COVID-19 Breakthrough Infections in Vaccinated Kidney Transplant Recipients
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Center-Level of COVID-19 Breakthrough Infection in Vaccinated KTRs
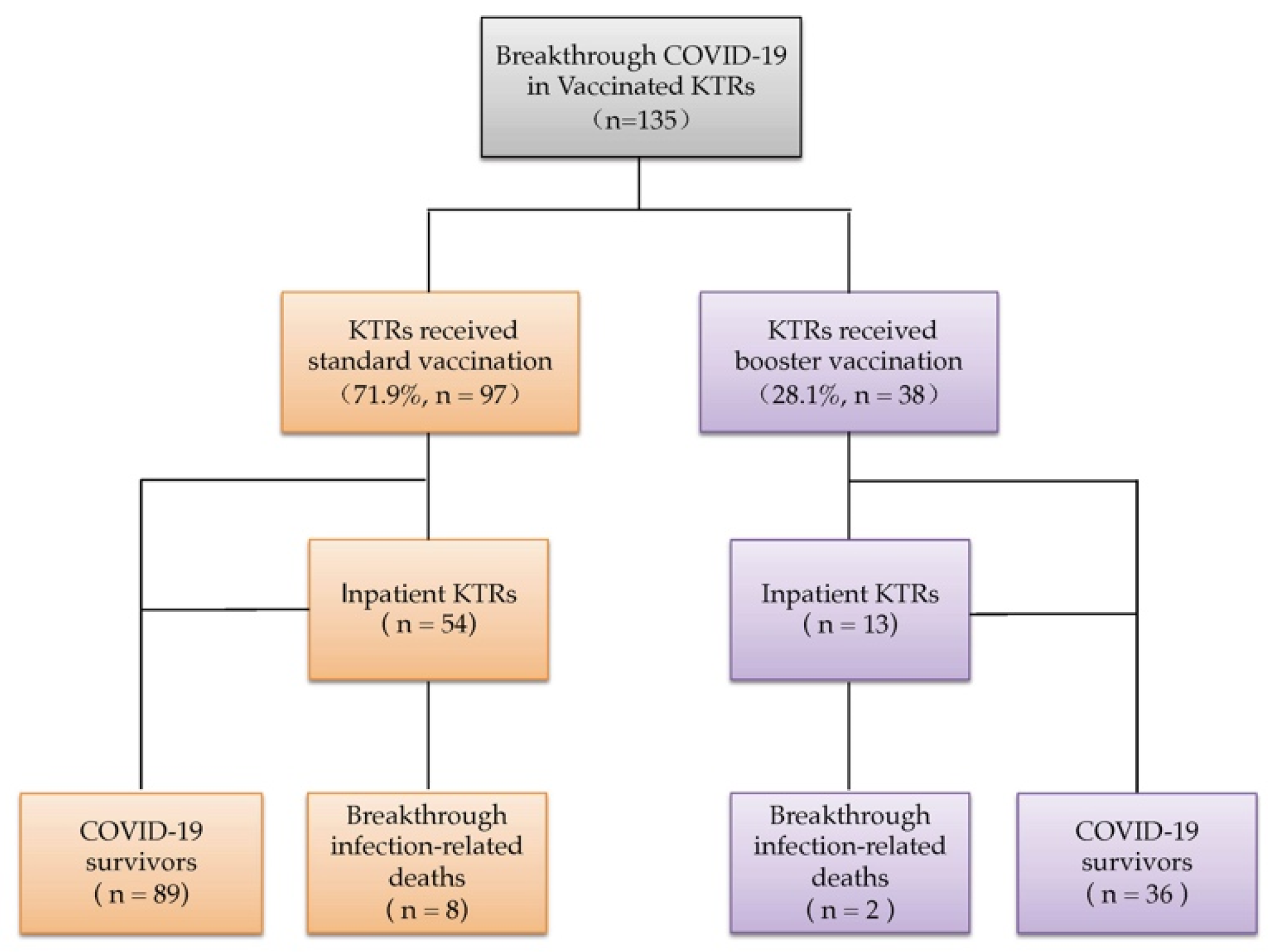
3.2. Characteristics of KTRs with Patient-Level Data and Comparison According to Vaccination Status
3.3. Comparison between COVID-19 Breakthrough Infection-Related Deaths and Survival
3.4. Comparison between Inpatients and Outpatients with COVID-19 Breakthrough Infections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elias, M.; Pievani, D.; Randoux, C.; Louis, K.; Denis, B.; Delion, A.; Le Goff, O.; Antoine, C.; Greze, C.; Pillebout, E.; et al. COVID-19 Infection in Kidney Transplant Recipients: Disease Incidence and Clinical Outcomes. J. Am. Soc. Nephrol. 2020, 31, 2413–2423. [Google Scholar] [CrossRef] [PubMed]
- Cristelli, M.P.; Viana, L.A.; Dantas, M.T.C.; Martins, S.B.S.; Fernandes, R.; Nakamura, M.R.; Santos, D.; Taddeo, J.B.; Azevedo, V.F.; Foresto, R.D.; et al. The Full Spectrum of COVID-19 Development and Recovery among Kidney Transplant Recipients. Transplantation 2021, 105, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Akalin, E.; Azzi, Y.; Bartash, R.; Seethamraju, H.; Parides, M.; Hemmige, V.; Ross, M.; Forest, S.; Goldstein, Y.D.; Ajaimy, M.; et al. COVID-19 and Kidney Transplantation. N. Engl. J. Med. 2020, 382, 2475–2477. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.; Campbell, J.; Lambourg, E.; Watters, C.; O’Neil, M.; Almond, A.; Buck, K.; Carr, E.J.; Clark, L.; Cousland, Z.; et al. The Impact of Vaccination on Incidence and Outcomes of SARS-CoV-2 Infection in Patients with Kidney Failure in Scotland. J. Am. Soc. Nephrol. 2022, 33, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Tau, N.; Yahav, D.; Schneider, S.; Rozen-Zvi, B.; Abu Sneineh, M.; Rahamimov, R. Severe consequences of COVID-19 infection among vaccinated kidney transplant recipients. Am. J. Transplant. 2021, 21, 2910–2912. [Google Scholar] [CrossRef]
- Ali, N.M.; Alnazari, N.; Mehta, S.A.; Boyarsky, B.; Avery, R.K.; Segev, D.L.; Montgomery, R.A.; Stewart, Z.A. Development of COVID-19 Infection in Transplant Recipients after SARS-CoV-2 Vaccination. Transplantation 2021, 105, e104–e106. [Google Scholar] [CrossRef]
- Grupper, A.; Rabinowich, L.; Schwartz, D.; Schwartz, I.F.; Ben-Yehoyada, M.; Shashar, M.; Katchman, E.; Halperin, T.; Turner, D.; Goykhman, Y.; et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transplant. 2021, 21, 2719–2726. [Google Scholar] [CrossRef]
- Danthu, C.; Hantz, S.; Dahlem, A.; Duval, M.; Ba, B.; Guibbert, M.; El Ouafi, Z.; Ponsard, S.; Berrahal, I.; Achard, J.M.; et al. Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 32, 2153–2158. [Google Scholar] [CrossRef]
- Bertrand, D.; Hamzaoui, M.; Lemee, V.; Lamulle, J.; Hanoy, M.; Laurent, C.; Lebourg, L.; Etienne, I.; Lemoine, M.; Le Roy, F.; et al. Antibody and T Cell Response to SARS-CoV-2 Messenger RNA BNT162b2 Vaccine in Kidney Transplant Recipients and Hemodialysis Patients. J. Am. Soc. Nephrol. 2021, 32, 2147–2152. [Google Scholar] [CrossRef]
