Abstract
Background: The COVID-19 pandemic has imposed a challenge on global healthcare and has tremendously impacted everyone’s lives. Vaccination is one of the most effective and vital strategies to halt the pandemic. However, new-onset and relapsed kidney diseases have been reported after COVID-19 vaccination. This narrative review was conducted to collect published data and generalize some hypotheses for the pathogenesis of renal side effects of COVID-19 vaccines. Methods: A systematic literature search of articles reporting renal adverse reactions, including in adults and children, in the PubMed and Web of Science databases until August 2022 was performed. Results: A total of 130 cases reporting a renal adverse reaction following COVID-19 vaccination from 90 articles were included in this review, of which 90 (69%) were new-onset kidney diseases, while 40 (31%) were relapsed kidney diseases. The most frequent renal side effects of COVID-19 vaccination were minimal change disease (52 cases), IgA nephropathy (48 cases), antineutrophil cytoplasmic autoantibody vasculitis (16 cases), and acute interstitial nephritis (12 cases). Other renal side effects occurred at a much lower frequency. Follow-up data were available for 105 patients, and 100 patients (95%) responded to the treatments. Conclusions: The number of reported cases is far less than the hundreds of millions of vaccinations, and the benefit of COVID-19 vaccination far outweighs its risks. This review will assist healthcare professionals, particularly nephrologists, who should be aware of these side effects and recognize them early and treat them efficiently.
1. Introduction
With the ongoing COVID-19 pandemic, vaccination programs are being rolled out worldwide to prevent COVID-19 and alleviate the severity of the disease. Over the past two years, multiple COVID-19 vaccines have been granted emergency use authorization, including mRNA vaccines (such as Pfizer-BioNTec and Moderna), adenovirus-vectored vaccines (such as Oxford-AstraZeneca and Janssen), recombinant vaccines (Novavax), and inactivated vaccines (CoronaVac) [1].
At present, short-term side effects are usually mild and self-limiting and mostly involve local injection symptoms such as pain, swelling and urticarial eruptions, and systemic symptoms of fever and headache [1,2]. However, since mass scale vaccination, a growing number of severe and required hospitalization adverse reactions to vaccinations has been reported, including neurological side effects [3], myocarditis [4], and autoimmune disease [5]. Furthermore, newly diagnosed, or relapsed kidney diseases have been reported in adults and children [6,7,8].
Given the emerging evidence between kidney diseases and COVID-19 vaccination, the risk of renal side effects has sparked public concern. Here, this narrative review was conducted to collect the renal side effects in the published data and discuss the plausible mechanism of action triggered by COVID-19 vaccination.
2. Methods
A systematic literature search was performed in the PubMed and Web of Science databases before August 2022 using the search terms “COVID-19 Vaccine” OR “COVID-19 Vaccination” OR “ SARS-CoV-2 vaccine” OR” SARS-CoV-2 Vaccination” AND “kidney” OR “Renal”. After the search was complete, all duplicates were removed and papers reporting the same case were also excluded. The titles and abstracts of the remaining studies were reviewed to ensure that they presented the association between renal disease and COVID-19 vaccination. Then the full-text screening, based on the relevance of the renal side effects of COVID-19 vaccination was selected. We only selected case or case series reports, other types of articles were excluded. Case or case series reporting new onset or relapse kidney histopathology in both native and transplanted kidneys following COVID-19 vaccination were included, and patients who had suffered from COVID-19 were excluded. Finally, 90 papers and 130 cases that developed renal side effects (128 cases of native kidneys and 2 cases of transplanted kidneys) were included in this systematic review [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98]. We then extracted patient demographics (age and sex), medical history, vaccine type, number of vaccine doses given, baseline characteristics, laboratories upon presentation, onset of symptoms, timing of symptom onset, treatments, and outcomes. The study selection process was carried out using the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines (Figure 1).
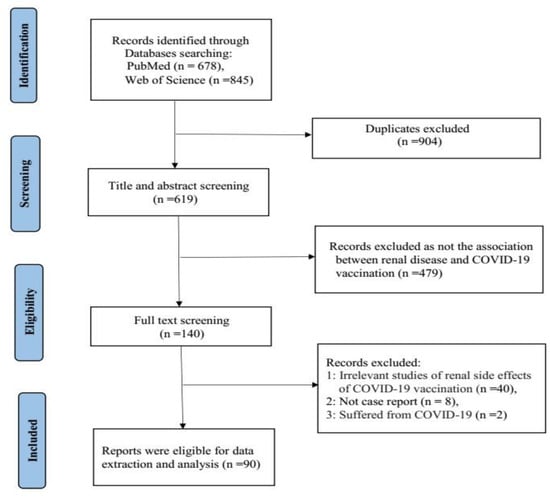
Figure 1.
PRISMA flow diagram.
Continuous data are presented as medians and ranges, while categorical data are presented as numbers and percentages.
3. Results
3.1. Overall
The renal side effects of COVID-19 vaccination collected from the literature mainly include minimal change disease (MCD), IgA nephropathy (IgAN), antineutrophil cytoplasmic autoantibody (ANCA) vasculitis, and acute interstitial nephritis (AIN). A total of 128 patients are summarized in Table 1, including 89 (70%) cases of newly diagnosed renal involvement and 39 (30%) cases of relapse. Of these, 52 (41%) cases were diagnosed with MCD, which is by far the most frequent renal side effect of COVID-19 vaccination, and the second most common pathology was IgAN (48/128, 37.5%), followed by 16 (12.5%) ANCA and 12 (9.0%) AIN. The median age was 42 (range 12–85) years, and 53% (68 of 128 cases) of patients were male.

Table 1.
Clinical characteristics of patients with MCD, IgAN, ANCA or AIN following COVID-19 vaccination.
Some others included membranous nephropathy (MN) in 8 cases, anti-glomerular basement membrane (anti-GBM) nephritis in 3 cases, focal segmental glomerulosclerosis (FSGS) in 3 cases, lupus nephritis in 3 cases and granulomatous vasculitis in 1 case. (Table 2) Renal side effects develop after any of the commercially available COVID-19 vaccinations, but 56% (72/128) of patients received the BNT162b2 (Pfizer) vaccine, followed by 30% (38/128) receiving the mRNA-1273 (Moderna) vaccine. In addition, 10.2% (13/128) received adenovirus vector (AstraZeneca) vaccine, 1.5% (2/128) received adenovirus vector (Janssen) vaccine, and another 2.3% (3/128) received inactivated vaccine (CoronaVac). Of these, 39% (50/128) of patients developed symptoms after the first dose, while 61% (78/128) of patients developed symptoms after the second dose. However, most reports did not describe whether the second dose of COVID-19 vaccines was the same as the first dose.

Table 2.
Summary of other cases of renal side effects following COVID-19 vaccination.
