Identifying Modifiable Predictors of COVID-19 Vaccine Side Effects: A Machine Learning Approach
Abstract
:1. Introduction
2. Materials and Methods
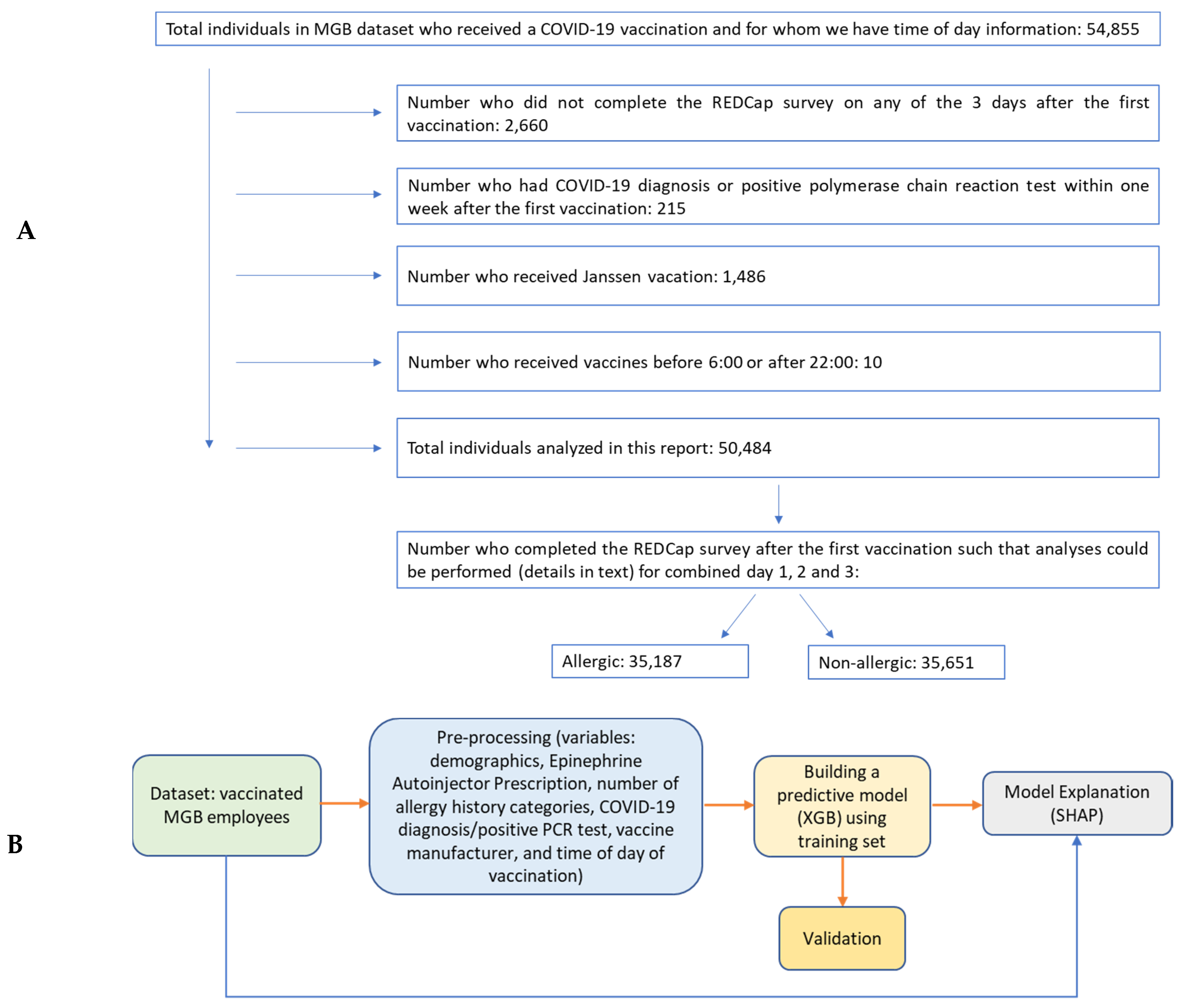
2.1. Data
- Allergic symptoms: (Yes/No) (i) Rash or itching; (ii) Hives; (iii) Swollen lips, tongue, eyes, or face; (iv) Respiratory symptoms (wheezing, chest tightness, or shortness of breath).
- Non-allergic symptoms: (None/lower severity/higher severity) (i) New headache; (ii) New fatigue; (iii) Joint pain; (iv) Muscle pain; (v) Fever.
