A Scoping Review of Three Dimensions for Long-Term COVID-19 Vaccination Models: Hybrid Immunity, Individual Drivers of Vaccinal Choice, and Human Errors
Abstract
:1. Introduction
2. Immunity: Variants, Waning Effect, and Hybrid Cases
3. Vaccinal Choice
3.1. Drivers of Vaccinal Choice
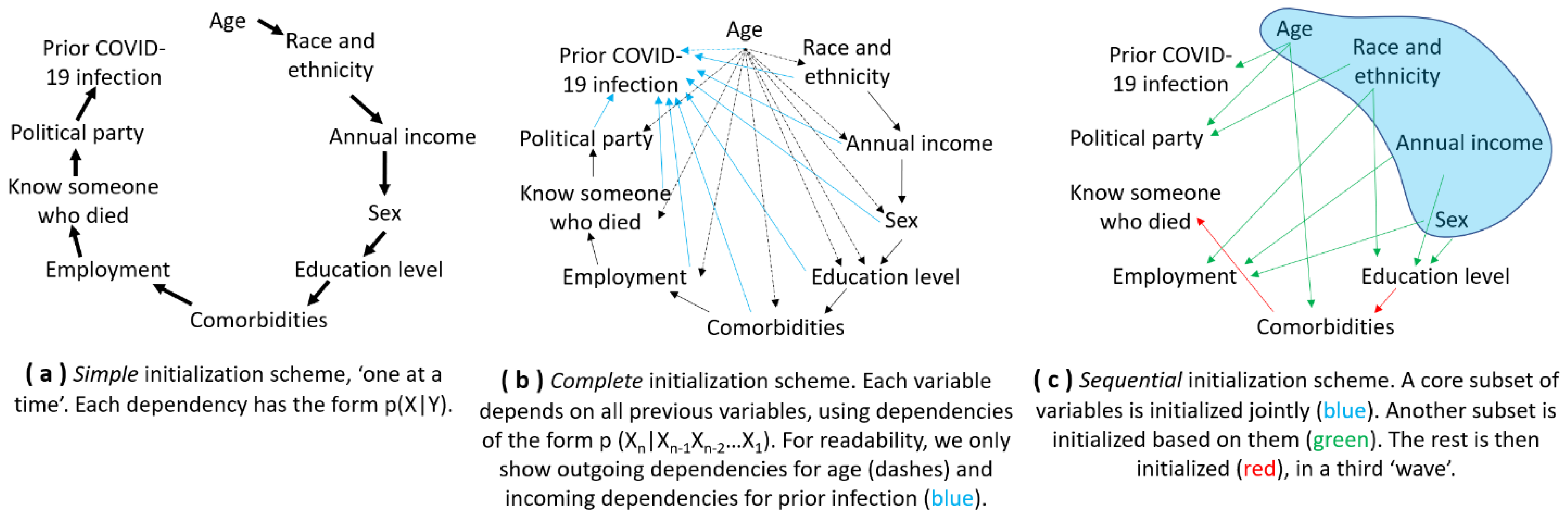
3.2. Capturing Drivers in a Model: The Role of Data and Sequential Agent Initialization
| 1st Wave of Initialization of Four Categorical Features Jointly [80] | ||||||
|---|---|---|---|---|---|---|
| Ref | Factors | Age | Income | Race and Ethnicity | Sex | |
| 2nd wave of initialization | [88,89] | Bachelor’s degree | ✓ | ✓ | ✓ | |
| [90,91] | Political party 1 | ✓ | ✓ | |||
