Glucomannan as a Dietary Supplement for Treatment of Breast Cancer in a Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Propagation
2.2. Experimental Mice
2.3. Tumor Stock Preparation
2.4. Tumor Antigen Lysate Preparation
2.5. Vaccine Mixture with Glucomannan
2.6. Tumor Transplantation to the Experimental Mice
2.7. Experimental Groups and Immunization
2.8. IFN-γ, TNF-α, IL-2, IL-4, and IL-17 Cytokine Assay
2.9. CTL Activity
2.10. Tumor Growth Measurement
2.11. Real-Time PCR Analysis of FOXP-3 and TGF-β Gene Expression in the Breast Tumor Microenvironment
2.11.1. RNA Extraction from Breast Tumors
2.11.2. Synthesis of cDNA
2.11.3. Real-Time PCR Analysis
2.12. Statistical Analysis
3. Results
3.1. TH1 Cytokine Response
3.2. TH2, TH17, and CTL Cytokine Response
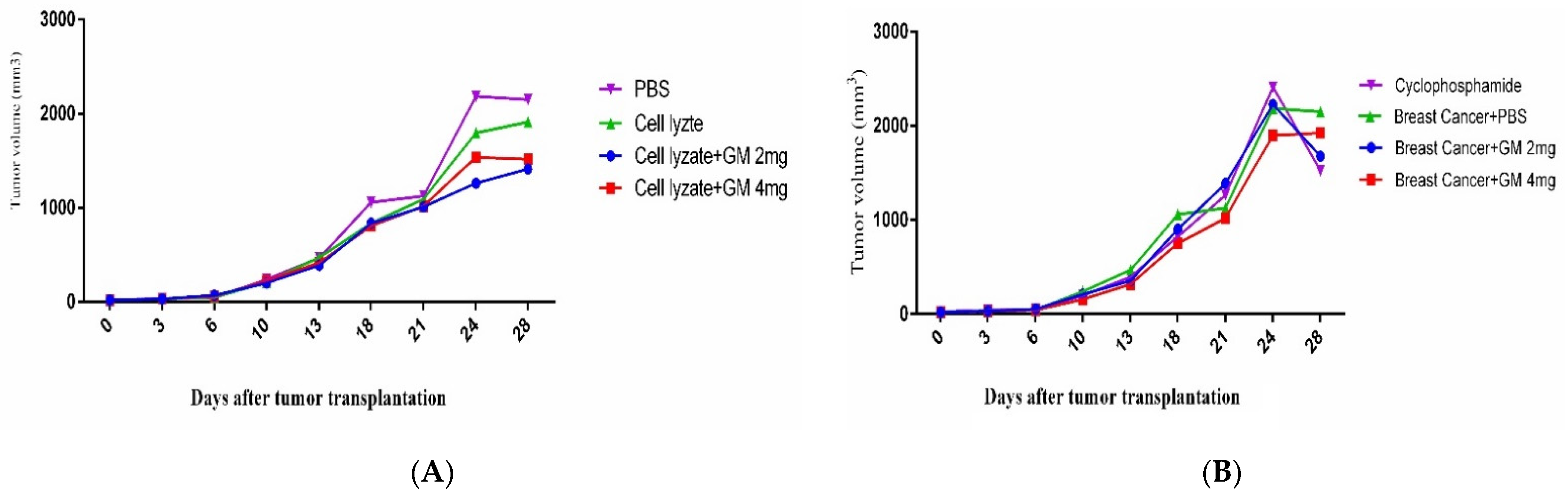
3.3. Tumor Growth Change
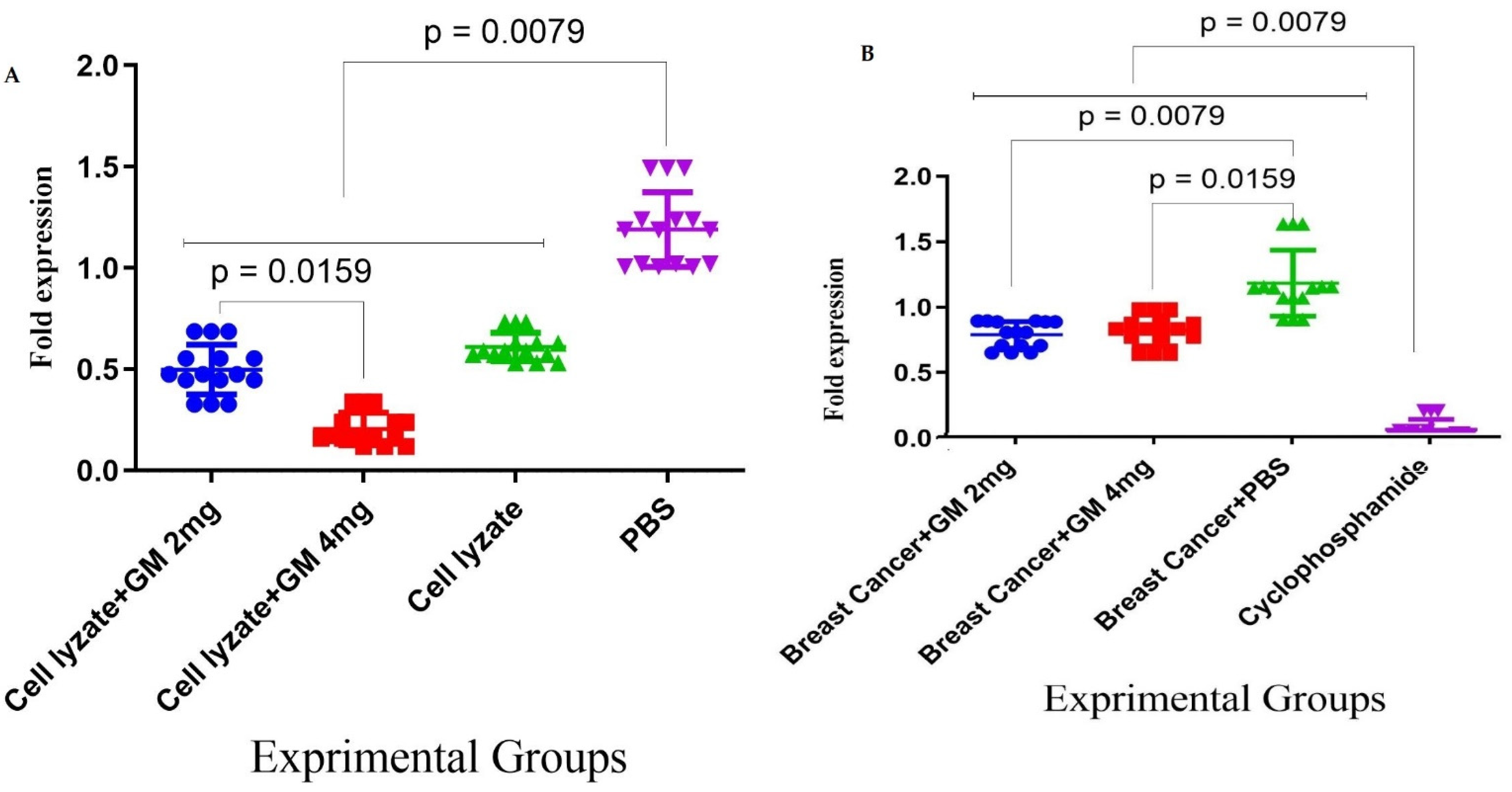
3.4. Foxp3 and TGF-β Genes Expression in Cohort I
3.5. Foxp3 and TGF-β Gene Expression in Cohort II
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hulka, B.S.; Stark, A.T. Breast cancer: Cause and prevention. Lancet 1995, 346, 883–887. [Google Scholar] [CrossRef]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Koczkodaj, P.; Sulkowska, U.; Gotlib, J.; Mańczuk, M. Breast cancer mortality trends in Europe among women in perimenopausal and postmenopausal age (45+). Arch. Med. Sci. 2019, 16, 146–156. [Google Scholar] [CrossRef]
- van Dam, F.S.; Boogerd, W.; Schagen, S.B.; Muller, M.J.; Droogleever Fortuyn, M.E.; Wall, E.v.; Rodenhuis, S. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: High-dose versus standard-dose chemotherapy. JNCI J. Natl. Cancer Inst. 1998, 90, 210–218. [Google Scholar] [CrossRef]
- Yates, J.S.; Mustian, K.M.; Morrow, G.R.; Gillies, L.J.; Padmanaban, D.; Atkins, J.N.; Issell, B.; Kirshner, J.J.; Colman, L.K. Prevalence of complementary and alternative medicine use in cancer patients during treatment. Support. Care Cancer 2005, 13, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Hryniuk, W. Correlation of dose intensity and prognosis in adjuvant chemotherapy: An extended controversy. In Adjuvant Therapy of Primary Breast Cancer; Springer: Berlin/Heidelberg, Germany, 1989; pp. 17–24. [Google Scholar]
- Curtis, R.E.; Boice, J.D., Jr.; Stovall, M.; Bernstein, L.; Greenberg, R.S.; Flannery, J.T.; Schwartz, A.G.; Weyer, P.; Moloney, W.C.; Hoover, R.N. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N. Engl. J. Med. 1992, 326, 1745–1751. [Google Scholar] [CrossRef]