2.2. Pre-Processing
2.3. Machine Learning Model
2.4. Evaluation
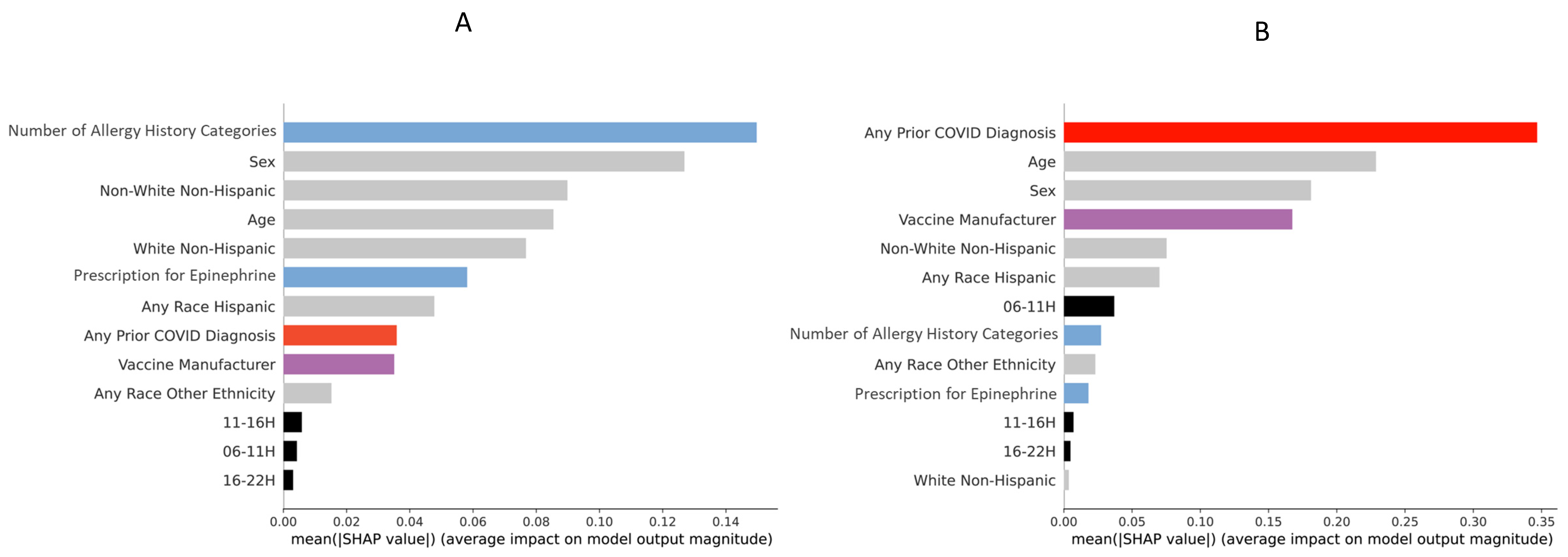
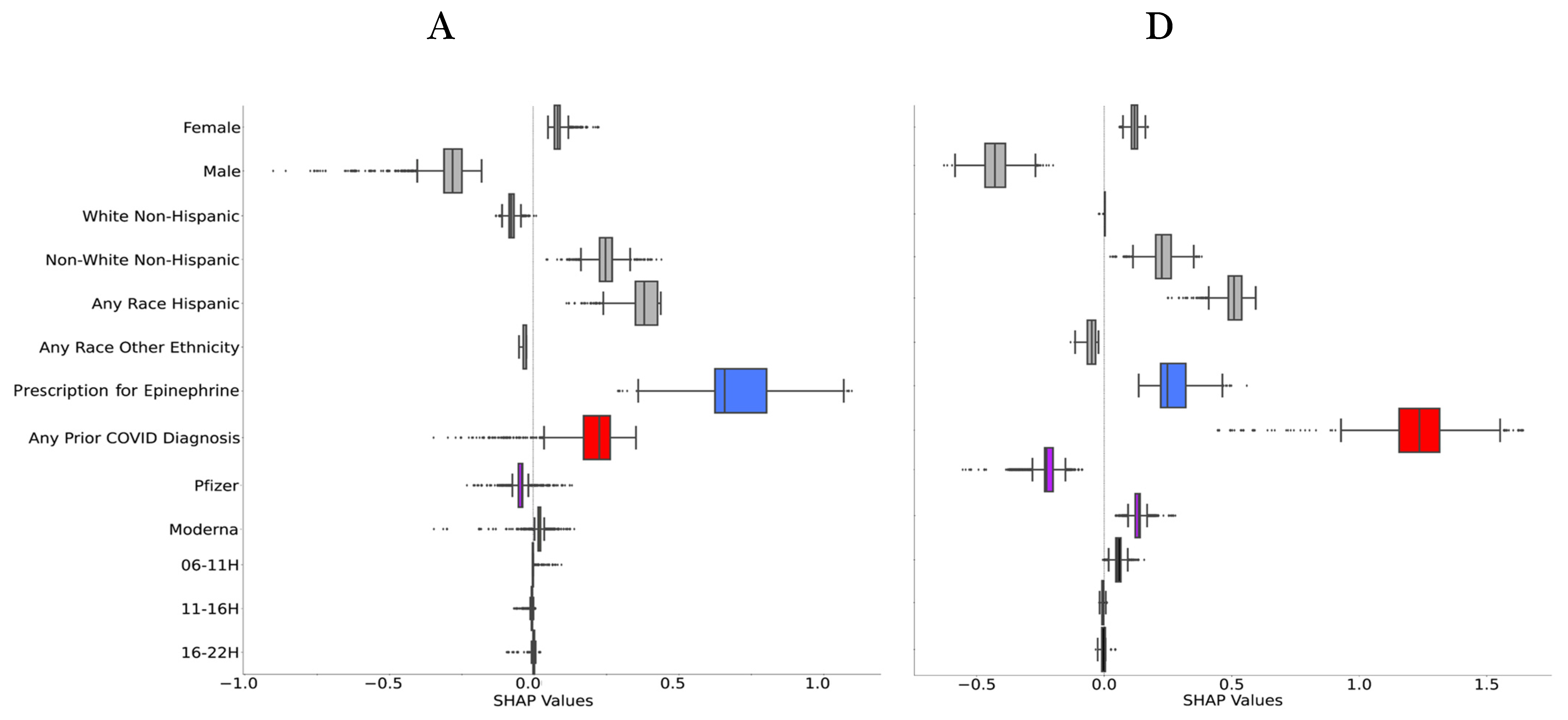
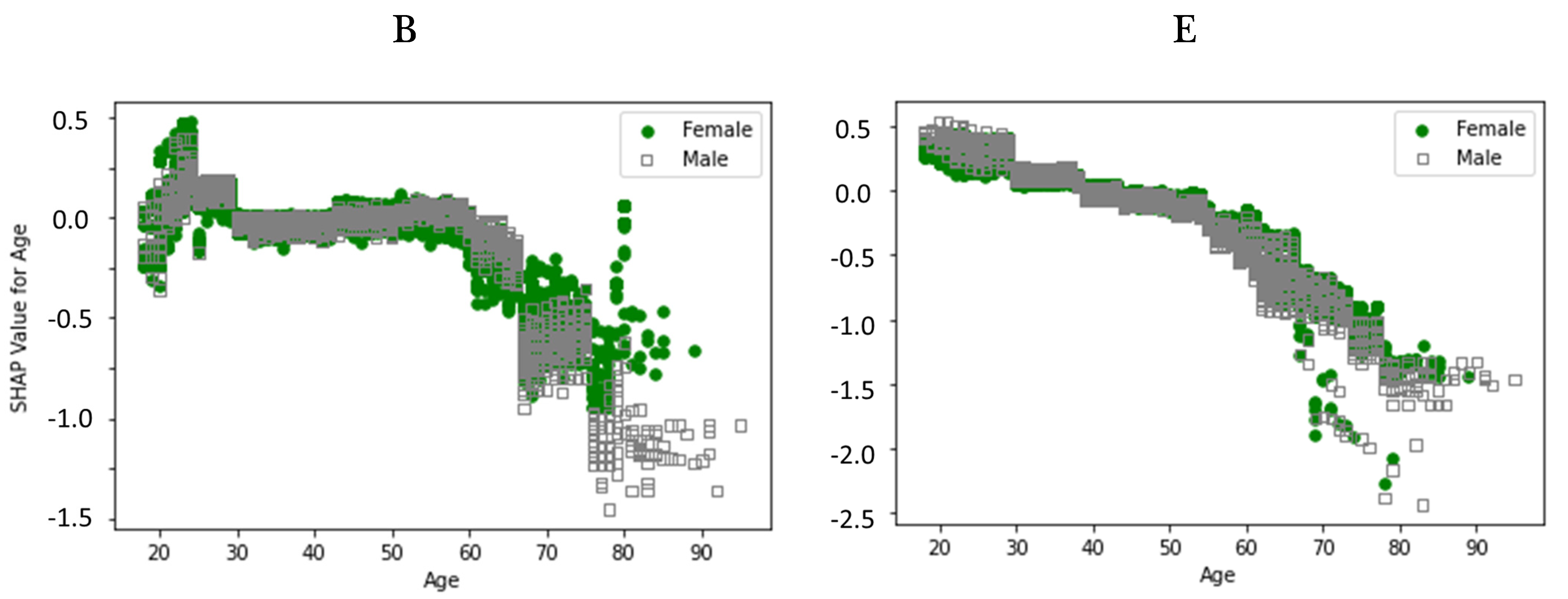
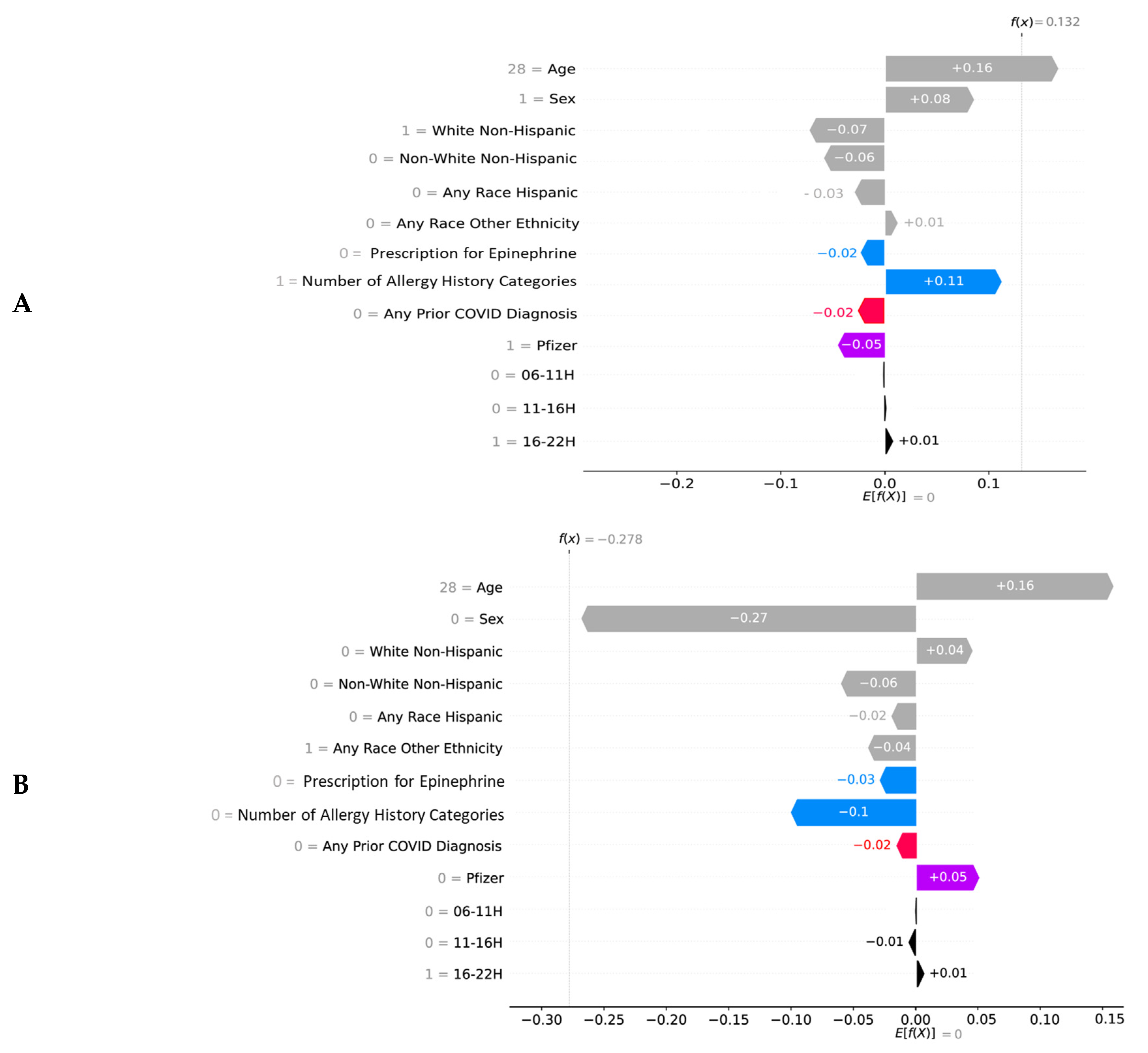
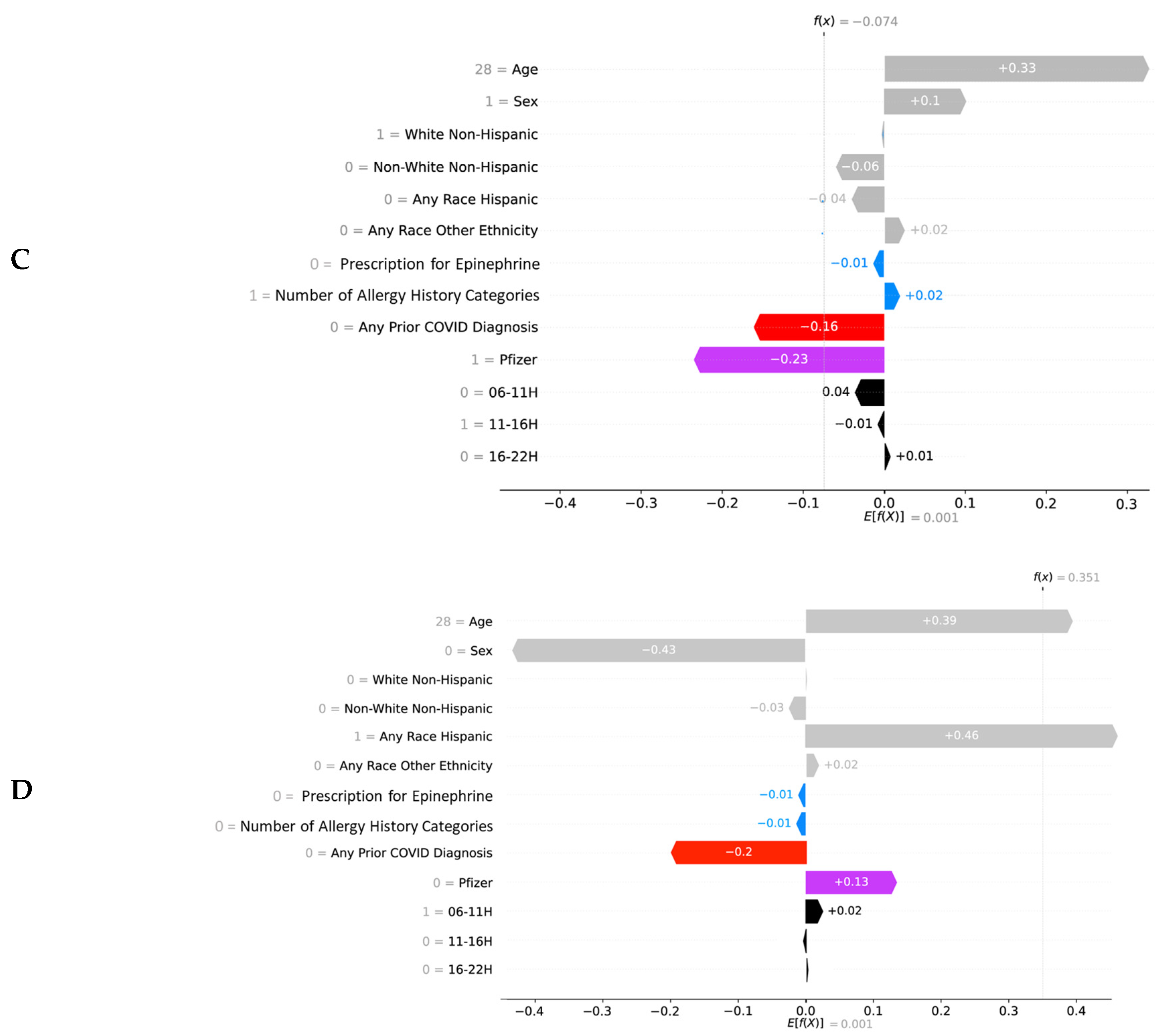
2.5. Explainability
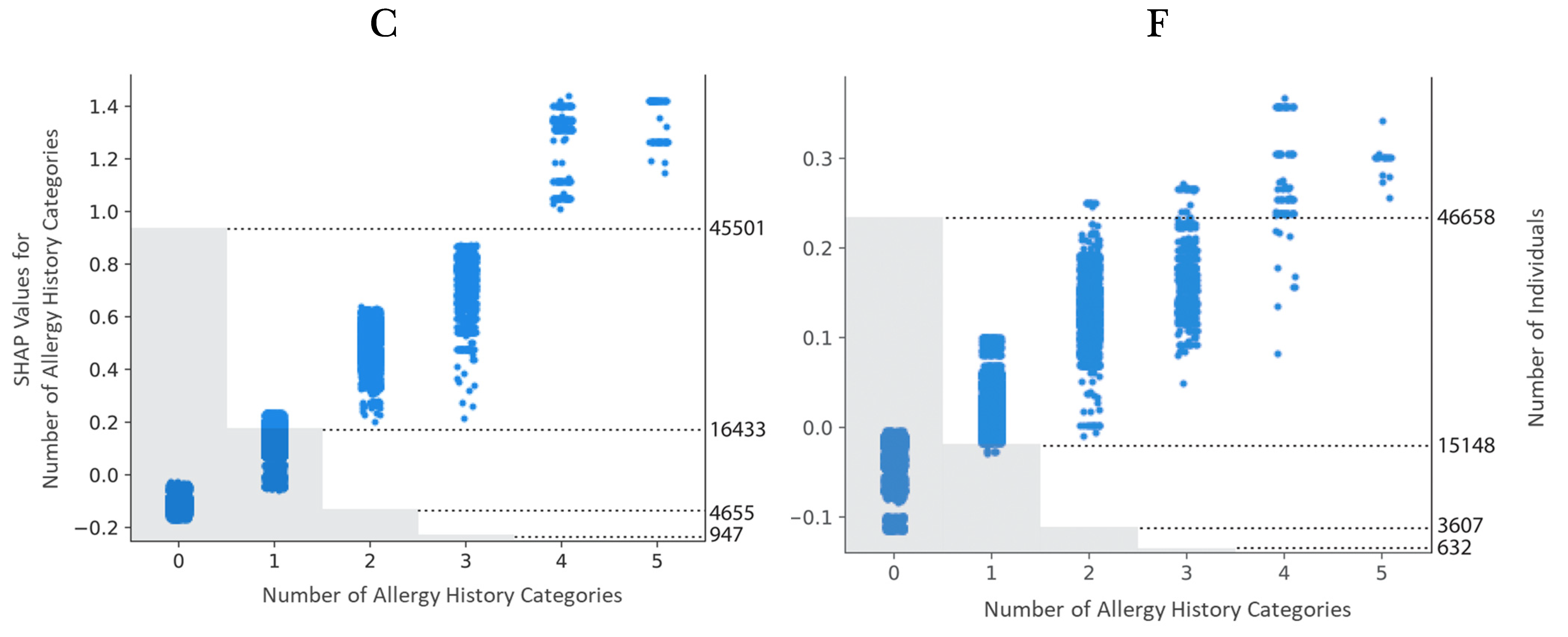
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC COVID-19 Vaccines Work. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/work.html (accessed on 1 August 2022).
- Coronavirus (COVID-19) Vaccinations. 2022. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 1 August 2022).
- Azarpanah, H.; Farhadloo, M.; Vahidov, R.; Pilote, L. Vaccine hesitancy: Evidence from an adverse events following immunization database, and the role of cognitive biases. BMC Public Health 2021, 21, 1686. [Google Scholar] [CrossRef]
- Biswas, N.; Mustapha, T.; Khubchandani, J.; Price, J.H. The nature and extent of COVID-19 vaccination hesitancy in healthcare workers. J. Community Health 2021, 46, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, E.S.; Wickner, P.G.; West, L.R.; Banerji, A.; Blumenthal, K.G.; Centi, A.J.; Gottlieb, A.; Hashimoto, D.M.; Kim, E.; Kim, M.; et