3.2. Minimal Change Disease (MCD)
MCD was the most common pathological type of renal side effect reported following COVID-19 vaccination, with a total of 52 cases (38 new and 14 relapse). The median age was 44 (14–83) years, with a male predominance (31/52, 60%). Approximately 28.8% of patients did not have any medical history of chronic illness, while 21% of patients had a history of hypertension, diabetes, or dyslipidemia. However, 76.9% (40/52) of patients developed edema, which is the most common symptom after vaccination. A total of 28 (53.8%) patients had clinical symptoms after the first dose of vaccine, of which 22 were new cases and 6 were relapses, with a median onset time of 7 (1–46) days. On the other hand, 24 (46.2%) patients presented after the second dose of vaccine, of which 16 were new cases and 8 were relapses, with a median onset time of 8 (2–88) days. Thirty-nine (75%) patients received steroid therapy, 5 patients received steroids and cyclophosphamide or mycophenolate mofetil or cyclosporine combination immunosuppression therapy, 3 patients underwent plasmapheresis or hemodialysis, 3 patients received rituximab, and the other 2 patients received conservative treatment. Follow-up data were available for 42 patients; in total, 41 (97.6%) patients responded well (28 patients achieved complete remission, 6 patients achieved partial remission, 7 patients improved) to the treatments, and 1 had no response [11] (Table 1).
3.3. IgA Nephropathy (IgAN)
IgAN was the second most frequent complication of renal side effects after COVID-19 vaccination. There were 48 patients with IgAN reported in the literature, of which 29 (60.4%) were new cases and 19 (39.6%) were relapse, with a median age of 33 (12–79) years, and approximately 64.6% of cases had a history of abnormal urine or kidney disease. The most frequent manifestation following COVID-19 vaccination was gross hematuria (43/48, 89.6%). In addition, 14 (29.2%) patients presented with fever, headache, nausea, vomiting, anorexia, or diarrhea, and 10 (20.8%) patients presented with acute kidney injury or renal failure, while proteinuria was observed in 7 (14.6%) cases. Most cases (35/48, 72.9%) occurred after the second dose, of which 19 were new cases and 16 were relapses, and the median time between COVID-19 vaccination injection and onset of symptoms was 2 (1–42) days. In contrast, 13 (27.1%) patients who developed clinical symptoms were reported following the first dose, of which 10 were new cases and 3 were relapses, with a median onset time of 4 (1–61) days. In these cases, 62.5% of patients received the Pfizer vaccine, 31.3% of patients received the Moderna vaccine, 4.2% of patients received the AstraZeneca vaccine, and the other 2.1% received CoronaVac. Twelve out of 48 patients (25.0%) were managed with steroids, 4 received combination immunosuppression therapy, and 3 patients received plasmapheresis or hemodialysis. Approximately half of the patients 24 (50.0%) were treated conservatively, and the other 4 (8.3%) patients symptoms subsided spontaneously. Forty patients had available follow-up data; of these, 37 (92.5%) patients responded to the treatments, and 3 had no response [41,47,93] (Table 1).
3.4. Anti-Glomerular Basement Membrane (Anti-GBM) and Anti-Neutrophil Cytoplasmic Autoantibody (ANCA) Vasculitis
There were ten cases with new-onset ANCA vasculitis, including 3 cases associated with proteinase 3 (PR3) and 7 cases of myeloperoxidase (MPO)-associated vasculitis, while six cases were ANCA vasculitis relapses. Eleven patients with ANCA vasculitis developed symptoms after the second dose of vaccine, with a median onset time of 14 (1–60) days, and 5 patients developed symptoms after the first dose, with a median onset time of 7 days. The clinical presentation was acute kidney injury or renal failure in 3 cases, 2 cases presenting with hematuria and 2 cases with proteinuria. Based on kidney biopsy, all ten new-onset patients confirmed crescentic glomerulonephritis and were treated with standard immunosuppression, of which 5 patients received steroids combined with plasmapheresis or hemodialysis, and 5 patients received combination immunosuppression therapy (4 patients were treated with rituximab and steroids). Eleven patients had available follow-up data, and 10 improved (Table 1).
Three cases of new-onset anti-GBM nephritis have been reported following COVID-19 vaccination. One case had no medical history and presented with fever, anorexia, and gross hematuria two weeks after the second dose of the Moderna vaccination, and the other case with a past medical history of hyperlipidemia developed macroscopic hematuria a day after the second dose of the Pfizer vaccination. In both cases, kidney histopathology revealed diffusely crescentic glomerulonephritis as well as linear staining of GBM for IgG. The two patients developed acute kidney injury and received immunosuppression therapy and plasma exchange; one patient remained on dialysis [54], while the other patient had no follow-up records [70]. The third patient was newly diagnosed with atypical anti-GBM nephritis accompanied by hypertension one week after the first Pfizer vaccination. The patient had not yet responded to immunosuppression therapy, and his serum creatinine level continued to rise [41] (Table 2).
3.5. Acute Interstitial Nephritis (AIN)
In the literature, 12 patients with a median age of 44 (12–77) years developed new-onset AIN, of which 8 patients developed symptoms following the second administration of vaccination with a median onset time of 14 (2–42) days, 4 patients developed symptoms after the first dose, and the median onset time was 14.5 (2–28) days. All 12 patients presented with acute kidney injury, and 5 patients presented with fever, anorexia, nausea, vomiting, or pain. Seven (58.3%) patients received steroid therapy, 4 (33.3%) patients were treated with hemodialysis, and they responded to the treatments. In addition, one patient with renal insufficiency gradually improved with supportive care (Table 1).
3.6. Membranous Nephropathy (MN)
MN developed in 8 patients, including 3 relapse patients who were associated with serum anti-phospholipase A2 receptor (PLA2R) antibody positivity and 5 new-onset patients, of which 1 new patient had neural epidermal growth factor-like 1 protein (NELL-1)-associated MN. Three new-onset patients developed nephrotic syndrome, of which 1 patient was treated with glucocorticoids, 2 patients received rituximab, and outcome data were not reported in these 3 patients [67]. One new-onset patient developed edema managed with conservative treatment, and no signs of spontaneous remission were observed within 60 days [83]. The other case of new-onset NELL-1-associated MN was treated conservatively with angiotensin converting enzyme inhibitor, and proteinuria significantly improved from 6.5 g/d to 0.4 g/d. [41]. Of 3 patients with PLA2R-associated MN, only 1 patient responded to tacrolimus with proteinuria, and serum albumin improved; 1 patient received obinutuzumab, but the outcome was unknown [41], and the other patient had no records of treatment or follow-up [55] (Table 2).
In addition, Gueguen et al. [84] reported a new case that developed PLA2R-associated MN after the first dose of Pfizer vaccination and achieved partial control after treatment with renin-angiotensin system blockade. However, edema worsened after the second dose of Moderna vaccination, and the patient achieved partial remission with rituximab treatment.
3.7. Others
There were 2 new-onset cases of focal segmental glomerulosclerosis (FSGS). One patient was in partial remission with persisting proteinuria and hyperlipoproteinemia after prednisolone therapy [37], and the other patient showed a tip-variant FSGS lesion that received glucocorticoid treatment with no follow-up data [67]. One patient with previous MCD but on repeat biopsy revealed a tip-variant FSGS lesion and achieved partial response to prednisone combined with tacrolimus therapy [41].
Kim et al. [91] reported a case of new-onset class III lupus nephritis with multiorgan involvement, and Zavala-Miranda et al. [82] reported a new-onset systemic lupus erythematosus beginning as class V lupus nephritis in a 23-year-old woman. The clinical symptoms of the 2 patients improved after immunosuppression treatment.