- Boedecker-Lips, S.C.; Lautem, A.; Runkel, S.; Klimpke, P.; Kraus, D.; Keil, P.; Holtz, S.; Tomalla, V.; Marczynski, P.; Boedecker, C.B.; et al. Six-Month Follow-Up after Vaccination with BNT162b2: SARS-CoV-2 Antigen-Specific Cellular and Humoral Immune Responses in Hemodialysis Patients and Kidney Transplant Recipients. Pathogens 2022, 11, 67. [Google Scholar] [CrossRef]
- Stumpf, J.; Siepmann, T.; Lindner, T.; Karger, C.; Schwobel, J.; Anders, L.; Faulhaber-Walter, R.; Schewe, J.; Martin, H.; Schirutschke, H.; et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg. Health Eur. 2021, 9, 100178. [Google Scholar] [CrossRef]
- Villanego, F.; Cazorla, J.M.; Vigara, L.A.; Garcia, T.; Trujillo, T.; Montiel, N.; Rodriquez-Iglesias, M.; Mazuecos, A. Protecting kidney transplant recipients against SARS-CoV-2 infection: A third dose of vaccine is necessary now. Am. J. Transplant. 2022, 22, 1275–1276. [Google Scholar] [CrossRef]
- Rincon-Arevalo, H.; Choi, M.; Stefanski, A.L.; Halleck, F.; Weber, U.; Szelinski, F.; Jahrsdorfer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol. 2021, 6, eabj1031. [Google Scholar] [CrossRef]
- Bertrand, D.; Hamzaoui, M.; Lemee, V.; Lamulle, J.; Laurent, C.; Etienne, I.; Lemoine, M.; Lebourg, L.; Hanoy, M.; Le Roy, F.; et al. Antibody and T-cell response to a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Kidney Int. 2021, 100, 1337–1340. [Google Scholar] [CrossRef]
- Ducloux, D.; Bamoulid, J.; Chabannes, M.; Colladant, M.; Munshi, A.; Roubiou, C.; Seibel, J.; Tachikart, A.; Yannaraki, M.; Crepin, T.; et al. Current vaccine strategies against SARS-CoV-2 only poorly protect kidney transplant recipients. J. Infect. 2022, 84, e34–e35. [Google Scholar] [CrossRef]
- Reindl-Schwaighofer, R.; Heinzel, A.; Mayrdorfer, M.; Jabbour, R.; Hofbauer, T.M.; Merrelaar, A.; Eder, M.; Regele, F.; Doberer, K.; Spechtl, P.; et al. Comparison of SARS-CoV-2 Antibody Response 4 Weeks After Homologous vs Heterologous Third Vaccine Dose in Kidney Transplant Recipients: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 165–171. [Google Scholar] [CrossRef]
- Benotmane, I.; Gautier, G.; Perrin, P.; Olagne, J.; Cognard, N.; Fafi-Kremer, S.; Caillard, S. Antibody Response After a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients With Minimal Serologic Response to 2 Doses. JAMA 2021, 326, 1063–1065. [Google Scholar] [CrossRef]
- McEvoy, C.M.; Lee, A.; Misra, P.S.; Lebovic, G.; Wald, R.; Yuen, D.A. Real-world Impact of 2-dose SARS-CoV-2 Vaccination in Kidney Transplant Recipients. Transplantation 2022, 106, e279–e280. [Google Scholar] [CrossRef]
- Aslam, S.; Adler, E.; Mekeel, K.; Little, S.J. Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transpl. Infect. Dis. 2021, 23, e13705. [Google Scholar] [CrossRef]
- Mehta, R.B.; Silveira, F.P. COVID-19 after two doses of mRNA vaccines in kidney transplant recipients. Am. J. Transplant. 2021, 21, 4102–4104. [Google Scholar] [CrossRef]
- Tsapepas, D.; Paget, K.; Mohan, S.; Cohen, D.J.; Husain, S.A. Clinically Significant COVID-19 Following SARS-CoV-2 Vaccination in Kidney Transplant Recipients. Am. J. Kidney Dis. 2021, 78, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Chenxi Song, C.; Christensen, J.; Kumar, D.; Vissichelli, N.; Morales, M.; Gupta, G. Early experience with SARS-CoV-2 mRNA vaccine breakthrough among kidney transplant recipients. Transpl. Infect. Dis. 2021, 23, e13654. [Google Scholar] [CrossRef] [PubMed]