- Shah, S.; Chen, B. Testing for HER2 in breast cancer: A continuing evolution. Pathol. Res. Int. 2011, 2011, 903202. [Google Scholar] [CrossRef]
- Behera, S.; Ray, R.C. Konjac glucomannan, a promising polysaccharide of Amorphophallus konjac K. Koch in health care. Int. J. Biol. Macromol. 2016, 92, 942–956. [Google Scholar] [CrossRef]
- Chua, M.; Baldwin, T.C.; Hocking, T.J.; Chan, K. Traditional uses and potential health benefits of Amorphophallus konjac K. Koch ex N.E.Br. J. Ethnopharmacol. 2010, 128, 268–278. [Google Scholar] [CrossRef]
- Tester, R.F.; Al-Ghazzewi, F.H. Mannans and health, with a special focus on glucomannans. Food Res. Int. 2013, 50, 384–391. [Google Scholar] [CrossRef]
- Ansil, P.; Wills, P.; Varun, R.; Latha, M. Cytotoxic and apoptotic activities of Amorphophallus campanulatus tuber extracts against human hepatoma cell line. Res. Pharm. Sci. 2015, 9, 269–277. [Google Scholar]
- Ansil, P.; Wills, P.; Varun, R.; Latha, M. Cytotoxic and apoptotic activities of Amorphophallus campanulatus (Roxb.) Bl. tuber extracts against human colon carcinoma cell line HCT-15. Saudi J. Biol. Sci. 2014, 21, 524–531. [Google Scholar] [CrossRef]
- Ansil, P.N.; Nitha, A.; Prabha, S.P.; Latha, M.S. Curative effect of Amorphophallus campanulatus (Roxb.) Blume. tuber on N-nitrosodiethylamine-induced hepatocellular carcinoma in rats. J. Environ. Pathol. Toxicol. Oncol. 2014, 33, 205–218. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, L.-Q.; Li, L.-J.; Lv, Y.; Chen, P.-F.; Pan, L. Suppression of gastric cancer by extract from the tuber of amorphophallus konjac via induction of apoptosis and autophagy. Oncol. Rep. 2017, 38, 1051–1058. [Google Scholar] [CrossRef]
- Cheong, H. Integrating autophagy and metabolism in cancer. Arch. Pharmacal Res. 2015, 38, 358–371. [Google Scholar] [CrossRef]
- Rice, R.Y. Botanical and Nonbotanical Products Used for Diabetes Comorbidities. In Complementary Health and Diabetes: A Focus on Dietary Supplements; American Diabetes Association: Arlington, VI, USA, 2021. [Google Scholar]
- Zhang, Y.-Q.; Xie, B.-J.; Gan, X. Advance in the applications of konjac glucomannan and its derivatives. Carbohydr. Polym. 2005, 60, 27–31. [Google Scholar] [CrossRef]
- Li, J.-y.; Sun, F.; Zhou, H.-f.; Yang, J.; Huang, C.; Fan, H. A systematic review exploring the anticancer activity and mechanisms of glucomannan. Front. Pharmacol. 2019, 10, 930. [Google Scholar] [CrossRef]