Tuschen et al. [81] reported a known case of class V lupus nephritis in remission presented with a flare up of lupus nephritis a week following the first dose of Pfizer vaccination. The patient received immunosuppressive therapy with mycophenolate mofetil and prednisolone, but her proteinuria was slow to resolve.
Gillion et al. [48] described a patient who felt unwell 4 weeks following the first dose vaccination. The patient underwent kidney biopsy because serum creatinine was 2.7 mg/dl, and a positron emission tomography scan showed findings suggestive of vasculitis. Histopathology revealed diffuse interstitial edema with noncaseating granulomas around small vessels. Serum creatinine returned to normal within 4 weeks after methylprednisolone treatment.
Chavarot et al. reported [97] that a 66-year-old kidney transplant patient underwent a 1-year protocol graft biopsy 8 weeks after the second vaccine dose of COVID-19 vaccination, and kidney biopsy staining revealed the presence of MN. Notably, staining was retrospectively negative in the 3-month protocol graft biopsy and in the native kidney nephrectomy specimen. The diagnosis of de novo post-transplant MN was firmly established.
Fulchiero et al. [98] present a 21-year-old male with a history of FSGS who underwent kidney transplant at 13 years of age and was stable for the following 8 years. He presented with edema, and kidney biopsy showed FSGS recurrence following two doses of COVID-19 vaccination.
4. Discussion
Some cases of renal side effects were observed following COVID-19 vaccination. This narrative review shows that most patients received mRNA vaccines associated with postvaccination kidney disease development. This can be attributed to the fact that Pfizer and Moderna vaccines have been more accessible and widely used under emergency authorization by the FDA, probably due to cell-mediated and antibody-mediated immune responses generated by the mRNA vaccines.
The approved Pfizer and Moderna mRNA vaccines contain purified modified mRNA and lipid nanoparticle (LNP) delivery systems, while AstraZeneca and Johnson vaccines contain adenovirus (AdV) vector systems [99,100,101,102]. The design of a vaccine requires a pathogen-specific immunogen and an adjuvant to stimulate immunity. An optimal adjuvant provides the necessary second signal for T-cell activation without inducing systemic inflammation. Typically, following mRNA vaccination, the mRNA serves as both immunogen and adjuvant and is recognized by various endosomal and cytosolic innate sensors and taken up by dendritic cells. Cytosolic sensing, such as melanoma differentiation-associated protein 5 (MDA5) and retinoic acid-inducible gene I (RIG-I), binds to single-stranded RNA and double-stranded RNA, while Toll-like receptor (TLR) 3/7 binds to single-stranded RNA in the endosome, resulting in interferon regulatory factor 3/7 and nuclear factor κB activation and the production of type I interferon (IFN-I) and multiple inflammatory mediators [103,104]. Unlike mRNA vaccination, AdV vector vaccines also contain inherent adjuvant properties, which reside with the virus particle that encases the DNA encoding the immunogen. Following injection, the particles target dendritic cells and macrophages and bind to TLR9, resulting in IFN-I secretion [105]. IFN-I induces the differentiation of CD4+ and CD8+ effector T cells, which thereby stimulates the production of inflammatory and cytotoxic mediators and activated CD4+ T follicular helper cells stimulate B-cell differentiation into plasma cells that secrete antibodies (Figure 2). The activation of antigen-specific effector T cells peaks one week after vaccination [106]. From our review, more than half of the patients with MCD were observed following the first dose of the COVID-19 vaccination, and the median time for symptom onset was seven days, which supports the hypothesis for the generation of T-cell-mediated injury in MCD, and other studies demonstrated cellular immune responses approximately 1 week after viral infection [107].
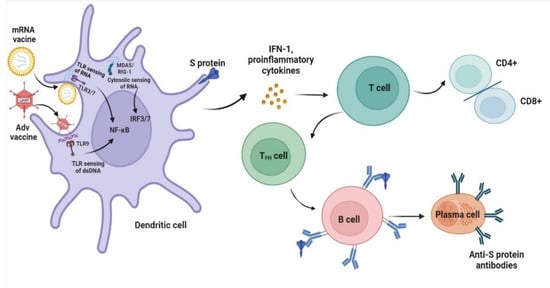
Figure 2.
Proposed mechanisms of minimal change disease caused by COVID-19 vaccination. After mRNA vaccines and adenovirus (AdV) vector vaccines enter dendritic cells, high levels of S protein are produced. In addition, innate sensors such as endosomal T Toll-like receptor (TLR3 and TLR7) sensing of RNA and TLR9 binding double-stranded DNA (dsDNA) activate nuclear factor κB (NF-κB), and cytosolic sensing of melanoma differentiation-associated protein 5 (MDA5) and retinoic acid-inducible gene I (RIG-I) activate IFN regulatory factor 3/7 (IRF3/7), leading to the production of type I interferon (IFN-I) and proinflammatory cytokines. The resultant activated dendritic cells present antigens and costimulatory molecules to S protein-specific naive T cells and promote the adaptive immune response of T and B cells. The figure refers to the pathogenesis of a normal immune reaction in contact with pathogens by Sprent et al. [104].
Furthermore, compared with conventional vaccines, mRNA vaccines provoke a stronger reaction of CD4+ T and CD8+ T cells and higher production of cytokines [103,108]. The resulting permeability factors can directly affect podocytes and alter the glomerular permeability barrier, leading to MCD [106].
IgA nephropathy is the most common form of primary glomerulonephritis worldwide and is characterized by diffuse mesangial deposition of immunoglobulin A1 (IgA1) in glomeruli [109]. The pathogenesis of IgAN is still unclear, and several studies have reported that IgAN is a condition with several hypothetical pathological mechanisms, including genetic factors influencing the encoding galactosylation of IgA1 and environmental triggers, such as bacterial or viral infection and alteration of microbiota and food antigens [110]. In recent years, a multihit mechanism has been widely accepted. Specifically, increased synthesis of galactose-deficient IgA1 as the first hit, production of antiglycan IgG and/or IgA1 autoantibodies as the second hit, and poorly galactosylated IgA1 and antiglycan IgG autoantibodies form immune complexes and deposit in the mesangial area of the glomerulus from the subsequent hits [111] The deposition of IgA1 immune complexes causes mesangial cell, podocyte, and tubular epithelial cell damage, leading to end-stage renal disease in patients with IgAN.
Although a previously reported influenza vaccine was associated with IgAN in both native and transplanted kidneys, IgAN is not frequently reported following other vaccinations [112]. However, IgAN is the second most common renal side effect after COVID-19 vaccination, and the explanation has not been fully established. In this review, among 29 patients with new-onset IgAN, 12 patients showed a history of hematuria, which could suggest that IgAN is pre-existing and exacerbated by vaccination. In addition, almost 90% of patients developed gross hematuria and proteinuria after the second dose. One hypothesis is that the production of excess anti-glycan antibodies has a cross-reaction with pre-existing poorly galactosylated IgA1 following COVID-19 vaccination. Furthermore, similar to what was reported for influenza vaccines, the COVID-19 mRNA vaccine relates to a spike in IgA and IgG production in healthy adults and further increases after the second vaccination, while vaccination also stimulates robust T-helper cell and B-cell responses [113]. Therefore, the production of excess pathogenic IgA is another explanation. Generally, within 2 days, approximately 30% of patients experience systemic symptoms such as fever and pain after vaccination, which suggests systemic cytokine-mediated attack. Thus, another explanation is that the receptor binding domain of the SARS-CoV-2 spike protein may act as a superantigen, activate the immune system and cause cytokine storms in which inflammatory factors, such as IL-6 and IL-10, rise sharply [8].