- Zahradka, I.; Petr, V.; Modos, I.; Magicova, M.; Dusek, L.; Viklicky, O. Association Between SARS-CoV-2 Messenger RNA Vaccines and Lower Infection Rates in Kidney Transplant Recipients: A Registry-Based Report. Ann. Intern. Med. 2022, 175, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Montagud-Marrahi, E.; Cucchiari, D.; Cuadrado-Payan, E.; Cofan, F.; Torregrosa, J.V.; Ventura-Aguiar, P.; Revuelta, I.; Bodro, M.; Pineiro, G.J.; Esforzado, N.; et al. SARS-CoV-2 Infection after Full Vaccination in Kidney Transplant Recipients. Transplantation 2021, 105, e278–e279. [Google Scholar] [CrossRef] [PubMed]
- Marinaki, S.; Xagas, E.; Tsoutsoura, P.; Katsaros, D.; Korogiannou, M.; Boletis, I.N. Occurrence of Severe SARS-CoV-2 Infection in Fully Vaccinated Solid Organ Transplant Recipients. Transplant. Proc. 2021, 54, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- Medina-Pestana, J.; Covas, D.T.; Viana, L.A.; Dreige, Y.C.; Nakamura, M.R.; Lucena, E.F.; Requiao-Moura, L.R.; Fortaleza, C.; Foresto, R.D.; Tedesco-Silva, H.; et al. Inactivated Whole-virus Vaccine Triggers Low Response against SARS-CoV-2 Infection among Renal Transplant Patients: Prospective Phase 4 Study Results. Transplantation 2022, 106, 853–861. [Google Scholar] [CrossRef]
- Rodriguez-Espinosa, D.; Montagud-Marrahi, E.; Cacho, J.; Arana, C.; Taurizano, N.; Hermida, E.; Del Risco-Zevallos, J.; Casals, J.; Rosario, A.; Cuadrado-Payan, E.; et al. Incidence of severe breakthrough SARS-CoV-2 infections in vaccinated kidney transplant and haemodialysis patients. J. Nephrol. 2022, 35, 769–778. [Google Scholar] [CrossRef]
- Basic-Jukic, N. Clinical consequences of the suboptimal effect of messenger RNA-based SARS-CoV-2 vaccine in renal transplant recipients. Ther. Apher. Dial. 2021, 26, 248–249. [Google Scholar] [CrossRef]
- Hamm, S.R.; Rezahosseini, O.; Moller, D.L.; Loft, J.A.; Poulsen, J.R.; Knudsen, J.D.; Pedersen, M.S.; Schonning, K.; Harboe, Z.B.; Rasmussen, A.; et al. Incidence and severity of SARS-CoV-2 infections in liver and kidney transplant recipients in the post-vaccination era: Real-life data from Denmark. Am. J. Transplant. 2022, 23, e13628. [Google Scholar] [CrossRef]
- Basic-Jukic, N.; Ivo, J. SARS-CoV-2 infection after two doses of mRNA vaccine in renal transplant recipients. Transpl. Infect. Dis. 2021, 23, e13628. [Google Scholar] [CrossRef]
- Wadei, H.M.; Gonwa, T.A.; Leoni, J.C.; Shah, S.Z.; Aslam, N.; Speicher, L.L. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am. J. Transplant. 2021, 21, 3496–3499. [Google Scholar] [CrossRef]
- Tsalouchos, A.; Rossolini, G.M.; Maggi, L.; Mazzoni, A.; Annunziato, F.; Dattolo, P.C. COVID-19 in a kidney transplant recipient after mRNA-based SARS-CoV-2 vaccination. Transpl. Infect. Dis. 2021, 23, e13649. [Google Scholar] [CrossRef]
- Wijtvliet, V.; Ledeganck, K.; Peeters, B.; Hellemans, R.; Abramowicz, D. SARS-CoV-2 breakthrough infections in vaccinated kidney transplant recipients: An issue of concern. Clin. Kidney J. 2021, 14, 2261–2262. [Google Scholar] [CrossRef]
- Malinis, M.; Cohen, E.; Azar, M.M. Effectiveness of SARS-CoV-2 vaccination in fully vaccinated solid organ transplant recipients. Am. J. Transplant. 2021, 21, 2916–2918. [Google Scholar] [CrossRef]