- Chen, B.; Xu, X.; Zheng, K.; Liu, L.; Yu, Y.; Xin, Y. Konjac glucomannan reverses multi-drug resistance of HepG2/5-FU cells by suppressing AKT signaling and increasing p53 expression. Oncol. Lett. 2020, 20, 2105–2112. [Google Scholar] [CrossRef]
- McCarty, M.F. Glucomannan minimizes the postprandial insulin surge: A potential adjuvant for hepatothermic therapy. Med. Hypotheses 2002, 58, 487–490. [Google Scholar] [CrossRef]
- Wu, C.; Qiu, S.; Liu, P.; Ge, Y.; Gao, X. Rhizoma Amorphophalli inhibits TNBC cell proliferation, migration, invasion and metastasis through the PI3K/Akt/mTOR pathway. J. Ethnopharmacol. 2018, 211, 89–100. [Google Scholar] [CrossRef]
- Sawai, S.; Mohktar, M.S.; Safwani, W.K.Z.W.; Ramasamy, T.S.; Mokhtar, M.S.; Zaman, W.S.W.K. Suppression of the Viability and Proliferation of HepG2 Hepatocellular Carcinoma Cell Line by Konjac Glucomannan. Anti-Cancer Agents Med. Chem. 2019, 18, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Miadoková, E.; Svidová, S.; Vlčková, V.; Dúhová, V.; Nad’ová, S.; Rauko, P.; Kogan, G. Diverse biomodulatory effects of glucomannan from Candida utilis. Toxicol. Vitr. 2006, 20, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Ansil, P.N.; Prabha, S.P.; Nitha, A.; Latha, M.S. Chemopreventive Effect of Amorphophallus campanulatus (Roxb.) blume tuber against aberrant crypt foci and cell proliferation in 1, 2-dimethylhydrazine induced colon carcinogenesis. Asian Pac. J. Cancer Prev. 2013, 14, 5331–5339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chorvatovičová, D.; Machová, E.; Šandula, J.; Kogan, G. Protective effect of the yeast glucomannan against cyclophosphamide-induced mutagenicity. Mutat. Res. /Genet. Toxicol. Environ. Mutagen. 1999, 444, 117–122. [Google Scholar] [CrossRef]
- Mizutani, T.; Mitsuoka, T. Effect of Konjac mannan on spontaneous liver tumorigenesis and fecal flora in C3HHe male mice. Cancer Lett. 1982, 17, 27–32. [Google Scholar] [CrossRef]
- Wu, W.-T.; Chen, H.-L. Effects of konjac glucomannan on putative risk factors for colon carcinogenesis in rats fed a high-fat diet. J. Agric. Food Chem. 2011, 59, 989–994. [Google Scholar] [CrossRef]
- Chong, E.S.L. A potential role of probiotics in colorectal cancer prevention: Review of possible mechanisms of action. World J. Microbiol. Biotechnol. 2014, 30, 351–374. [Google Scholar] [CrossRef]
- Klinder, A.; Glei, M.; Pool-Zobel, B.L. Prebiotics and reduction of risk of carcinogenesis: Review of experimental and human data. In Handbook of Prebiotics; CRC Press: Boca Raton, FL, USA, 2008; pp. 313–346. [Google Scholar]