| [92] | Diabetes | ✓ | ✓ | ✓ | ||
| [93,94] | Hypertension 2 | ✓ | ✓ | |||
| [95] | ≥1 dose of vaccine | ✓ | ✓ | |||
3.3. Extending an Existing Package: Example in COVASIM
4. Human Errors in Decision-Making
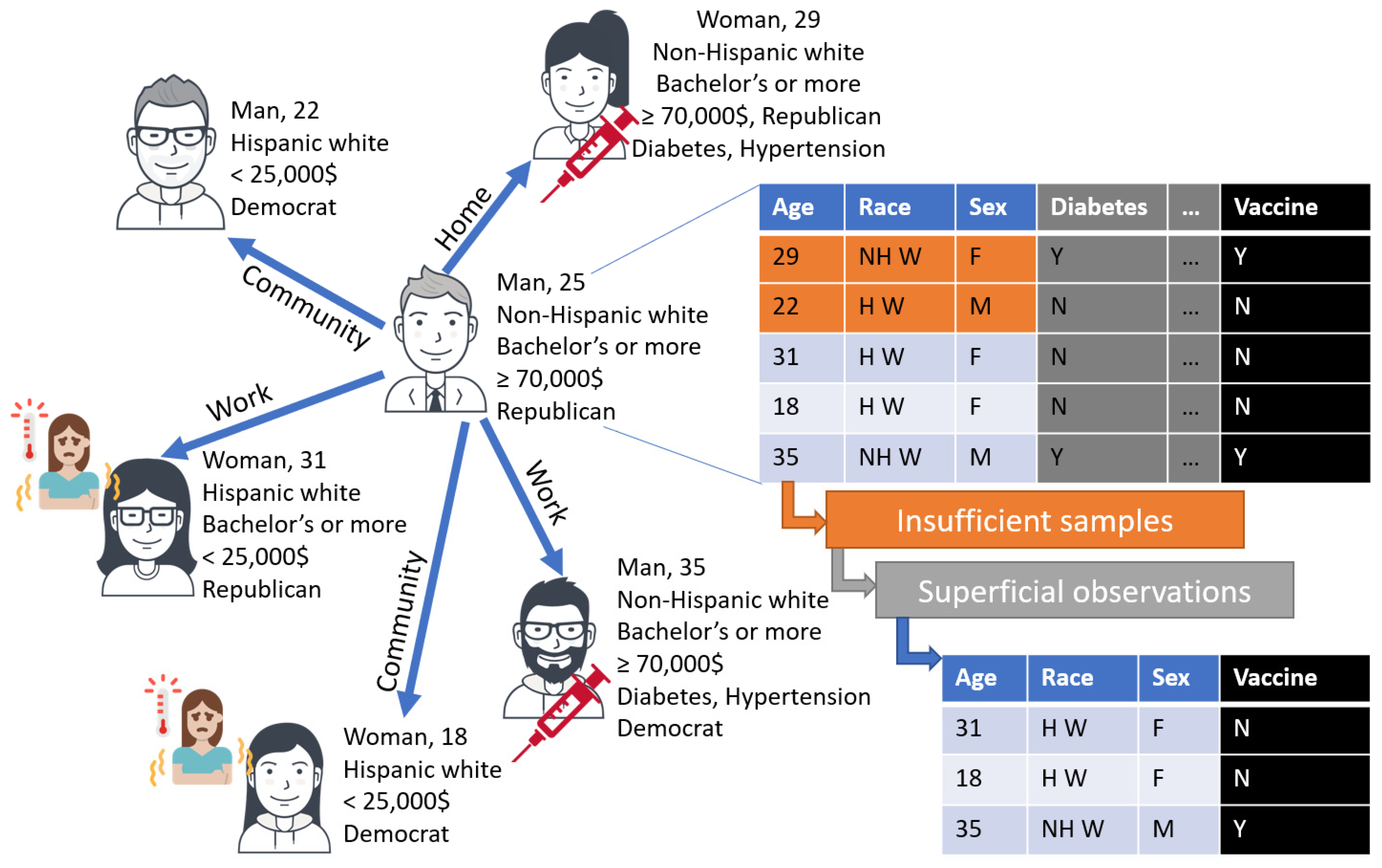
4.1. Limitations of Observations and Reflections
4.2. Operationalizing Human Errors in a Model: The Role of Machine Learning as a Filter
5. Discussion
5.1. Overview
5.2. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Axios/Ipsos. Most Americans Agree We Will Never Fully Be Rid of COVID-19. Last Updated: 2022-07-19. Available online: https://www.ipsos.com/en-us/news-polls/axios-ipsos-coronavirus-index (accessed on 2 August 2022).