- Loconsole, D.; Stea, E.D.; Sallustio, A.; Fonto, G.; Pronzo, V.; Simone, S.; Centrone, F.; Accogli, M.; Gesualdo, L.; Chironna, M. Severe COVID-19 by SARS-CoV-2 Lineage B.1.1.7 in Vaccinated Solid-Organ Transplant Recipients: New Preventive Strategies Needed to Protect Immunocompromised Patients. Vaccines 2021, 9, 806. [Google Scholar] [CrossRef]
- Chang, K.M.; Berlinrut, I.; Wallach, F.R. A case of severe COVID-19 despite full vaccination with mRNA-1273 SARS-CoV-2 vaccine (Moderna) in a kidney transplant recipient. Transpl. Infect. Dis. 2021, 23, e13710. [Google Scholar] [CrossRef]
- Meshram, H.S.; Kute, V.B.; Shah, N.; Chauhan, S.; Navadiya, V.V.; Patel, A.H.; Patel, H.V.; Engineer, D.; Banerjee, S.; Rizvi, J.; et al. COVID-19 in Kidney Transplant Recipients Vaccinated With Oxford-AstraZeneca COVID-19 Vaccine (Covishield): A Single-center Experience From India. Transplantation 2021, 105, e100–e103. [Google Scholar] [CrossRef]
- Anjan, S.; Natori, Y.; Fernandez Betances, A.A.; Agritelley, M.S.; Mattiazzi, A.; Arosemena, L.; Andrews, D.M.; Simkins, J.; Guerra, G.; Abbo, L.M. Breakthrough COVID-19 Infections after mRNA Vaccination in Solid Organ Transplant Recipients in Miami, Florida. Transplantation 2021, 105, e139–e141. [Google Scholar] [CrossRef]
- Chen, S.; Kowalewska, J.; McCune, T.R. COVID-19 Associated Collapsing FSGS in an APOL1 Homozygous Transplant Recipient after Successful COVID Vaccination: A Case Report. Transplant. Proc. 2022, 54, 1543–1546. [Google Scholar] [CrossRef]
- Almaghrabi, R.S.; Alhamlan, F.S.; Dada, A.; Al-Tawfiq, J.A.; Al Hroub, M.K.; Saeedi, M.F.; Alamri, M.; Alhothaly, B.; Alqasabi, A.; Al-Qahtani, A.A.; et al. Outcome of SARS-CoV-2 variant breakthrough infection in fully immunized solid organ transplant recipients. J. Infect. Public Health 2022, 15, 51–55. [Google Scholar] [CrossRef]
- Radcliffe, C.; Azar, M.M.; Cohen, E.; Tucker, M.; Grubaugh, N.D.; Malinis, M. Clinical effectiveness of additional primary SARS-CoV-2 vaccine doses for solid organ transplant recipients. Clin. Transplant. 2022, 36, e14601. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Ho, Q.Y.; Liew, I.T.; Thien, S.Y.; Chan, Y.F.Z.; Cherng, B.P.Z.; Wong, H.M.; Chua, Y.Y.; Kee, T.; Tan, T.T. Impact of COVID-19 infections among kidney transplant recipients. Ann. Acad. Med. Singap. 2022, 51, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Natori, Y.; Andrews, D.; Guerra, G. Rapid reinfection of severe acute respiratory syndrome coronavirus 2 confirmed with sequencing in a solid organ transplant recipient. Transpl. Infect. Dis. 2022, 24, e13840. [Google Scholar] [CrossRef] [PubMed]
- Benotmane, I.; Velay, A.; Gautier-Vargas, G.; Olagne, J.; Obrecht, A.; Cognard, N.; Heibel, F.; Braun-Parvez, L.; Keller, N.; Martzloff, J.; et al. Breakthrough COVID-19 cases despite prophylaxis with 150 mg of tixagevimab and 150 mg of cilgavimab in kidney transplant recipients. Am. J. Transplant. 2022, 22, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Fahim, P.; Nicolaysen, A.; Yabu, J.M.; Zuckerman, J.E. Osmotic Tubulopathy and Acute Thrombotic Microangiopathy in a Kidney Transplant Recipient with a Breakthrough SARS-CoV-2 Infection. Kidney Med. 2022, 4, 100492. [Google Scholar] [CrossRef]