al. Symptom monitoring after coronavirus disease 2019 (COVID-19) vaccination in a large integrated healthcare system: Separating symptoms from severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection. Infect. Control Hosp. Epidemiol. 2021, 1–8. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Robinson, L.B.; Camargo, C.A.; Shenoy, E.S.; Banerji, A.; Landman, A.B.; Wickner, P. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA 2021, 325, 1562–1565. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Freeman, E.E.; Saff, R.R.; Robinson, L.B.; Wolfson, A.R.; Foreman, R.K.; Hashimoto, D.; Banerji, A.; Li, L.; Anvari, S.; et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N. Engl. J. Med. 2021, 384, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.B.; Fu, X.; Hashimoto, D.; Wickner, P.; Shenoy, E.S.; Landman, A.B.; Blumenthal, K.G. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. JAMA Dermatol. 2021, 157, 1000–1002. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.B.; Landman, A.B.; Shenoy, E.S.; Hashimoto, D.; Fu, X.; Camargo, C.A.; Wickner, P.; Blumenthal, K.G. Allergic symptoms after mRNA COVID-19 vaccination and risk of incomplete vaccination. J. Allergy Clin. Immunol. Pract. 2021, 9, 3200–3202. [Google Scholar]
- Li, L.; Robinson, L.B.; Patel, R.; Landman, A.B.; Fu, X.; Shenoy, E.S.; Hashimoto, D.M.; Banerji, A.; Wickner, P.G.; Samarakoon, U.; et al. Association of Self-reported High-Risk Allergy History with Allergy Symptoms After COVID-19 Vaccination. JAMA Netw. Open 2021, 4, e2131034. [Google Scholar]
- Shavit, R.; Maoz-Segal, R.; Iancovici-Kidon, M.; Offengenden, I.; Yahia, S.H.; Maayan, D.M.; Lifshitz-Tunitsky, Y.; Niznik, S.; Frizinsky, S.; Deutch, M.; et al. Prevalence of allergic reactions after Pfizer-BioNTech COVID-19 vaccination among adults with high allergy risk. JAMA Netw. Open 2021, 4, e2122255. [Google Scholar]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Gajula, V.; Madathala, R.R.; Chennaiahgari, N.; Malayala, S.V. Non-life-threatening adverse effects with COVID-19 mRNA-1273 vaccine: A randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J. Med. Virol. 2021, 93, 4420–4429. [Google Scholar]