- Wen, W.; Chen, C.; Tang, J.; Wang, C.; Zhou, M.; Cheng, Y.; Zhou, X.; Wu, Q.; Zhang, X.; Feng, Z.; et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: A meta-analysis. Ann. Med. 2022, 54, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, Q. Safety and Efficacy of the Common Vaccines against COVID-19. Vaccines 2022, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics. Daily Updates of Totals by Week and State. Last Updated: 2022-08-02. Available online: https://www.cdc.gov/nchs/nvss/vsrr/covid19/index.htm (accessed on 2 August 2022).
- Lyttelton, T.; Zang, E. Occupations and Sickness-Related Absences during the COVID-19 Pandemic. J. Health Soc. Behav. 2022, 63, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Thurman, W.; Heitkemper, E.; Johnson, K.E. Challenges Facing Public Health Nursing Faculty in the United States: COVID-19 as a Catalyst for Change. Am. J. Public Health 2022, 112, S314–S320. [Google Scholar] [CrossRef] [PubMed]
- Carver-Thomas, D.; Burns, D.; Leung, M.; Ondrasek, N. Teacher Shortages During the Pandemic: How California Districts Are Responding; Learning Policy Institute: Palo Alto, CA, USA, 2022. [Google Scholar] [CrossRef]
- Jiskrova, G.K. Impact of COVID-19 pandemic on the workforce: From psychological distress to the Great Resignation. J. Epidemiol. Community Health 2022, 76, 525–526. [Google Scholar] [CrossRef]
- Schmid, S.R.; Melkote, S.N. Manufacturing and the Great Resignation. Mech. Eng. 2022, 144, 38–43. [Google Scholar] [CrossRef]
- Gavin, M.; Poorhosseinzadeh, M.; Arrowsmith, J. The transformation of work and employment relations: COVID-19 and beyond. Labour Ind. 2022, 32, 1–9. [Google Scholar] [CrossRef]
- Pronk, N.P. Addressing COVID-19 Disruptions at the Workplace While Preparing for a Postpandemic Future. ACSM’S Health Fit. J. 2022, 26, 52–55. [Google Scholar] [CrossRef]
- Varizi, A.; Beamish, R. COVID in California: Reinfections account for 1 in 7 coronavirus cases in state. San Francisco Chronicles, 1 August 2022. Available online: https://www.sfchronicle.com/bayarea/article/COVID-in-California-Latest-updates-about-the-17342154.php (accessed on 2 August 2022).
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Sharma, N.; Anwer, K.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Bhatia, S.; et al. There is nothing exempt from the peril of mutation—The Omicron spike. Biomed. Pharmacother. 2022, 148, 112756. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration (FDA); Office of Vaccines Research and Review. Fall 2022 COVID-19 Vaccine Strain Composition Selection Recommendation. Memorandum. 30 June 2022. Available online: https://www.fda.gov/media/159597/download (accessed on 3 August 2022).
- Saad, L. Roundup of Gallup COVID-19 Coverage. Updated 06/06/22. Available online: https://news.gallup.com/opinion/gallup/308126/roundup-gallup-covid-coverage.aspx (accessed on 3 August 2022).
- Tyson, A. 57% of Americans Say Masks Should Be Required on Airplanes and Public Transportation. Pew Research Center. Last updated: 05/11/22. Available online: https://www.pewresearch.org/fact-tank/2022/05/11/57-of-americans-say-masks-should-be-required-on-airplanes-and-public-transportation/ (accessed on 3 August 2022).