- Team, C.C.-V.B.C.I. COVID-19 Vaccine Breakthrough Infections Reported to CDC—United States, January 1-April 30, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 792–793. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Kumar, D.; Ferreira, V.H.; Hall, V.G.; Hu, Q.; Samson, R.; Ku, T.; Ierullo, M.; Majchrzak-Kita, B.; Tomlinson, G.; Gingras, A.C.; et al. Neutralization of SARS-CoV-2 Variants in Transplant Recipients After Two and Three Doses of mRNA-1273 Vaccine: Secondary Analysis of a Randomized Trial. Ann. Intern. Med. 2022, 175, 226–233. [Google Scholar] [CrossRef]
- Benning, L.; Morath, C.; Bartenschlager, M.; Kim, H.; Reineke, M.; Beimler, J.; Buylaert, M.; Nusshag, C.; Kalble, F.; Reichel, P.; et al. Neutralizing antibody response against the B.1.617.2 (delta) and the B.1.1.529 (omicron) variants after a third mRNA SARS-CoV-2 vaccine dose in kidney transplant recipients. Am. J. Transplant. 2022, 22, 1873–1883. [Google Scholar] [CrossRef]
- Caillard, S.; Thaunat, O.; Benotmane, I.; Masset, C.; Blancho, G. Antibody Response to a Fourth Messenger RNA COVID-19 Vaccine Dose in Kidney Transplant Recipients: A Case Series. Ann. Intern. Med. 2022, 175, 455–456. [Google Scholar] [CrossRef]
- Cassaniti, I.; Gregorini, M.; Bergami, F.; Arena, F.; Sammartino, J.C.; Percivalle, E.; Soleymaninejadian, E.; Abelli, M.; Ticozzelli, E.; Nocco, A.; et al. in Kidney Transplant Recipients. Vaccines 2022, 10, 921. [Google Scholar] [CrossRef]
- Shafqat, A.; Arabi, T.Z.; Sabbah, B.N.; Abdulkader, H.S.; Shafqat, S.; Razak, A.; Kashir, J.; Alkattan, K.; Yaqinuddin, A. Understanding COVID-19 Vaccines Today: Are T-cells Key Players? Vaccines 2022, 10, 904. [Google Scholar] [CrossRef]
- Reischig, T.; Kacer, M.; Vlas, T.; Drenko, P.; Kielberger, L.; Machova, J.; Topolcan, O.; Kucera, R.; Kormunda, S. Insufficient response to mRNA SARS-CoV-2 vaccine and high incidence of severe COVID-19 in kidney transplant recipients during pandemic. Am. J. Transplant. 2022, 22, 801–812. [Google Scholar] [CrossRef]
- Sattler, A.; Schrezenmeier, E.; Weber, U.A.; Potekhin, A.; Bachmann, F.; Straub-Hohenbleicher, H.; Budde, K.; Storz, E.; Pross, V.; Bergmann, Y.; et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J. Clin. Investig. 2021, 131, e150175. [Google Scholar] [CrossRef]
- Kantauskaite, M.; Muller, L.; Kolb, T.; Fischer, S.; Hillebrandt, J.; Ivens, K.; Andree, M.; Luedde, T.; Orth, H.M.; Adams, O.; et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am. J. Transplant. 2022, 22, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Lai, Q.; Poli, L.; Perrone, M.P.; Gaeta, A.; Rossi, M.; Mastroianni, C.M.; Garofalo, M.; Pretagostini, R. SARS-CoV-2 vaccination with BNT162B2 in renal transplant patients: Risk factors for impaired response and immunological implications. Clin. Transplant. 2022, 36, e14495. [Google Scholar] [CrossRef]
- Chavarot, N.; Morel, A.; Leruez-Ville, M.; Vilain, E.; Divard, G.; Burger, C.; Serris, A.; Sberro-Soussan, R.; Martinez, F.; Amrouche, L.; et al. Weak antibody response to three doses of mRNA vaccine in kidney transplant recipients treated with belatacept. Am. J. Transplant. 2021, 21, 4043–4051. [Google Scholar] [CrossRef]
- Chavarot, N.; Ouedrani, A.; Marion, O.; Leruez-Ville, M.; Vilain, E.; Baaziz, M.; Del Bello, A.; Burger, C.; Sberro-Soussan, R.; Martinez, F.; et al. Poor Anti-SARS-CoV-2 Humoral and T-cell Responses After 2 Injections of mRNA Vaccine in Kidney Transplant Recipients Treated With Belatacept. Transplantation 2021, 105, e94–e95. [Google Scholar] [CrossRef]