- Zhan, X.; Jia, L.; Niu, Y.; Qi, H.; Chen, X.; Zhang, Q.; Zhang, J.; Wang, Y.; Dong, L.; Wang, C. Targeted depletion of tumour-associated macrophages by an alendronate–glucomannan conjugate for cancer immunotherapy. Biomaterials 2014, 35, 10046–10057. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Shuai, Y.; Cui, X.; Nie, L. Controlled release of anticancer drug using graphene oxide as a drug-binding effector in konjac glucomannan/sodium alginate hydrogels. Colloids Surfaces B Biointerfaces 2014, 113, 223–229. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Gong, J.; Mu, R.; Li, Y.; Wu, C.; Pang, J. Fabrication of chitosan-coated konjac glucomannan/sodium alginate/graphene oxide microspheres with enhanced colon-targeted delivery. Int. J. Biol. Macromol. 2019, 131, 209–217. [Google Scholar] [CrossRef]
- Salazar-Onfray, F. Interleukin-10: A cytokine used by tumors to escape immunosurveillance. Cancer Immunol. Immunother. 1999, 16, 86–94. [Google Scholar] [CrossRef]
- Suzuki, S. Studies on the interferon-inducing activity of the yeast polysaccharide with reference to its antitumor effect in mice. Sci. Rep. Res. Inst. Tohoku Univ. Ser. C Med. Tohoku Daigaku 1983, 30, 56–64. [Google Scholar]
- Vázquez-Velasco, M.; González-Torres, L.; López-Gasco, P.; Bastida, S.; Benedí, J.; González-Muñoz, M.J.; Sánchez-Muniz, F.J. Effects of glucomannan/spirulina-surimi on liver oxidation and inflammation in Zucker rats fed atherogenic diets. J. Physiol. Biochem. 2015, 71, 611–622. [Google Scholar] [CrossRef]
- Gholami, A.; Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Chiang, W.-H.; Parvin, N. Current trends in chemical modifications of magnetic nanoparticles for targeted drug delivery in cancer chemotherapy. Drug Metab. Rev. 2020, 52, 205–224. [Google Scholar]
- Mahdavi, M.; Ebtekar, M.; Khorshid, H.R.K.; Azadmanesh, K.; Hartoonian, C.; Hassan, Z.M. ELISPOT analysis of a new CTL based DNA vaccine for HIV-1 using GM-CSF in DNA prime/peptide boost strategy: GM-CSF induced long-lived memory responses. Immunol. Lett. 2011, 140, 14–20. [Google Scholar] [CrossRef]
- Mahdavi, M.; Tajik, A.H.; Ebtekar, M.; Rahimi, R.; Adibzadeh, M.M.; Moozarmpour, H.R.; Beikverdi, M.S.; Olfat, S.; Hassan, Z.M.; Choopani, M.; et al. Granulocyte-macrophage colony-stimulating factor, a potent adjuvant for polarization to Th-17 pattern: An experience on HIV-1 vaccine model. Apmis 2017, 125, 596–603. [Google Scholar] [CrossRef]