- Lennon, R.P.; Block, R.; Schneider, E.C.; Zephrin, L.; Shah, A.; The African American Research Collaborative 2021 COVID Group. Underserved population acceptance of combination influenza-COVID-19 booster vaccines. Vaccine 2021, 40, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.; Woodcock, J.; Califf, R. COVID-19 Vaccination—Becoming Part of the New Normal. JAMA 2022, 327, 1863. [Google Scholar] [CrossRef]
- Abuhammad, S.; Khabour, O.F.; Alzoubi, K.H.; Hamaideh, S.; Alzoubi, B.A.; Telfah, W.S.; El-Zubi, F.K. The public’s attitude to and acceptance of periodic doses of the COVID-19 vaccine: A survey from Jordan. PLoS ONE 2022, 17, e0271625. [Google Scholar] [CrossRef]
- Reifferscheid, L.; Lee, J.S.W.; MacDonald, N.E.; Sadarangani, M.; Assi, A.; Lemaire-Paquette, S.; MacDonald, S.E. Transition to endemic: Acceptance of additional COVID-19 vaccine doses among Canadian adults in a national cross-sectional survey. medRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Weitzer, J.; Birmann, B.M.; Steffelbauer, I.; Bertau, M.; Zenk, L.; Caniglia, G.; Laubichler, M.D.; Steiner, G.; Schernhammer, E.S. Willingness to receive an annual COVID-19 booster vaccine in the German-speaking DA-CH region in Europe: A cross-sectional study. Lancet Reg. Health-Eur. 2022, 18, 100414. [Google Scholar] [CrossRef]
- Lee, R.C.; Hu, H.; Kawaguchi, E.S.; Kim, A.E.; Soto, D.W.; Shanker, K.; Klausner, J.D.; Van Orman, S.; Unger, J.B. COVID-19 booster vaccine attitudes and behaviors among university students and staff in the United States: The USC Trojan pandemic research Initiative. Prev. Med. Rep. 2022, 28, 101866. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yang, C.; Wang, T.; Coltey, E.; Jin, Y.; Liu, Y.; Jiang, R.; Fan, Z.; Song, X.; Shibasaki, R.; et al. A Survey on Data-Driven COVID-19 and Future Pandemic Management. ACM Comput. Surv. 2022. [Google Scholar] [CrossRef]
- Bilal, U.; Mullachery, P.H.; Schnake-Mahl, A.; Rollins, H.; McCulley, E.; Kolker, J.; Barber, S.; Roux, A.V.D. Heterogeneity in Spatial Inequities in COVID-19 Vaccination Across 16 Large US Cities. Am. J. Epidemiol. 2022, 191, 1546–1556. [Google Scholar] [CrossRef]
- Masters, N.B.; Zhou, T.; Meng, L.; Lu, P.-J.; Kriss, J.L.; Black, C.; Omari, A.; Boone, K.; Weiss, D.; Carter, R.J.; et al. Geographic Heterogeneity in Behavioral and Social Drivers of COVID-19 Vaccination. Am. J. Prev. Med. 2022. [Google Scholar] [CrossRef]
- Saldaña, J.; Scoglio, C. Influence of heterogeneous age-group contact patterns on critical vaccination rates for herd immunity to SARS-CoV-2. Sci. Rep. 2022, 12, 2640. [Google Scholar] [CrossRef] [PubMed]
- Lorig, F.; Johansson, E.; Davidsson, P. Agent-Based Social Simulation of the Covid-19 Pandemic: A Systematic Review. J. Artif. Soc. Soc. Simul. 2021, 24, 5. [Google Scholar] [CrossRef]
- Giabbanelli, P.J.; Badham, J.; Castellani, B.; Kavak, H.; Mago, V.; Negahban, A.; Swarup, S. Opportunities and Challenges in Developing COVID-19 Simulation Models: Lessons from Six Funded Projects. In Proceedings of the 2021 Annual Modeling and Simulation Conference (ANNSIM), Fairfax, VA, USA, 19–22 July 2021. [Google Scholar] [CrossRef]