- Gonzalez-Dias, P.; Lee, E.K.; Sorgi, S.; de Lima, D.S.; Urbanski, A.H.; Silveira, E.L.; Nakaya, H.I. Methods for predicting vaccine immunogenicity and reactogenicity. Hum. Vaccines Immunother. 2020, 16, 269–276. [Google Scholar] [CrossRef]
- Pondo, T.; Rose, C.E.; Martin, S.W.; Keitel, W.A.; Keyserling, H.L.; Babcock, J.; Parker, S.; Jacobson, R.M.; Poland, G.A.; McNeil, M.M. Evaluation of sex, race, body mass index and pre-vaccination serum progesterone levels and post-vaccination serum anti-anthrax protective immunoglobulin G on injection site adverse events following anthrax vaccine adsorbed (AVA) in the CDC AVA human clinical trial. Vaccine 2014, 32, 3548–3554. [Google Scholar] [PubMed] [Green Version]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- Allada, R.; Bass, J. Circadian mechanisms in medicine. N. Engl. J. Med. 2021, 384, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Long, J.E.; Drayson, M.T.; Taylor, A.E.; Toellner, K.M.; Lord, J.M.; Phillips, A.C. Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine 2016, 34, 2679–2685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Balfe, P.; Eyre, D.W.; Lumley, S.F.; O’Donnell, D.; Warren, F.; Crook, D.W.; Jeffery, K.; Matthews, P.C.; Klerman, E.B.; et