It has been reported that ANCA-associated vasculitis occurs after injection of influenza and rabies vaccines based on viral mRNAs [114]. Moreover, a significantly reduced ANCA response after treatment with ribonuclease vaccines was observed. This encourages the question of whether ANCA vasculitis and RNA vaccines have a direct relationship. Although the occurrence of ANCA vasculitis disease after COVID-19 vaccination has been described, whether it more frequently occurs following mRNA vaccines is required to establish further real-world study. According to the report, a hyperactive immune and an autoimmune response toward SARS-CoV-2 cause ANCA and anti-GBM vasculitis [115,116,117]. Booster COVID-19 mRNA vaccination primes a notably enhanced innate immune response compared with primary immunization [118]. The heightened innate immune response following booster vaccination could be responsible for triggering the observed PR3 and MPO autoantibodies [51]. In ANCA-associated vasculitis, TLRs play crucial roles in initiating autoimmunity and inflammation, which are primarily attributed to TLR-2-induced Th17 autoimmunity, while TLR-9 promotes Th1 autoimmunity [119]. Interestingly, the activation of TLR2 in the immunodominant cytotoxic T lymphocyte response to the spike glycoprotein of SARS-CoV-2, which is also produced by COVID-19 vaccines, was described [120].
AIN is one of the leading causes of acute kidney injury, histopathologically characterized by the presence of inflammatory cell infiltrate in the interstitium, resulting in local edema and destruction of tubular basement membrane and interstitial architecture. Many causes of AIN are known, the most frequently triggered by immuno-allergic reactions to drug therapy that mainly involve nonsteroidal anti-inflammatory drugs and antibiotics; in addition, infections, toxins, and vasculitis can induce AIN [121]. In the review, all twelve patients who did not take any over-the-counter medications, including nonsteroidal anti-inflammatory drugs or herbal remedies, developed AIN after COVID-19 vaccination and responded well to steroid treatment. The mechanisms underlying the association of AIN with COVID-19 vaccination remain elusive. However, molecular mimicry could be applied to demonstrate the association between AIN and COVID-19 vaccination. Molecular mimicry refers to a similarity between certain pathogenic elements contained in the vaccine and human proteins with consecutive immune cross-reactivity [122]. Mira et al. [87] reported a case of AIN development after two doses of Pfizer vaccine that considered a drug-induced hypersensitivity reaction. The lymphocyte transformation test was positive for both vaccine solution and polyethylene glycol (PEG) excipient-indicated T cells, particularly for PEG-specific T cells involved in a type IV hypersensitivity reaction.
Although these may be a causative mechanism resulting from COVID-19 vaccination, granulomatous vasculitis, membranous nephropathy, and lupus nephritis will be required to further research the validity of these associations.
Transplant recipients have not been included in vaccine trials to date. Therefore, vaccine safety, efficacy, and durability profiles have not been measured in these patients. The immunosuppressed condition in these patients may cause a lower anti-SARS-CoV-2 antibody response, depending on the period since transplantation, the intensity of immunosuppression, and the type of transplantation. There would appear to be a slight chance of stimulating immunologic rejection reactions via the vaccination-induced immune response.
The first core issue is proof with certainty that the COVID-19 vaccine resulted in the development of new kidney disease or relapse of kidney disease. The time point of onset and exclusive diagnosis are useful for clinical diagnosis, and renal biopsy may be considered. Most new cases treated with steroid therapy can be improved. For patients with chronic kidney disease, closely observing the condition of basic diseases and monitoring disease activity after vaccination are important for identification of recurrence, and steroid combination immunosuppression can be rapidly relieved.
This review has certain limitations. First, not all patients experiencing renal side effect may have been included, because the search was performed in two databases and epidemiological investigations are lacking, so we cannot determine the true incidence of renal side effects after COVID-19 vaccination. Second, the mechanisms that we have discussed about the vaccine-related association only combine hypotheses from case reports, which have not been proven.
5. Conclusions
In conclusion, some studies reporting renal side effects appear to be associated with COVID-19 vaccination and we discussed the likely immune-mediated hypotheses of kidney disease following COVID-19 vaccination. Further research is warranted to better understand the causes and mechanisms of kidney disease after COVID-19 vaccination. All we can recommend at this point is that if some symptoms, such as hematuria, foamy urine, and edema, can be detected in an early phase, patients will benefit from a timely treatment.
Healthcare professionals should keep a watchful eye for these side effects and recognize them early and treat them efficiently.
Author Contributions
J.Z.: Conceptualization, writing original draft and editing; J.C.: Investigation, formal analysis, and data curation; Q.Y.: Conceptualization: supervision, editing, review, and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Key Project of Provincial Ministry Construction, Health Science and Technology Project Plan of Zhejiang Province (WKJ-ZJ-2128), Key Laboratory of Women’s Reproductive Health Research of Zhejiang Province (No. ZDFY2020-RH-0006), the National Natural Science Foundation of China (Grant/Award Number: U20A20351) and Key Research and Development Plan of Zhejiang Province (Grant/Award Number: 2021C03079).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Liu, Y.; Ye, Q. Safety and Efficacy of the Common Vaccines against COVID-19. Vaccines 2022, 10, 513. [Google Scholar] [CrossRef]