- Ståhlberg, A.; Zoric, N.; Åman, P.; Kubista, M. Quantitative real-time PCR for cancer detection: The lymphoma case. Expert Rev. Mol. Diagn. 2005, 5, 221–230. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Wickert, L.; Steinkrüger, S.; Abiaka, M.; Bolkenius, U.; Purps, O.; Schnabel, C.; Gressner, A.M. Quantitative monitoring of the mRNA expression pattern of the TGF-β-isoforms (β1, β2, β3) during transdifferentiation of hepatic stellate cells using a newly developed real-time SYBR Green PCR. Biochem. Biophys. Res. Commun. 2002, 295, 330–335. [Google Scholar] [CrossRef]
- Hermawan, A.; Putri, H.; Utomo, R.Y. Comprehensive bioinformatics study reveals targets and molecular mechanism of hesperetin in overcoming breast cancer chemoresistance. Mol. Divers. 2020, 24, 933–947. [Google Scholar] [CrossRef]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef]
- Sauter, E.R. Cancer prevention and treatment using combination therapy with natural compounds. Expert Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef]
- Du, Q.; Liu, J.; Ding, Y. Recent progress in biological activities and health benefits of konjac glucomannan and its derivatives. Bioact. Carbohydrates Diet. Fibre 2021, 26, 100270. [Google Scholar] [CrossRef]
- Geiger, J.D.; Hutchinson, R.J.; Hohenkirk, L.F.; McKenna, E.A.; Yanik, G.A.; Levine, J.; Chang, A.E.; Braun, T.M.; Mulé, J.J. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001, 61, 8513–8519. [Google Scholar]
- Prins, R.M.; Wang, X.; Soto, H.; Young, E.; Lisiero, D.N.; Fong, B.; Everson, R.; Yong, W.H.; Lai, A.; Li, G.; et al. Comparison of Glioma-associated Antigen Peptide-loaded Versus Autologous Tumor Lysate-loaded Dendritic Cell Vaccination in Malignant Glioma Patients. J. Immunother. 2013, 36, 152–157. [Google Scholar] [CrossRef]
- González, F.E.; Gleisner, A.; Falcón-Beas, F.; Osorio, F.; López, M.N.; Salazar-Onfray, F. Tumor cell lysates as immunogenic sources for cancer vaccine design. Hum. Vaccines Immunother. 2014, 10, 3261–3269. [Google Scholar] [CrossRef]
- Bercovici, N.; Haicheur, N.; Massicard, S.; Vernel-Pauillac, F.; Adotevi, O.; Landais, D.; Gorin, I.; Robert, C.; Prince, H.M.; Grob, J.-J.; et al. Analysis and Characterization of Antitumor T-cell Response After Administration of Dendritic Cells Loaded with Allogeneic Tumor Lysate to Metastatic Melanoma Patients. J. Immunother. 2008, 31, 101–112. [Google Scholar] [CrossRef]