al. Time of day of vaccination affects SARS-CoV-2 antibody responses in an observational study of health care workers. J. Biol. Rhythms 2022, 37, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Langlois, P.; White, R.; Glezen, W. Diurnal variation in human response to influenza vaccination? A pilot study of 125 volunteers. Ann. Rev. Chronopharmacol. 1986, 3, 123. [Google Scholar]
- Langlois, P.H.; Smolensky, M.H.; Glezen, W.P.; Keitel, W.A. Diurnal variation in responses to influenza vaccine. Chronobiol. Int. 1995, 12, 28–36. [Google Scholar] [CrossRef]
- Moncada-Torres, A.; van Maaren, M.C.; Hendriks, M.P.; Siesling, S.; Geleijnse, G. Explainable machine learning can outperform Cox regression predictions and provide insights in breast cancer survival. Sci Rep. 2021, 11, 6968. [Google Scholar] [CrossRef]
- Hu, L.; Liu, B.; Ji, J.; Li, Y. Tree-Based Machine Learning to Identify and Understand Major Determinants for Stroke at the Neighborhood Level. J. Am. Heart Assoc. 2020, 9, e016745. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Nair, B.; Vavilala, M.S.; Horibe, M.; Eisses, M.J.; Adams, T.; Liston, D.E.; Low, D.K.-W.; Newman, S.-F.; Kim, J.; et al. Explainable machine-learning predictions for the prevention of hypoxaemia during surgery. Nat. Biomed. Eng. 2018, 2, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. methodology and workflow process for providing translational research informatics support. J. Biomed. Informat. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A., Jr.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: Current evidence and suggested approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Geurkink, Y.; Boone, J.; Verstockt, S.; Bourgois, J.G. Machine Learning-Based Identification of the Strongest Predictive Variables of Winning and Losing in Belgian Professional Soccer. Appl. Sci. 2021, 11, 2378. [Google Scholar] [CrossRef]
- Chan, T.M.; Li, Y.; Chiau, C.C.; Zhu, J.; Jiang, J.; Huo, Y. Imbalanced target prediction with pattern discovery on clinical data repositories. BMC Med. Inform. Decis. Mak. 2017, 17, 47. [Google Scholar] [CrossRef]
- Circadian Rhythms. Available online: https://nigms.nih.gov/education/fact-sheets/Pages/circadian-rhythms.aspx (accessed on 1 July 2022).