- Li, J.; Giabbanelli, P.J. Identifying Synergistic Interventions to Address COVID-19 Using a Large Scale Agent-Based Model. In Proceedings of the International Conference on Computational Science, Krakow, Poland, 16–18 June 2021; pp. 655–662. [Google Scholar] [CrossRef]
- Colomer, M.À.; Margalida, A.; Alòs, F.; Oliva-Vidal, P.; Vilella, A.; Fraile, L. Modelling the SARS-CoV-2 outbreak: Assessing the usefulness of protective measures to reduce the pandemic at population level. Sci. Total Environ. 2021, 789, 147816. [Google Scholar] [CrossRef]
- Ghaffarzadegan, N.; Childs, L.M.; Täuber, U.C. Diverse Computer Simulation Models Provide Unified Lessons on University Operation during a Pandemic. BioScience 2020, 71, 113–114. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, S.; Zheng, Z.; Lu, J. Optimizing spatial allocation of COVID-19 vaccine by agent-based spatiotemporal simulations. GeoHealth 2021, 5, e2021GH000427. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Giabbanelli, P. Returning to a Normal Life via COVID-19 Vaccines in the United States: A Large-scale Agent-Based Simulation Study. JMIR Med. Inform. 2021, 9, e27419. [Google Scholar] [CrossRef] [PubMed]
- Romero-Brufau, S.; Chopra, A.; Ryu, A.J.; Gel, E.; Raskar, R.; Kremers, W.; Anderson, K.S.; Subramanian, J.; Krishnamurthy, B.; Singh, A.; et al. Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: Simulation agent based modeling study. BMJ 2021, 373, n1087. [Google Scholar] [CrossRef] [PubMed]
- Truszkowska, A.; Zino, L.; Butail, S.; Caroppo, E.; Jiang, Z.P.; Rizzo, A.; Porfiri, M. Predicting the Effects of Waning Vaccine Immunity Against COVID-19 through High-Resolution Agent-Based Modeling. Adv. Theory Simul. 2022, 5, 2100521. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.L.; Le Rutte, E.A.; Richter, M.; Penny, M.A.; Shattock, A.J. COVID-19 vaccine booster strategies in light of emerging viral variants: Frequency, timing, and target groups. medRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.D.; Pell, B.; Nelson, P. A Mathematical Study of COVID-19 Spread by Vaccination Status in Virginia. Appl. Sci. 2022, 12, 1723. [Google Scholar] [CrossRef]
- Caetano, C.; Morgado, M.L.; Patricio, P.; Leite, A.; Machado, A.; Torres, A.; Pereira, J.F.; Namorado, S.; Sottomayor, A.; Peralta, A.; et al. Measuring the impact of COVID-19 vaccination and immunity waning: A modelling study for Portugal. medRxiv 2021. [Google Scholar] [CrossRef]
- Kerr, C.C.; Stuart, R.M.; Mistry, D.; Abeysuriya, R.G.; Rosenfeld, K.; Hart, G.R.; Núñez, R.C.; Cohen, J.A.; Selvaraj, P.; Hagedorn, B.; et al. Covasim: An agent-based model of COVID-19 dynamics and interventions. PLoS Comput. Biol. 2021, 17, e1009149. [Google Scholar] [CrossRef]
- Hinch, R.; Probert, W.J.; Nurtay, A.; Kendall, M.; Wymant, C.; Hall, M.; Lythgoe, K.; Bulas Cruz, A.; Zhao, L.; Stewart, A.; et al. OpenABM-Covid19—An agent-based model for non-pharmaceutical interventions against COVID-19 including contact tracing. PLoS Comput. Biol. 2021, 17, e1009146. [Google Scholar] [CrossRef]