- Furukawa, K.; Tanemura, M.; Miyoshi, E.; Eguchi, H.; Nagano, H.; Matsunami, K.; Nagaoka, S.; Yamada, D.; Asaoka, T.; Noda, T.; et al. A practical approach to pancreatic cancer immunotherapy using resected tumor lysate vaccines processed to express α-gal epitopes. PLoS ONE 2017, 12, e0184901. [Google Scholar] [CrossRef]
- Ashrafi, S.; Shapouri, R.; Shirkhani, A.; Mahdavi, M. Anti-tumor effects of propranolol: Adjuvant activity on a transplanted murine breast cancer model. Biomed. Pharmacother. 2018, 104, 45–51. [Google Scholar] [CrossRef]
- Chen, N.; Zhu, P.; Du, T.; Han, K.; Wang, D.; Ye, J.; Xiao, S.; Ye, X.; Wang, Y. Preparation of Modified Konjac Glucomannan Nanoparticles and their Application as Vaccine Adjuvants to Promote Ovalbumin-Induced Immune Response in Mice. Pharm. Res. 2018, 35, 105. [Google Scholar] [CrossRef]
- Suzuki, H.; Oomizu, S.; Yanase, Y.; Onishi, N.; Uchida, K.; Mihara, S.; Ono, K.; Kameyoshi, Y.; Hide, M. Hydrolyzed konjac glucomannan suppresses IgE production in mice B cells. Int. Arch. Allergy Immunol. 2010, 152, 122–130. [Google Scholar] [CrossRef]
- Onitake, T.; Ueno, Y.; Tanaka, S.; Sagami, S.; Hayashi, R.; Nagai, K.; Hide, M.; Chayama, K. Pulverized konjac glucomannan ameliorates oxazolone-induced colitis in mice. Eur. J. Nutr. 2015, 54, 959–969. [Google Scholar] [CrossRef]
- Oomizu, S.; Onishi, N.; Suzuki, H.; Ueda, K.; Mochizuki, M.; Morimoto, K.; Kawamoto, S.; Ono, K.; Kameyoshi, Y.; Hide, M. Oral administration of pulverized Konjac glucomannan prevents the increase of plasma immunoglobulin E and immunoglobulin G levels induced by the injection of syngeneic keratinocyte extracts in BALB/c mice. Clin. Exp. Allergy 2006, 36, 102–110. [Google Scholar] [CrossRef]
- Onishi, N.; Kawamoto, S.; Suzuki, H.; Santo, H.; Aki, T.; Shigeta, S.; Hashimoto, K.; Hide, M.; Ono, K. Dietary Pulverized Konjac Glucomannan Suppresses Scratching Behavior and Skin Inflammatory Immune Responses in NC/Nga Mice. Int. Arch. Allergy Immunol. 2007, 144, 95–104. [Google Scholar] [CrossRef]
- Ashrafi, S.; Shapouri, R.; Mahdavi, M. Immunological consequences of immunization with tumor lysate vaccine and propranolol as an adjuvant: A study on cytokine profiles in breast tumor microenvironment. Immunol. Lett. 2017, 181, 63–70. [Google Scholar] [CrossRef]
- Kumano, N.; Suzuki, S.; Ishikawa, T.; Koinumaru, S.; Konno, K. Antitumor effect of the yeast polysaccharide preparation in syngeneic mouse tumor models. Tohoku J. Exp. Med. 1985, 146, 89–96. [Google Scholar] [CrossRef]