- Osmanodja, B.; Ronicke, S.; Budde, K.; Jens, A.; Hammett, C.; Koch, N.; Seelow, E.; Waiser, J.; Zukunft, B.; Bachmann, F.; et al. Serological Response to Three, Four and Five Doses of SARS-CoV-2 Vaccine in Kidney Transplant Recipients. J. Clin. Med. 2022, 11, 2565. [Google Scholar] [CrossRef]
- Regele, F.; Heinzel, A.; Hu, K.; Raab, L.; Eskandary, F.; Fae, I.; Zelzer, S.; Bohmig, G.A.; Bond, G.; Fischer, G.; et al. Stopping of Mycophenolic Acid in Kidney Transplant Recipients for 2 Weeks Peri-Vaccination Does Not Increase Response to SARS-CoV-2 Vaccination-A Non-randomized, Controlled Pilot Study. Front. Med. 2022, 9, 914424. [Google Scholar] [CrossRef]
- Masset, C.; Ville, S.; Garandeau, C.; Le Borgne, F.; Letellier, T.; Cantarovich, D.; Meurette, A.; Guillot-Gueguen, C.; Bentoumi-Loaec, M.; Giral, M.; et al. Observations on improving COVID-19 vaccination responses in kidney transplant recipients: Heterologous vaccination and immunosuppression modulation. Kidney Int. 2022, 101, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Ducloux, D.; Courivaud, C. REGEN-Cov antibody combination to prevent COVID-19 infection in kidney transplant recipient without detectable antibody response to optimal vaccine scheme. Kidney Int. 2022, 101, 645–646. [Google Scholar] [CrossRef] [PubMed]
| No. of Vaccinated (n = 16,820) | Country | Vaccine Type | No. of Infections (n = 633) | No. of Dead (n = 75) | BI Rate (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| BNT162b2 | mRNA-1273 | Jansen | CoronaVac | ChAdOx1 | Other | |||||
| 1402 [18] | Canada | 1048 | 253 | 48 | 53 | 3 | 1 | 0.21 | ||
| 341 [19] | USA | 341 (mRNA vaccines > 95%) | 1 | 0 | 0.30 | |||||
| 1680 [20] | USA | 1680 | 8 | 0 | 0.48 | |||||
| 904 [21] | USA | 658 | 229 | 17 | 7 | 1 | 0.8 | |||
| 843 [12] | Spain | 843 | 15 | 1 | 1.78 | |||||
| 380 [22] | USA | 380 | 7 | 0 | 1.8 | |||||
| 1509 [23] | Czech Rep. | 1274 | 235 | 33 | 8 | 2.18 | ||||
| 800 [24] | Spain | 800 | 21 | 1 | 3.0 | |||||
| 372 [25] | Greece | 372 | 13 | 1 | 3.49 | |||||
| 3340 [26] | Brazil | 3340 | 135 | 30 | 4.04 | |||||
| 1034 [27] | Spain | 7 | 1027 | 44 | 4 | 4.26 | ||||
| 164 [28] | Croatia | 164 | 8 | 0 | 4.88 | |||||
| 3201 [4] | Scotland | 962 | 2239 | 259 | 26 | 8.09 | ||||
| 850 [29] | Denmark | 850 | 79 | 2 | 9.29 | |||||
| Study | Case (n) | Country | Age (Y) | Sex (M/F) | Years Since KT | Maintenance IS | No. of Hospilization | Vaccine Type | No. of Dose | Days from Last Dose to COVID-19 | No. of Deaths |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Basic [30] | 1 | Croatia | 46 | 1/0 | 14 | TAC/MMF/Steroid | 1 | BNT162b2 | 2 | 14 | 0 |
| Wadei [31] | 1 | USA | 60 | 1/0 | 1.3 | TAC/MMF/Steroid | 1 | mRNA-1273 | 2 | 44 | 0 |
| Tsapepas [21] | 7 | USA | 29–68 | 4/3 | 1.5–18 | TAC/MPA/Steroid (2/7) TAC/MPA (4/7) S/MPA (1/7) | 2 | mRNA-1273 (2/7) BNT162b2 (5/7) | 2 | 17–46 | 1 |
| Tsalouchos [32] | 1 | Italy | 49 | 0/1 | NA | TAC/MMF/Steroid | NA | BNT162b2 | 2 | 23 | 0 |
| Tau [5] | 14 | Israel | 26–85 | 10/4 | 0.6–21 | TAC/MMF/Steroid (11/14) | 8 | BNT162b2 | 2 | 25–85 | 4 |
| TAC/Steroid (2/14) | |||||||||||
| S/MMF/Steroid (1/14) | |||||||||||