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Neurological side effects of SARS-CoV-2 vaccinations. Acta Neurol. Scand. 2022, 145, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Mehta, J.L. COVID-19 Vaccine and Myocarditis. Am. J. Cardiol. 2021, 157, 146–148. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.M.; Shuai, Z.W.; Ye, D.Q.; Pan, H.F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef]
- Wu, H.H.L.; Kalra, P.A.; Chinnadurai, R. New-Onset and Relapsed Kidney Histopathology Following COVID-19 Vaccination: A Systematic Review. Vaccines 2021, 9, 1252. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rao, M.; Xu, G. New-Onset Acute Kidney Disease Post COVID-19 Vaccination. Vaccines 2022, 10, 742. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, G. New-Onset IgA nephropathy Following COVID-19 Vaccination. QJM Mon. J. Assoc. Physicians 2022. [Google Scholar] [CrossRef]
- Morlidge, C.; El-Kateb, S.; Jeevaratnam, P.; Thompson, B. Relapse of minimal change disease following the AstraZeneca COVID-19 vaccine. Kidney Int. 2021, 100, 459. [Google Scholar] [CrossRef]
- Komaba, H.; Wada, T.; Fukagawa, M. Relapse of Minimal Change Disease Following the Pfizer-BioNTech COVID-19 Vaccine. Am. J. Kidney Dis. 2021, 78, 469–470. [Google Scholar] [CrossRef] [PubMed]
- D’Agati, V.D.; Kudose, S.; Bomback, A.S.; Adamidis, A.; Tartini, A. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int. 2021, 100, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, L.; Sapojnikov, M.; Wechsler, A.; Varadi-Levi, R.; Zamir, D.; Tobar, A.; Levin-Iaina, N.; Fytlovich, S.; Yagil, Y. Minimal Change Disease Following the Pfizer-BioNTech COVID-19 Vaccine. Am. J. Kidney Dis. 2021, 78, 142–145. [Google Scholar] [CrossRef]
- Leclerc, S.; Royal, V.; Lamarche, C.; Laurin, L.P. Minimal Change Disease With Severe Acute Kidney Injury Following the Oxford-AstraZeneca COVID-19 Vaccine: A Case Report. Am. J. Kidney Dis. 2021, 78, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.J.; Gianotten, S.; van der Meijden, W.A.G. An Additional Case of Minimal Change Disease Following the Pfizer-BioNTech COVID-19 Vaccine. Am. J. Kidney Dis. 2021, 78, 312. [Google Scholar] [CrossRef] [PubMed]
- Kervella, D.; Jacquemont, L.; Chapelet-Debout, A.; Deltombe, C.; Ville, S. Minimal change disease relapse following SARS-CoV-2 mRNA vaccine. Kidney Int. 2021, 100, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Ingram, A.; Shao, T. Minimal Change Disease After First Dose of Pfizer-BioNTech COVID-19 Vaccine: A Case Report and Review of Minimal Change Disease Related to COVID-19 Vaccine. Can. J. Kidney Health Dis. 2021, 8, 20543581211058271. [Google Scholar] [CrossRef]
- Weijers, J.; Alvarez, C.; Hermans, M.M.H. Post-vaccinal minimal change disease. Kidney Int. 2021, 100, 459–461. [Google Scholar] [CrossRef]
- Holzworth, A.; Couchot, P.; Cruz-Knight, W.; Brucculeri, M. Minimal change disease following the Moderna mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021, 100, 463–464. [Google Scholar] [CrossRef]
- Salem, F.; Rein, J.L.; Yu, S.M.; Abramson, M.; Cravedi, P.; Chung, M. Report of Three Cases of Minimal Change Disease Following the Second Dose of mRNA SARS-CoV-2 COVID-19 Vaccine. Kidney Int. Rep. 2021, 6, 2523–2524. [Google Scholar] [CrossRef]
- Nakazawa, E.; Uchimura, T.; Hirai, Y.; Togashi, H.; Oyama, Y.; Inaba, A.; Shiga, K.; Ito, S. New-onset pediatric nephrotic syndrome following Pfizer-BioNTech SARS-CoV-2 vaccination: A case report and literature review. CEN Case Rep. 2022, 11, 242–246. [Google Scholar] [CrossRef]
- Lim, J.H.; Han, M.H.; Kim, Y.J.; Kim, M.S.; Jung, H.Y.; Choi, J.Y.; Cho, J.H.; Kim, C.D.; Kim, Y.L.; Park, S.H. New-onset Nephrotic Syndrome after Janssen COVID-19 Vaccination: A Case Report and Literature Review. J. Korean Med. Sci. 2021, 36, e218. [Google Scholar] [CrossRef] [PubMed]
- Mancianti, N.; Guarnieri, A.; Tripodi, S.; Salvo, D.P.; Garosi, G. Minimal change disease following vaccination for SARS-CoV-2. J. Nephrol. 2021, 34, 1039–1040. [Google Scholar] [CrossRef]
- Schwotzer, N.; Kissling, S.; Fakhouri, F. Letter regarding “Minimal change disease relapse following SARS-CoV-2 mRNA vaccine”. Kidney Int. 2021, 100, 458–459. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Fugo, K.; Yamazaki, K.; Terawaki, H. Minimal change disease soon after Pfizer-BioNTech COVID-19 vaccination. Clin. Kidney J. 2021, 14, 2606–2607. [Google Scholar] [CrossRef] [PubMed]
- Anupama, Y.J.; Patel, R.G.N.; Vankalakunti, M. Nephrotic Syndrome Following ChAdOx1 nCoV-19 Vaccine Against SARScoV-2. Kidney Int. Rep. 2021, 6, 2248. [Google Scholar] [CrossRef] [PubMed]
- Dirim, A.B.; Safak, S.; Andac, B.; Garayeva, N.; Demir, E.; Artan, A.S.; Ozluk, Y.; Kilicaslan, I.; Oto, O.A.; Ozturk, S.; et al. Minimal change disease following vaccination with CoronaVac. Clin. Kidney J. 2021, 14, 2268–2269. [Google Scholar] [CrossRef] [PubMed]
- Thappy, S.; Thalappil, S.R.; Abbarh, S.; Al-Mashdali, A.; Akhtar, M.; Alkadi, M.M. Minimal change disease following the Moderna COVID-19 vaccine: First case report. BMC Nephrol. 2021, 22, 376. [Google Scholar] [CrossRef]
- Alhosaini, M.N. A Case of Minimal Change Disease after SARS-CoV-2 Vaccination under the Age of 18. Avicenna J. Med. 2022, 12, 31–33. [Google Scholar] [CrossRef]
- Pella, E.; Sarafidis, P.A.; Alexandrou, M.E.; Stangou, M.; Nikolaidou, C.; Kosmidis, D.; Papagianni, A. De novo Minimal Change Disease in an Adolescent after Pfizer-BioNTech COVID-19 Vaccination: A Case Report. Case Rep. Nephrol. Dial. 2022, 12, 44–49. [Google Scholar] [CrossRef]
- Mochizuki, R.I.; Takahashi, N.; Ikenouchi, K.; Shoda, W.; Kuyama, T.; Takahashi, D. A de novo case of minimal change disease following the first dose of the Moderna mRNA-1273 SARS-CoV-2 vaccine without relapse after the second dose. CEN Case Rep. 2022, 18, 1–5. [Google Scholar] [CrossRef]
- Biradar, V.; Konnur, A.; Gang, S.; Hegde, U.; Rajapurkar, M.; Patel, H.; Pandey, S.N.; Soni, S. Adult-onset nephrotic syndrome following coronavirus disease vaccination. Clin. Kidney J. 2022, 15, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Unver, S.; Haholu, A.; Yildirim, S. Nephrotic syndrome and acute kidney injury following CoronaVac anti-SARS-CoV-2 vaccine. Clin. Kidney J. 2021, 14, 2608–2611. [Google Scholar] [CrossRef] [PubMed]