- Ruben, M.D.; Hogenesch, J.B.; Smith, D.F. Sleep and circadian medicine: Time of day in the neurologic clinic. Neurol. Clin. 2019, 37, 615–629. [Google Scholar] [CrossRef]
- Ruben, M.D.; Smith, D.F.; FitzGerald, G.A.; Hogenesch, J.B. Dosing time matters. Science 2019, 365, 547–549. [Google Scholar] [CrossRef]
- Caruana, R.; Lou, Y.; Gehrke, J.; Koch, P.; Sturm, M.; Elhadad, N. Intelligible models for healthcare: Predicting pneumonia risk and hospital 30-day readmission. In Proceedings of the 21th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, Sydney, Australia, 10–13 August 2015; pp. 1721–1730. [Google Scholar]
- Wiens, J.; Shenoy, E.S. Machine learning for healthcare: On the verge of a major shift in healthcare epidemiology. Clin. Infect. Dis. 2018, 66, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Spiegel, K.; Sheridan, J.F.; Van Cauter, E. Effect of sleep deprivation on response to immunizaton. JAMA 2002, 288, 1471–1472. [Google Scholar] [CrossRef]
- Lange, T.; Dimitrov, S.; Bollinger, T.; Diekelmann, S.; Born, J. Sleep after vaccination boosts immunological memory. J. Immunol. 2011, 187, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Prather, A.A.; Hall, M.; Fury, J.M.; Ross, D.C.; Muldoon, M.F.; Cohen, S.; Marsland, A.L. Sleep and antibody response to hepatitis B vaccination. Sleep 2012, 35, 1063–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, T.; Perras, B.; Fehm, H.L.; Born, J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom. Med. 2003, 65, 831–835. [Google Scholar] [CrossRef] [PubMed]
| Variables | N | (%) |
|---|---|---|
| Age in Years | ||
| Age Group 1 (18–40) | 25,213 | 50 |
| Age Group 2 (41–60) | 18,529 | 37 |
| Age Group 3 (61–95) | 6742 | 13 |
| Total | 50,484 | 100 |
| Sex | ||
| Female | 36,801 | 73 |
| Male | 13,683 | 27 |
| Total | 50,484 | 100 |
| Race/Ethnicity | ||
| White/Non-Hispanic | 28,408 | 56 |
| Non-White/Non-Hispanic | 8066 | 16 |
| Any Race/Hispanic | 2662 | 5 |
| Any Race/Other Ethnicity | 11,348 | 23 |
| Total | 50,484 | 100 |
| Prescription History | ||
| Epinephrine Autoinjector Prescription | 1246 | 2 |
| Allergy History | ||
| Any History of Allergy | 14,197 | 28 |
| COVID-19 Diagnosis/Positive PCR Test | ||
| Any Before Vaccination 1 | 3797 | 8 |
| Vaccine Manufacturer | ||
| Pfizer | 20,324 | 40 |
| Moderna | 30,160 | 60 |
| Total | 50,484 | 100 |
| Clock Time of Vaccine Administration/Appointment | ||
| Time 1 (6:00–10:59) | 17,254 | 34 |
| Time 2 (11:00–15:59) | 22,367 | 44 |
| Time 3 (16:00–21:59) | 10,863 | 22 |
| Total | 50,484 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbaspour, S.; Robbins, G.K.; Blumenthal, K.G.; Hashimoto, D.; Hopcia, K.; Mukerji, S.S.; Shenoy, E.S.; Wang, W.; Klerman, E.B. Identifying Modifiable Predictors of COVID-19 Vaccine Side Effects: A Machine Learning Approach. Vaccines 2022, 10, 1747. https://doi.org/10.3390/vaccines10101747
Abbaspour S, Robbins GK, Blumenthal KG, Hashimoto D, Hopcia K, Mukerji SS, Shenoy ES, Wang W, Klerman EB. Identifying Modifiable Predictors of COVID-19 Vaccine Side Effects: A Machine Learning Approach. Vaccines. 2022; 10(10):1747. https://doi.org/10.3390/vaccines10101747
Chicago/Turabian StyleAbbaspour, Sara, Gregory K. Robbins, Kimberly G. Blumenthal, Dean Hashimoto, Karen Hopcia, Shibani S. Mukerji, Erica S. Shenoy, Wei Wang, and Elizabeth B. Klerman. 2022. "Identifying Modifiable Predictors of COVID-19 Vaccine Side Effects: A Machine Learning Approach" Vaccines 10, no. 10: 1747. https://doi.org/10.3390/vaccines10101747
APA StyleAbbaspour, S., Robbins, G. K., Blumenthal, K. G., Hashimoto, D., Hopcia, K., Mukerji, S. S., Shenoy, E. S., Wang, W., & Klerman, E. B. (2022). Identifying Modifiable Predictors of COVID-19 Vaccine Side Effects: A Machine Learning Approach. Vaccines, 10(10), 1747. https://doi.org/10.3390/vaccines10101747