| Wijtvliet [33] | 1 | Belgium | 23 | 0/1 | 1.4 | S/MMF/Steroid | 1 | BNT162b2 | 2 | 41 | 0 |
| Malinis [34] | 2 | USA | 65–80 | 0/1 | 2.2–6.3 | Bela/MMF/Steroid (1/2) | 0 | mRNA-1273 (1/2) | 2 | 18–63 | 0 |
| Bela (1/2) | BNT162b2 (1/2) | ||||||||||
| Chenxi [22] | 7 | USA | 49–77 | 5/2 | 1.8–6.2 | TAC/MMF/Steroid (6/7) | 4 | mRNA-1273 (2/7) | 2 | 16–75 | 0 |
| TAC/Steroid (1/7) | BNT162b2 (5/7) | ||||||||||
| Loconsole [35] | 1 | Italy | 48 | 0/1 | 6.0 | TAC/MMF/Steroid | 1 | BNT162b2 | 2 | 71 | 0 |
| Aslam [19] | 1 | USA | 67 | 1/0 | 6 | CsA/Steroid | 1 | BNT162b2 | 2 | 72 | 0 |
| Chang [36] | 1 | USA | 73 | 1/0 | 1.3 | TAC/MMF/Steroid | 1 | mRNA-1273 | 2 | 107 | 0 |
| Mehta [20] | 8 | USA | 36–73 | 5/3 | 0.3–20.7 | TAC/MMF/Steroid (4/8) TAC/Steroid (1/8) TAC/MMF (1/8) TAC/AZA (1/8) CsA/MMF (1/8) | I3 | mRNA-1273 (4/8) BNT162b2 (4/8) | 2 | 20–77 | 0 # |
| Meshram [37] | 1 | India | 71 | 1/0 | 16 | CsA/MPA/Steroid | 1 | ChAdOx1 | 2 | 20 | 1 |
| Ali [6] | 8 | USA | 27–72 | NA | 0.2–4.4 | TAC/MMF/Steroid (6/8) | 3 | mRNA-1273 (2/8) | 2 | 18–45 | 0 |
| TAC/MPA/Steroid (1/8) | BNT162b2 (5/8) | ||||||||||
| Bela/MPA/Steroid (1/8) | Jansen (1/8) | ||||||||||
| Anjan [38] | 12 | USA | 32–81 | 6/6 | NA | TAC/MMF/Steroid (5/12) | 5 | BNT162b2 (12/12) | 2 | 15–96 | 0 |
| TAC/MMF (5/12) | |||||||||||
| TAC/Bela/Steroid (1/12) | |||||||||||
| TAC/Bela (1/12) | |||||||||||
| Chen [39] | 1 | USA | 57 | 0/1 | 23 | MMF/Steroid | 1 | mRNA-1273 | 2 | 42 | 0 |
| Almaghrabi [40] | 3 | Saudi Arabia | 42–69 | 2/1 | 5.0–27.0 | TAC/MMF/Steroid (2/3) | 3 | BNT162b2 (2/3) | 2 | 21–150 | 1 |
| S (1/3) | ChAdOx1 (1/3) | ||||||||||
| Marinaki [25] | 13 | Greece | 21–72 | 11/2 | 1.2–15.7 | TAC/MMF/Steroid (11/13) | 7 | mRNA-1273 (3/13) | 2 | 57–115 | 1 |
| CsA/MMF/Steroid (2/13) | BNT162b2 (10/13) | ||||||||||
| Radcliffe [41] | 4 | USA | 43–73 | 4/0 | 1.7–11.6 | TAC/MMF/Steroid (4/4) | 1 | mRNA-1273 (1/4) | 3 | 43–73 | 0 |
| BNT162b2 (3/4) | |||||||||||
| Chung [42] | 7 | Singapore | 34–69 | 3/4 | 1.8–31 | Aza/CsA/Steroid (2/7) | 7 | BNT162b2 | 2 | 35–175 | 0 |
| TAC/MMF(MPA)/Steroid (2/7) | |||||||||||
| CsA/MMF/Steroid (1/7) | |||||||||||
| TAC/Steroid (1/7) | |||||||||||
| EVR/Steroid (1/7) | |||||||||||
| Natori [43] | 1 | USA | 66 | 0/1 | 10 | TAC/MMF | 1 | BNT162b2 | 3 | 90 | 0 |
| Benotmane [44] | 39 | France | 19–79 | 23/16 | 0.1–32.9 | TAC/MMF(MPA)/Steroid (23/39) MMF(MPA)/Bela/Steroid (8/39) CsA/MMF(MPA) (2/39) CsA/MMF(MPA)/Steroid (2/39) TAC/MMF(MPA) (2/39) TAC/Steroid (1/39) CsA/Steroid (1/39) | 6 | mRNA-1273 (22/39) BNT162b2 (15/39) mRNA-1273 & BNT162b2 (2/39) | 2 (6/39) 3 (27/39) 4 (6/39) | 49–351 | 2 |
| Fahim [45] | 1 | USA | 45 | 0/1 | 2.2 | TAC/MMF/Steroid | 1 | mRNA-1273 | 2 | 120 | 0 |
| Variables | All (n = 135) | Booster Vaccination (n = 38) | Standard Vaccination (n = 97) | p |
|---|---|---|---|---|
| Males, n (%) a | 78 (61.4) | 23 (60.5) | 55 (61.8) | 0.89 |
| Age (y), median [IQR] | 59.0 [49.0, 69.0] | 60.5 [51.0, 72.0] | 58.0 [48.0, 68.0] | 0.32 |
| Years since KT (y), median [IQR] b | 3.9 [1.7, 8.6] | 3.5 [1.5, 7.7] | 4.0 [2.0, 9.4] | 0.54 |
| Maintenance IS at diagnosis of COVID-19 | ||||
| Tacrolimus, n (%) | 105 (77.8) | 29 (76.3) | 76 (78.4) | 0.80 |
| MMF/MPA, n (%) | 112 (83.0) | 37 (97.4) | 75 (77.3) | <0.01 |
| Steroid, n (%) | 116 (85.9) | 35 (92.1) | 81 (83.5) | 0.20 |