- Wu, W.-T.; Cheng, H.-C.; Chen, H.-L. Ameliorative effects of konjac glucomannan on human faecal β-glucuronidase activity, secondary bile acid levels and faecal water toxicity towards Caco-2 cells. Br. J. Nutr. 2011, 105, 593–600. [Google Scholar] [CrossRef]



| Cohort-I | |||
| Route of Administration | Groups | N a | |
| subcutaneously | Group 1: Naïve mice immunized with 100 µg of tumor lysate vaccine + 2 mg glucomannan. | 17 | |
| subcutaneously | Group 2: Naïve mice immunized with 100 µg of tumor lysate vaccine + 4 mg glucomannan. | 17 | |
| subcutaneously | Group 3: Naïve mice immunized with 100µg of tumor lysate vaccine + 100 mg glucomannan. | 17 | |
| subcutaneously | Group 4: Naïve mice immunized with PBS. | 17 | |
| Cohort-II | |||
| Route of Administration | Groups | N a | |
| orally | Group 1: Tumor-bearing mice were fed with 2 mg glucomannan. | 17 | |
| orally | Group 2: Tumor-bearing mice were fed with 4 mg glucomannan. | 17 | |
| orally | Group 3: Tumor-bearing mice were fed with 200 µg cyclophosphamide daily as the positive control. | 17 | |
| orally | Group 4: Tumor-bearing mice were fed with PBS buffer daily as the negative control. | 17 |
| Gene | Primer Sequence |
|---|---|
| TGF-β | Forward: 5′-CCGCATCTCCTGCTAATGTTG-3 Revers: 5′-AATAGGCGGCATCCAAAGC-3′ |
| Foxp3 | Forward: 5′-F: CAGCTGCCTACAGTGCCCCTAG-3′ Revers: 5′-CATTTGCCAGCAGTGGGTAG-3′ |
| β-actin | Forward: 5’-TGGAATCCTGTGGCATCCATGAAAC-3′ Revers: 5’-TAAAACGCAGCTCAGTAACAGTCCG-3’ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, N.; Jahantigh, H.R.; Noorbazargan, H.; Yazdi, M.H.; Mahdavi, M. Glucomannan as a Dietary Supplement for Treatment of Breast Cancer in a Mouse Model. Vaccines 2022, 10, 1746. https://doi.org/10.3390/vaccines10101746
Ahmadi N, Jahantigh HR, Noorbazargan H, Yazdi MH, Mahdavi M. Glucomannan as a Dietary Supplement for Treatment of Breast Cancer in a Mouse Model. Vaccines. 2022; 10(10):1746. https://doi.org/10.3390/vaccines10101746
Chicago/Turabian StyleAhmadi, Nioosha, Hamid Reza Jahantigh, Hassan Noorbazargan, Mohammad Hossein Yazdi, and Mehdi Mahdavi. 2022. "Glucomannan as a Dietary Supplement for Treatment of Breast Cancer in a Mouse Model" Vaccines 10, no. 10: 1746. https://doi.org/10.3390/vaccines10101746
APA StyleAhmadi, N., Jahantigh, H. R., Noorbazargan, H., Yazdi, M. H., & Mahdavi, M. (2022). Glucomannan as a Dietary Supplement for Treatment of Breast Cancer in a Mouse Model. Vaccines, 10(10), 1746. https://doi.org/10.3390/vaccines10101746