- Jongvilaikasem, P.; Rianthavorn, P. Minimal change disease and acute interstitial nephritis following SARS-CoV-2 BNT162b2 vaccination. Pediatr. Nephrol. 2022, 37, 1419–1421. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; An, W.S.; Rha, S.H.; Kim, S.E.; Lee, S.M. Minimal change glomerulonephritis following the second dose of the Moderna COVID-19 vaccine. QJM Mon. J. Assoc. Physicians 2022, 115, 490–491. [Google Scholar] [CrossRef] [PubMed]
- Abdulgayoom, M.; Albuni, M.K.; Abdelmahmuod, E.; Murshed, K.; Eldeeb, Y. Minimal change nephrotic syndrome four days after the administration of Pfizer-BioNTech COVID-19 vaccine-a new side effect or coincidence? Clin. Case Rep. 2021, 9, e05003. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, K.; Cohen, A.W.S.; Weerasinghe, N.; Vilayur, E. Report of two cases of minimal change disease following vaccination for COVID–19. Nephrology 2022, 27, 111–112. [Google Scholar] [CrossRef]
- Dormann, H.; Knüppel-Ruppert, A.; Amann, K.; Erley, C. Nephrotic Syndrome After Vaccination Against COVID-19: Three New Cases From Germany. Dtsch. Arztebl. Int. 2021, 118, 662–663. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Kageyama, M.; Iwase, M.; Ueda, A. A young adult with nephrotic syndrome following COVID-19 vaccination. CEN Case Rep. 2022, 11, 397–398. [Google Scholar] [CrossRef]
- Hartley, J.L.; Bailey, N.; Sharma, A.; Shawki, H. Nephrotic syndrome with minimal change disease after the Pfizer-BioNTech COVID-19 vaccine: Two cases. BMJ Case Rep. 2022, 15, e244638. [Google Scholar] [CrossRef]
- Chandra, P.; Roldao, M.; Drachenberg, C.; Santos, P.; Washida, N.; Clark, A.; Bista, B.; Mitsuna, R.; Yango, A. Minimal change disease and COVID-19 vaccination: Four cases and review of literature. Clin. Nephrol. Case Stud. 2022, 10, 54–63. [Google Scholar] [CrossRef]
- Klomjit, N.; Alexander, M.P.; Fervenza, F.C.; Zoghby, Z.; Garg, A.; Hogan, M.C.; Nasr, S.H.; Minshar, M.A.; Zand, L. COVID-19 Vaccination and Glomerulonephritis. Kidney Int. Rep. 2021, 6, 2969–2978. [Google Scholar] [CrossRef] [PubMed]
- Sekar, A.; Campbell, R.; Tabbara, J.; Rastogi, P. ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney Int. 2021, 100, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, M.T.; Birkenbach, M.P.; Lynch, M. ANCA-Associated Vasculitis Following Pfizer-BioNTech COVID-19 Vaccine. Am. J. Kidney Dis. 2021, 78, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Feghali, E.J.; Zafar, M.; Abid, S.; Santoriello, D.; Mehta, S. De-novo Antineutrophil Cytoplasmic Antibody-Associated Vasculitis Following the mRNA-1273 (Moderna) Vaccine for COVID-19. Cureus 2021, 13, e19616. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, H.Y.; Lu, C.C.; Lin, S.H. Case Report: Anti-neutrophil Cytoplasmic Antibody-Associated Vasculitis With Acute Renal Failure and Pulmonary Hemorrhage May Occur After COVID-19 Vaccination. Front. Med. 2021, 8, 765447. [Google Scholar] [CrossRef]
- Kim, B.C.; Kim, H.S.; Han, K.H.; Han, S.Y.; Jo, H.A. A Case Report of MPO-ANCA-Associated Vasculitis Following Heterologous mRNA1273 COVID-19 Booster Vaccination. J. Korean Med. Sci. 2022, 37, e204. [Google Scholar] [CrossRef]
- Anderegg, M.A.; Liu, M.; Saganas, C.; Montani, M.; Vogt, B.; Huynh-Do, U.; Fuster, D.G. De novo vasculitis after mRNA-1273 (Moderna) vaccination. Kidney Int. 2021, 100, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Gillion, V.; Jadoul, M.; Demoulin, N.; Aydin, S.; Devresse, A. Granulomatous vasculitis after the AstraZeneca anti-SARS-CoV-2 vaccine. Kidney Int. 2021, 100, 706–707. [Google Scholar] [CrossRef]
- Dube, G.K.; Benvenuto, L.J.; Batal, I. Antineutrophil Cytoplasmic Autoantibody-Associated Glomerulonephritis Following the Pfizer-BioNTech COVID-19 Vaccine. Kidney Int. Rep. 2021, 6, 3087–3089. [Google Scholar] [CrossRef]
- Villa, M.; Díaz-Crespo, F.; Pérez de José, A.; Verdalles, Ú.; Verde, E.; Almeida Ruiz, F.; Acosta, A.; Mijaylova, A.; Goicoechea, M. A case of ANCA-associated vasculitis after AZD1222 (Oxford-AstraZeneca) SARS-CoV-2 vaccination: Casualty or causality? Kidney Int. 2021, 100, 937–938. [Google Scholar] [CrossRef]
- Hakroush, S.; Tampe, B. Case Report: ANCA-Associated Vasculitis Presenting With Rhabdomyolysis and Pauci-Immune Crescentic Glomerulonephritis After Pfizer-BioNTech COVID-19 mRNA Vaccination. Front. Immunol. 2021, 12, 762006. [Google Scholar] [CrossRef] [PubMed]
- Kudose, S.; Friedmann, P.; Albajrami, O.; D’Agati, V.D. Histologic correlates of gross hematuria following Moderna COVID-19 vaccine in patients with IgA nephropathy. Kidney Int. 2021, 100, 468–469. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.; Herrera Hernandez, L.P.; Bu, L.; Kizilbash, S.; Najera, L.; Rheault, M.N.; Czyzyk, J.; Kouri, A.M. IgA nephropathy presenting as macroscopic hematuria in 2 pediatric patients after receiving the Pfizer COVID-19 vaccine. Kidney Int. 2021, 100, 705–706. [Google Scholar] [CrossRef]
- Sacker, A.; Kung, V.; Andeen, N. Anti-GBM nephritis with mesangial IgA deposits after SARS-CoV-2 mRNA vaccination. Kidney Int. 2021, 100, 471–472. [Google Scholar] [CrossRef]
- Aydın, M.F.; Yıldız, A.; Oruç, A.; Sezen, M.; Dilek, K.; Güllülü, M.; Yavuz, M.; Ersoy, A. Relapse of primary membranous nephropathy after inactivated SARS-CoV-2 virus vaccination. Kidney Int. 2021, 100, 464–465. [Google Scholar] [CrossRef] [PubMed]
- Perrin, P.; Bassand, X.; Benotmane, I.; Bouvier, N. Gross hematuria following SARS-CoV-2 vaccination in patients with IgA nephropathy. Kidney Int. 2021, 100, 466–468. [Google Scholar] [CrossRef]
- Negrea, L.; Rovin, B.H. Gross hematuria following vaccination for severe acute respiratory syndrome coronavirus 2 in 2 patients with IgA nephropathy. Kidney Int. 2021, 99, 1487. [Google Scholar] [CrossRef] [PubMed]
- Yokote, S.; Ueda, H.; Shimizu, A.; Okabe, M.; Yamamoto, K.; Tsuboi, N.; Yokoo, T. IgA nephropathy with glomerular capillary IgA deposition following SARS-CoV-2 mRNA vaccination: A report of three cases. CEN Case Rep. 2022, 13, 1–7. [Google Scholar] [CrossRef]