| Belacept, n (%) | 13 (9.6) | 6 (15.8) | 7 (7.2) | 0.19 |
| CNIs, n (%) | 117 (86.7) | 32 (84.2) | 85 (87.6) | 0.60 |
| Triple maintenance IS, n (%) | 98 (72.6) | 34 (89.5) | 64 (66.0) | <0.01 |
| Days from last dose to COVID-19 (d), median [IQR] c | 74.5 [37.0, 164.8] | 218.0 [149.3, 267.0] | 53.0 [35.0, 91.0] | <0.01 |
| Hospitalized, n (%) d | 67 (50.0) | 13 (34.2) | 54 (56.3) | 0.02 |
| Dead, n (%) | 10 (7.5) | 2 (5.3) | 8 (8.3) | 0.72 |
| Variables | BI-Related Deaths (n = 10) | COVID-19 Survivors (n = 125) | p |
|---|---|---|---|
| Males, n (%) a | 8 (80.0) | 70 (59.8) | 0.32 |
| Age (y), median [IQR] | 69.5 [64.3, 71.3] | 57.5 [48.0, 68.0] | 0.02 |
| Age ≥ 60 y, n (%) | 9 (90.0) | 57 (45.6) | <0.01 |
| Years since KT (y), median [IQR] b | 7.2 [2.8, 13.8] | 3.9 [1.6, 7.8] | 0.26 |
| Maintenance IS at diagnosis of COVID-19 | |||
| Tacrolimus, n (%) | 6 (60.0) | 99 (79.2) | 0.23 |
| Mycophenolate, n (%) | 9 (90.0) | 103 (82.4) | 1.00 |
| Steroid, n (%) | 10 (100.0) | 106 (84.8) | 0.36 |
| Belacept, n (%) | 2 (20.0) | 11 (8.8) | 0.25 |
| CNIs, n (%) | 7 (70.0) | 110 (88.0) | 0.13 |
| Triple maintenance IS, n (%) | 9 (90.0) | 89 (71.2) | 0.28 |
| Days from last dose to COVID-19 (d), median [IQR] c | 69.0 [24.0, 152.5] | 75.0 [39.0, 175.0] | 0.49 |
| Booster vaccination, n (%) | 2 (20.0) | 36 (28.8) | 0.73 |
| Variables | Inpatients (n = 67) | Outpatients (n = 67) | p |
|---|---|---|---|
| Males, n (%) a | 39 (65.0) | 34 (57.6) | 0.41 |
| Age (y), median [IQR] | 65.0 [53.0, 71.0] | 53.0 [43.0, 63.0] | |
| Age ≥ 60 y, n (%) | 44 (65.7) | 22 (32.8) | <0.01 |
| Years since KT (y), median [IQR] b | 3.8 [1.6, 9.0] | 4.0 [1.7, 8.2] | 0.67 |
| Booster vaccination, n (%) | 13 (19.4) | 25 (37.3) | 0.02 |
| Maintenance IS at diagnosis of COVID-19 | |||
| Tacrolimus, n (%) | 48 (71.6) | 56 (83.6) | 0.10 |
| MMF/MPA, n (%) | 52 (77.6) | 59 (88.1) | 0.11 |
| Steroid, n (%) | 59 (88.1) | 56 (83.6) | 0.46 |
| Belacept, n (%) | 5 (7.5) | 8 (11.9) | 0.38 |
| CNIs, n (%) | 58 (86.6) | 58 (86.6) | 1.00 |
| Triple maintenance IS, n (%) | 48 (71.6) | 49 (73.1) | 0.85 |
| Days from last dose to COVID-19 (d), median [IQR] c | 74.5 [35.0, 150.5] | 77.0 [40.0, 222.0] | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Weng, R.; Liu, F.; Xie, Y.; Jin, Y.; Li, Q.; Huang, G.; Chen, J.; Wang, J.; Shen, H.; et al. COVID-19 Breakthrough Infections in Vaccinated Kidney Transplant Recipients. Vaccines 2022, 10, 1911. https://doi.org/10.3390/vaccines10111911
Zhang X, Weng R, Liu F, Xie Y, Jin Y, Li Q, Huang G, Chen J, Wang J, Shen H, et al. COVID-19 Breakthrough Infections in Vaccinated Kidney Transplant Recipients. Vaccines. 2022; 10(11):1911. https://doi.org/10.3390/vaccines10111911
Chicago/Turabian StyleZhang, Xiaojing, Ruopeng Weng, Fei Liu, Yi Xie, Yanyan Jin, Qiuyu Li, Guoping Huang, Junyi Chen, Jingjing Wang, Huijun Shen, and et al. 2022. "COVID-19 Breakthrough Infections in Vaccinated Kidney Transplant Recipients" Vaccines 10, no. 11: 1911. https://doi.org/10.3390/vaccines10111911
APA StyleZhang, X., Weng, R., Liu, F., Xie, Y., Jin, Y., Li, Q., Huang, G., Chen, J., Wang, J., Shen, H., Fu, H., & Mao, J. (2022). COVID-19 Breakthrough Infections in Vaccinated Kidney Transplant Recipients. Vaccines, 10(11), 1911. https://doi.org/10.3390/vaccines10111911