- Gaudou, B.; Huynh, N.Q.; Philippon, D.; Brugière, A.; Chapuis, K.; Taillandier, P.; Larmande, P.; Drogoul, A. COMOKIT: A Modeling Kit to Understand, Analyze, and Compare the Impacts of Mitigation Policies Against the COVID-19 Epidemic at the Scale of a City. Front. Public Health 2020, 8, 563247. [Google Scholar] [CrossRef] [PubMed]
- Aylett-Bullock, J.; Cuesta-Lazaro, C.; Quera-Bofarull, A.; Icaza-Lizaola, M.; Sedgewick, A.; Truong, H.; Curran, A.; Elliott, E.; Caulfield, T.; Fong, K.; et al. June: Open-source individual-based epidemiology simulation. R. Soc. Open Sci. 2021, 8, 210506. [Google Scholar] [CrossRef] [PubMed]
- Reguly, I.Z.; Csercsik, D.; Juhász, J.; Tornai, K.; Bujtár, Z.; Horváth, G.; Keömley-Horváth, B.; Kós, T.; Cserey, G.; Iván, K.; et al. Microsimulation based quantitative analysis of COVID-19 management strategies. PLoS Comput. Biol. 2022, 18, e1009693. [Google Scholar] [CrossRef]
- Kerr, C.C.; Mistry, D.; Stuart, R.M.; Rosenfeld, K.; Hart, G.R.; Núñez, R.C.; Cohen, J.A.; Selvaraj, P.; Abeysuriya, R.G.; Jastrzębski, M.; et al. Controlling COVID-19 via test-trace-quarantine. Nat. Commun. 2021, 12, 2993. [Google Scholar] [CrossRef]
- Cohen, J.A.; Stuart, R.M.; Rosenfeld, K.; Lyons, H.; White, M.; Kerr, C.; Klein, D.J.; Famulare, M. Mechanistic modeling of SARS-CoV-2 immune memory, variants, and vaccines. medRxiv, 2021; preprint. [Google Scholar]
- Scott, N.; Palmer, A.; Delport, D.; Abeysuriya, R.; Stuart, R.M.; Kerr, C.C.; Mistry, D.; Klein, D.J.; Sacks-Davis, R.; Heath, K.; et al. Modelling the impact of relaxing COVID-19 control measures during a period of low viral transmission. Med. J. Aust. 2020, 214, 79–83. [Google Scholar] [CrossRef]
- Abeysuriya, R.G.; Delport, D.; Stuart, R.M.; Sacks-Davis, R.; Kerr, C.C.; Mistry, D.; Klein, D.J.; Hellard, M.; Scott, N. Preventing a cluster from becoming a new wave in settings with zero community COVID-19 cases. BMC Infect. Dis. 2022, 22, 232. [Google Scholar] [CrossRef]
- Sanz-Leon, P.; Stevenson, N.J.; Stuart, R.M.; Abeysuriya, R.G.; Pang, J.C.; Lambert, S.B.; Kerr, C.C.; Roberts, J.A. Risk of sustained SARS-CoV-2 transmission in Queensland, Australia. Sci. Rep. 2022, 12, 6309. [Google Scholar] [CrossRef]
- Hamer, D.H.; White, L.F.; Jenkins, H.E.; Gill, C.J.; Landsberg, H.E.; Klapperich, C.; Bulekova, K.; Platt, J.; Decarie, L.; Gilmore, W.; et al. Assessment of a COVID-19 Control Plan on an Urban University Campus During a Second Wave of the Pandemic. JAMA Netw. Open 2021, 4, e2116425. [Google Scholar] [CrossRef]
- Panovska-Griffiths, J.; Kerr, C.C.; Waites, W.; Stuart, R.M.; Mistry, D.; Foster, D.; Klein, D.J.; Viner, R.M.; Bonell, C. Modelling the potential impact of mask use in schools and society on COVID-19 control in the UK. Sci. Rep. 2021, 11, 8747. [Google Scholar] [CrossRef]
- Krivorotko, O.; Sosnovskaia, M.; Vashchenko, I.; Kerr, C.; Lesnic, D. Agent-based modeling of COVID-19 outbreaks for New York state and UK: Parameter identification algorithm. Infect. Dis. Model. 2021, 7, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.D.; Stuart, R.M.; Nguyen, T.V.; Luong, Q.C.; Tran, Q.D.; Pham, T.Q.; Phan, L.T.; Dang, T.Q.; Tran, D.N.; Do, H.T.; et al. Estimating and mitigating the risk of COVID-19 epidemic rebound associated with reopening of international borders in Vietnam: A modelling study. Lancet Glob. Health 2021, 9, e916–e924. [Google Scholar] [CrossRef]