- Uchiyama, Y.; Fukasawa, H.; Ishino, Y.; Nakagami, D.; Kaneko, M.; Yasuda, H.; Furuya, R. Sibling cases of gross hematuria and newly diagnosed IgA nephropathy following SARS-CoV-2 vaccination. BMC Nephrol. 2022, 23, 216. [Google Scholar] [CrossRef]
- Rahim, S.E.G.; Lin, J.T.; Wang, J.C. A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination. Kidney Int. 2021, 100, 238. [Google Scholar] [CrossRef]
- Martinez Valenzuela, L.; Oliveras, L.; Gomà, M.; Quiros, E.; Antón-Pámpols, P.; Gómez-Preciado, F.; Fulladosa, X.; Cruzado, J.M.; Torras, J.; Draibe, J. Th1 Cytokines Signature in 2 Cases of IgA Nephropathy Flare after mRNA-Based SARS-CoV-2 Vaccine: Exploring the Pathophysiology. Nephron 2022, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abramson, M.; Mon-Wei Yu, S.; Campbell, K.N.; Chung, M.; Salem, F. IgA Nephropathy After SARS-CoV-2 Vaccination. Kidney Med. 2021, 3, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Niel, O.; Florescu, C. IgA nephropathy presenting as rapidly progressive glomerulonephritis following first dose of COVID-19 vaccine. Pediatr. Nephrol. 2022, 37, 461–462. [Google Scholar] [CrossRef]
- Abdel-Qader, D.H.; Hazza Alkhatatbeh, I.; Hayajneh, W.; Annab, H.; Al Meslamani, A.Z.; Elmusa, R.A. IgA nephropathy in a pediatric patient after receiving the first dose of Pfizer-BioNTech COVID-19 vaccine. Vaccine 2022, 40, 2528–2530. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kikuchi, E.; Nagasawa, M.; Oshiba, A.; Shimoda, M. An adolescent girl diagnosed with IgA nephropathy following the first dose of the COVID-19 vaccine. CEN Case Rep. 2022, 11, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Yoshida, K.; Ichikawa, D.; Shibagaki, Y.; Yazawa, M. Abrupt worsening of occult IgA nephropathy after the first dose of SARS-CoV-2 vaccination. CEN Case Rep. 2022, 11, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, R.; Lalloni, S.; Marchisio, M.; Oddone, V.; De Simone, E.; Del Vecchio, G.; Sciascia, S.; Roccatello, D. New Onset Biopsy-Proven Nephropathies after COVID Vaccination. Am. J. Nephrol. 2022, 53, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.K.; Chan, K.W. Gross haematuria after mRNA COVID-19 vaccination in two patients with histological and clinical diagnosis of IgA nephropathy. Nephrology 2022, 27, 110–111. [Google Scholar] [CrossRef]
- Leong, L.C.; Hong, W.Z.; Khatri, P. Reactivation of minimal change disease and IgA nephropathy after COVID-19 vaccination. Clin. Kidney J. 2022, 15, 569–570. [Google Scholar] [CrossRef]
- Tan, H.Z.; Tan, R.Y.; Choo, J.C.J.; Lim, C.C.; Tan, C.S.; Loh, A.H.L.; Tien, C.S.; Tan, P.H.; Woo, K.T. Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int. 2021, 100, 469–471. [Google Scholar] [CrossRef]
- Horino, T.; Sawamura, D.; Inotani, S.; Ishihara, M.; Komori, M.; Ichii, O. Newly diagnosed IgA nephropathy with gross haematuria following COVID-19 vaccination. QJM Mon. J. Assoc. Physicians 2022, 115, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Geara, A.S.; Han, S.; Hogan, J.J.; Coppock, G. Need for symptom monitoring in IgA nephropathy patients post COVID-19 vaccination. Clin. Nephrol. 2022, 97, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Morisawa, K.; Honda, M. Two patients presenting IgA nephropathy after COVID-19 vaccination during a follow-up for asymptomatic hematuria. Pediatr. Nephrol. 2022, 37, 1695–1696. [Google Scholar] [CrossRef] [PubMed]
- Nihei, Y.; Kishi, M.; Suzuki, H.; Koizumi, A.; Yoshida, M.; Hamaguchi, S.; Iwasaki, M.; Fukuda, H.; Takahara, H.; Kihara, M.; et al. IgA Nephropathy with Gross Hematuria Following COVID-19 mRNA Vaccination. Intern. Med. 2022, 61, 1033–1037. [Google Scholar] [CrossRef]
- Horino, T. IgA nephropathy flare-up following SARS-CoV-2 vaccination. QJM Mon. J. Assoc. Physicians 2021, 114, 735–736. [Google Scholar] [CrossRef]
- Watanabe, S.; Zheng, S.; Rashidi, A. IgA nephropathy relapse following COVID-19 vaccination treated with corticosteroid therapy: Case report. BMC Nephrol. 2022, 23, 135. [Google Scholar] [CrossRef]
- Udagawa, T.; Motoyoshi, Y. Macroscopic hematuria in two children with IgA nephropathy remission following Pfizer COVID-19 vaccination. Pediatr. Nephrol. 2022, 37, 1693–1694. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, M.S.; Kim, Y.J.; Han, M.H.; Jung, H.Y.; Choi, J.Y.; Cho, J.H.; Kim, C.D.; Kim, Y.L.; Park, S.H. New-Onset Kidney Diseases after COVID-19 Vaccination: A Case Series. Vaccines 2022, 10, 302. [Google Scholar] [CrossRef]
- Plasse, R.; Nee, R.; Gao, S.; Olson, S. Acute kidney injury with gross hematuria and IgA nephropathy after COVID-19 vaccination. Kidney Int. 2021, 100, 944–945. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, E.Y. Renal side effects of COVID-19 vaccines in patients with immunoglobulin A nephropathy. Kidney Res. Clin. Pract. 2022, 41, 124–127. [Google Scholar] [CrossRef]
- Tuschen, K.; Bräsen, J.H.; Schmitz, J.; Vischedyk, M.; Weidemann, A. Relapse of class V lupus nephritis after vaccination with COVID-19 mRNA vaccine. Kidney Int. 2021, 100, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Miranda, M.F.; González-Ibarra, S.G.; Pérez-Arias, A.A.; Uribe-Uribe, N.O.; Mejia-Vilet, J.M. New-onset systemic lupus erythematosus beginning as class V lupus nephritis after COVID-19 vaccination. Kidney Int. 2021, 100, 1340–1341. [Google Scholar] [CrossRef] [PubMed]
- Da, Y.; Goh, G.H.; Khatri, P. A case of membranous nephropathy following Pfizer-BioNTech mRNA vaccination against COVID-19. Kidney Int. 2021, 100, 938–939. [Google Scholar] [CrossRef]
- Gueguen, L.; Loheac, C.; Saidani, N.; Khatchatourian, L. Membranous nephropathy following anti-COVID-19 mRNA vaccination. Kidney Int. 2021, 100, 1140–1141. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kang, K.S.; Han, K.H. Two adolescent cases of acute tubulointerstitial nephritis after second dose of COVID-19 mRNA vaccine. Hum. Vaccines Immunother. 2022, 18, 2059308. [Google Scholar] [CrossRef] [PubMed]
- Dheir, H.; Sipahi, S.; Cakar, G.C.; Yaylaci, S.; Hacibekiroglu, T.; Karabay, O. Acute tubulointerstitial nephritis after COVID-19 m-RNA BNT162b2 vaccine. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6171–6173. [Google Scholar] [PubMed]
