. [Google Scholar] [PubMed]
- Tsai, C.M. The analysis of lipopolysaccharide (endotoxin) in meningococcal polysaccharide vaccines by silver staining following SDS-PAGE. J. Biol. Stand. 1986, 14, 25–33. [Google Scholar] [CrossRef]
- Pyrogens (2.6.8.). European Pharmacopoeia, 8th ed.; European Directorate for the Quality of Medicines & Healthcare, Council of Europe: Strasbourg, France, 2013; pp. 183–184. [Google Scholar]
- Wadolkowski, E.A.; Burris, J.A.; O’Brien, A.D. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 1990, 58, 2438–2445. [Google Scholar] [CrossRef] [PubMed]


| Preparation | Immunization Scheme | Infection on Day 45, Strain of E. coli | Survival Rate (%) | ||
|---|---|---|---|---|---|
| 1st Injection | 2nd Injection | 3rd Injection | |||
| E. coli O157 Ac3-S-LPS | Day 0 | Day 30 | - | O157 | 60 |
| Day 0 | Day 10 | Day 20 | O157 | 70 | |
| E. coli O104 Ac3-S-LPS | Day 0 | Day 30 | - | O104 | 60 |
| Day 0 | Day 10 | Day 20 | O104 | 100 | |
| E. coli (O157+O104) Ac3-S-LPS | Day 0 | Day 30 | - | O157 | 100 |
| Day 0 | Day 10 | Day 20 | O104 | 100 | |
| Control (sterile saline) | Day 0 | - | - | O157 | 0 |
| Day 0 | - | - | O104 | 0 | |
| Preparation | Infection on Day 45, Strain of E. coliStr | E. coli O157 or O104 Fecal Cell Count (lg CFU/g), Mean ± SD | ||||
|---|---|---|---|---|---|---|
| 2nd Day | 5th Day | 7th Day | 9th Day | 12th Day | ||
| E. coli O157 Ac3-S-LPS | O157 | 8.83 ± 0.03 * | 8.52 ± 0.02 * | 8.48 ± 0.04 * | 8.34 ± 0.02 * | 8.16 ± 0.01 * |
| E. coli O104 Ac3-S-LPS | O104 | 9.13 ± 0.02 * | 8.98 ± 0.04 * | 9.00 ± 0.01 * | 8.79 ± 0.02 * | 8.57 ± 0.03 * |
| E. coli (O157+O104) Ac3-S-LPS | O157 | 8.60 ± 0.01 * | 8.51 ± 0.01 * | 8.29 ±0.01 * | 8.09 ± 0.03 * | 8.04 ± 0.03 * |
| O104 | 9.3 ± 0.01 * | 9.0 ± 0.02 * | 8.6 ±0.03 * | 8.4 ± 0.04 * | 8.2 ± 0.02 * | |
| Control (sterile saline) | O157 | 9.55 ± 0.02 | 9.33 ± 0.03 | 9.27 ± 0.02 | 8.99 ± 0.05 | 8.81 ± 0.01 |
| O104 | 9.8 ± 0.04 | 9.5 ± 0.03 | 9.5± 0.02 | 9.0 ± 0.01 | 8.9 ± 0.04 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyatlov, I.A.; Svetoch, E.A.; Mironenko, A.A.; Eruslanov, B.V.; Firstova, V.V.; Fursova, N.K.; Kovalchuk, A.L.; Lvov, V.L.; Aparin, P.G. Molecular Lipopolysaccharide Di-Vaccine Protects from Shiga-Toxin Producing Epidemic Strains of Escherichia coli O157:H7 and O104:H4. Vaccines 2022, 10, 1854. https://doi.org/10.3390/vaccines10111854
Dyatlov IA, Svetoch EA, Mironenko AA, Eruslanov BV, Firstova VV, Fursova NK, Kovalchuk AL, Lvov VL, Aparin PG. Molecular Lipopolysaccharide Di-Vaccine Protects from Shiga-Toxin Producing Epidemic Strains of Escherichia coli O157:H7 and O104:H4. Vaccines. 2022; 10(11):1854. https://doi.org/10.3390/vaccines10111854
Chicago/Turabian StyleDyatlov, Ivan A., Edward A. Svetoch, Anna A. Mironenko, Boris V. Eruslanov, Victoria V. Firstova, Nadezhda K. Fursova, Alexander L. Kovalchuk, Vyacheslav L. Lvov, and Petr G. Aparin. 2022. "Molecular Lipopolysaccharide Di-Vaccine Protects from Shiga-Toxin Producing Epidemic Strains of Escherichia coli O157:H7 and O104:H4" Vaccines 10, no. 11: 1854. https://doi.org/10.3390/vaccines10111854
APA StyleDyatlov, I. A., Svetoch, E. A., Mironenko, A. A., Eruslanov, B. V., Firstova, V. V., Fursova, N. K., Kovalchuk, A. L., Lvov, V. L., & Aparin, P. G. (2022). Molecular Lipopolysaccharide Di-Vaccine Protects from Shiga-Toxin Producing Epidemic Strains of Escherichia coli O157:H7 and O104:H4. Vaccines, 10(11), 1854. https://doi.org/10.3390/vaccines10111854