- Mira, F.S.; Costa Carvalho, J.; de Almeida, P.A.; Pimenta, A.C.; Alen Coutinho, I.; Figueiredo, C.; Rodrigues, L.; Sousa, V.; Ferreira, E.; Pinto, H.; et al. A Case of Acute Interstitial Nephritis After Two Doses of the BNT162b2 SARS-CoV-2 Vaccine. Int. J. Nephrol. Renov. Dis 2021, 14, 421–426. [Google Scholar] [CrossRef]
- Rieckmann, S.; Seibert, F.S.; Hogeweg, M.; Bertram, S.; Doevelaar, A.A.N.; Amann, K.; Babel, N.; Westhoff, T.H. Acute interstitial nephritis after vaccination with BNT162b2. J. Nephrol. 2022, 35, 779–782. [Google Scholar] [CrossRef]
- Tan, F.S.; Kabir, M.E.; Bhandari, S. Acute interstitial nephritis after COVID-19 vaccination. BMJ Case Rep. 2022, 15, e246841. [Google Scholar] [CrossRef]
- Hong, L.-Y.; Lee, C.-H.; Chiu, I.J. De novo podocytopathy following moderna COVID-19 vaccine: A case report and racial disproportionality in adverse effect reports. Front. Med. 2022, 9, 844004. [Google Scholar] [CrossRef]
- Kim, H.J.; Jung, M.; Lim, B.J.; Han, S.H. New-onset class III lupus nephritis with multi-organ involvement after COVID-19 vaccination. Kidney Int. 2022, 101, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Marampudi, S.; Beshai, R.; Banker, G. Reactivation of minimal change disease after pfizer vaccine against COVID-19. J. Osteopath. Med. 2022, 122, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Ran, E.; Wang, M.; Wang, Y.; Liu, R.; Yi, Y.; Liu, Y. New-onset crescent IgA nephropathy following the CoronaVac vaccine: A case report. Medicine 2022, 101, e30066. [Google Scholar] [CrossRef] [PubMed]
- Fillon, A.; Sautenet, B.; Barbet, C.; Moret, L.; Thillard, E.M.; Jonville-Béra, A.P.; Halimi, J.M. De novo and relapsing necrotizing vasculitis after COVID-19 vaccination. Clin. Kidney J. 2022, 15, 560–563. [Google Scholar] [CrossRef]
- David, R.; Hanna, P.; Lee, K.; Ritchie, A. Relapsed ANCA associated vasculitis following Oxford AstraZeneca ChAdOx1-S COVID-19 vaccination: A case series of two patients. Nephrology 2022, 27, 109–110. [Google Scholar] [CrossRef]
- Schaubschlager, T.; Rajora, N.; Diep, S.; Kirtek, T.; Cai, Q.; Hendricks, A.R.; Shastri, S.; Zhou, X.J.; Saxena, R. De novo or recurrent glomerulonephritis and acute tubulointerstitial nephritis after COVID-19 vaccination: A report of six cases from a single center. Clin. Nephrol. 2022, 97, 289–297. [Google Scholar] [CrossRef]
- Chavarot, N.; Padden, M.; Amrouche, L.; Malard, S.; Scemla, A.; Sberro-Soussan, R.; Leon, J.; Legendre, C.; Duong, J.P.; Zuber, J.; et al. De novo posttransplant membranous nephropathy following BNT162b2 mRNA COVID-19 vaccine in a kidney transplant recipient. Am. J. Transplant. 2022. [Google Scholar] [CrossRef]
- Fulchiero, R.; Amaral, S. Focal segmental glomerulosclerosis recurrence in a young adult with kidney transplant after mRNA COVID-19 vaccination. Pediatr. Nephrol. 2022, 37, 2217. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Sprent, J.; King, C. COVID-19 vaccine side effects: The positives about feeling bad. Sci. Immunol. 2021, 6, eabj9256. [Google Scholar] [CrossRef]
- Sayedahmed, E.E.; Elkashif, A.; Alhashimi, M.; Sambhara, S.; Mittal, S.K. Adenoviral Vector-Based Vaccine Platforms for Developing the Next Generation of Influenza Vaccines. Vaccines 2020, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, M.; Massella, L.; Ruggiero, B.; Emma, F. Minimal Change Disease. Clin. J. Am. Soc. Nephrol. CJASN 2017, 12, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Schena, F.P.; Nistor, I. Epidemiology of IgA Nephropathy: A Global Perspective. Semin. Nephrol. 2018, 38, 435–442. [Google Scholar] [CrossRef]
- Lai, K.N. Pathogenesis of IgA nephropathy. Nat. Rev. Nephrol. 2012, 8, 275–283. [Google Scholar] [CrossRef]
- Lai, K.N.; Tang, S.C.; Schena, F.P.; Novak, J.; Tomino, Y.; Fogo, A.B.; Glassock, R.J. IgA nephropathy. Nat. Rev. Dis. Prim. 2016, 2, 16001. [Google Scholar] [CrossRef]
- Patel, C.; Shah, H.H. Vaccine-associated kidney diseases: A narrative review of the literature. Saudi J. Kidney Dis. Transplant. 2019, 30, 1002–1009. [Google Scholar] [CrossRef]
- Wisnewski, A.V.; Campillo Luna, J.; Redlich, C.A. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS ONE 2021, 16, e0249499. [Google Scholar] [CrossRef] [PubMed]
- Jeffs, L.S.; Nitschke, J.; Tervaert, J.W.; Peh, C.A.; Hurtado, P.R. Viral RNA in the influenza vaccine may have contributed to the development of ANCA-associated vasculitis in a patient following immunisation. Clin. Rheumatol. 2016, 35, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Uppal, N.N.; Kello, N.; Shah, H.H.; Khanin, Y.; De Oleo, I.R.; Epstein, E.; Sharma, P.; Larsen, C.P.; Bijol, V.; Jhaveri, K.D. De Novo ANCA-Associated Vasculitis With Glomerulonephritis in COVID-19. Kidney Int. Rep. 2020, 5, 2079–2083. [Google Scholar] [CrossRef] [PubMed]
- Vlachoyiannopoulos, P.G.; Magira, E.; Alexopoulos, H.; Jahaj, E.; Theophilopoulou, K.; Kotanidou, A.; Tzioufas, A.G. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann. Rheum. Dis. 2020, 79, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Prendecki, M.; Clarke, C.; Cairns, T.; Cook, T.; Roufosse, C.; Thomas, D.; Willicombe, M.; Pusey, C.D.; McAdoo, S.P. Anti-glomerular basement membrane disease during the COVID-19 pandemic. Kidney Int. 2020, 98, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef]
- Summers, S.A.; Steinmetz, O.M.; Gan, P.Y.; Ooi, J.D.; Odobasic, D.; Kitching, A.R.; Holdsworth, S.R. Toll-like receptor 2 induces Th17 myeloperoxidase autoimmunity while Toll-like receptor 9 drives Th1 autoimmunity in murine vasculitis. Arthritis Rheum. 2011, 63, 1124–1135. [Google Scholar] [CrossRef]
- Kumar, N.; Admane, N.; Kumari, A.; Sood, D.; Grover, S.; Prajapati, V.K.; Chandra, R.; Grover, A. Cytotoxic T-lymphocyte elicited vaccine against SARS-CoV-2 employing immunoinformatics framework. Sci. Rep. 2021, 11, 7653. [Google Scholar] [CrossRef]
- Ruebner, R.L.; Fadrowski, J.J. Tubulointerstitial Nephritis. Pediatr. Clin. N. Am. 2019, 66, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Segal, Y.; Shoenfeld, Y. Vaccine-induced autoimmunity: The role of molecular mimicry and immune crossreaction. Cell. Mol. Immunol. 2018, 15, 586–594. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).