- Cattaneo, A.; Vitali, A.; Mazzoleni, M.; Previdi, F. An agent-based model to assess large-scale COVID-19 vaccination campaigns for the Italian territory: The case study of Lombardy region. Comput. Methods Programs Biomed. 2022, 224, 107029. [Google Scholar] [CrossRef] [PubMed]
- Lutz, C.B.; Giabbanelli, P.J. When Do We Need Massive Computations to Perform Detailed COVID-19 Simulations? Adv. Theory Simul. 2021, 5, 2100343. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Deng, X.; Yang, J.; Sun, K.; Liu, H.; Chen, Z.; Peng, C.; Chen, X.; Wu, Q.; Zou, J.; et al. Modeling transmission of SARS-CoV-2 omicron in China. Nat. Med. 2022, 28, 1468–1475. [Google Scholar] [CrossRef]
- Pell, B.; Johnston, M.D.; Nelson, P. A data-validated temporary immunity model of COVID-19 spread in Michigan. Math. Biosci. Eng. 2022, 19, 10122–10142. [Google Scholar] [CrossRef]
- Clarke, K.E.; Jones, J.M.; Deng, Y.; Nycz, E.; Lee, A.; Iachan, R.; Gundlapalli, A.V.; Hall, A.J.; MacNeil, A. Seroprevalence of Infection-Induced SARS-CoV-2 Antibodies—United States, September 2021–February 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 606. [Google Scholar] [CrossRef]
- Cohen, J.A.; Stuart, R.M.; Panovska-Griffiths, J.; Mudimu, E.; Abeysuriya, R.G.; Kerr, C.C.; Famulare, M.; Klein, D.J. The changing impact of vaccines in the COVID-19 pandemic. medRxiv 2022. [Google Scholar] [CrossRef]
- Lee, S.; Zabinsky, Z.B.; Wasserheit, J.N.; Ross, J.M.; Chen, S.; Liu, S. COVID-19 Endemic Plan: Impact of Vaccination and Non-pharmaceutical Interventions with Viral Variants and Waning Immunity Using an Agent-Based Simulation. medRxiv 2022. [Google Scholar] [CrossRef]
- Feng, A.; Obolski, U.; Stone, L.; He, D. Modelling COVID-19 Vaccine Breakthrough Infections in Highly Vaccinated Israel-the effects of waning immunity and third vaccination dose. medRxiv 2022. [Google Scholar] [CrossRef]
- Pepper, M. Immunity to SARS-CoV-2 infection. Immunol. Rev. 2022, 309, 5. [Google Scholar] [CrossRef]
- Epsi, N.J.; Richard, S.A.; Lindholm, D.A.; Mende, K.; Ganesan, A.; Huprikar, N.; Lalani, T.; Fries, A.C.; Maves, R.C.; Colombo, R.E.; et al. Understanding ‘hybrid immunity’: Comparison and predictors of humoral immune responses to SARS-CoV-2 infection and COVID-19 vaccines. Clin. Infect. Dis. 2022, ciac392. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N. Engl. J. Med. 2022, 387, 21–34. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Lamothe, P.A.; Woodruff, M.C.; Saini, A.S.; Faliti, C.E.; Sanz, I.; Lee, F.E.H. COVID-19 and plasma cells: Is there long-lived protection? Immunol. Rev. 2022, 309, 40–63. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Sharma, A.R.; Dhama, K.; Agoramoorthy, G.; Chakraborty, C. Hybrid immunity against COVID-19 in different countries with a special emphasis on the Indian scenario during the Omicron period. Int. Immunopharmacol. 2022, 108, 108766. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol. Rev. 2022, 310, 27–46. [Google Scholar] [CrossRef]
- Bates, T.A.; McBride, S.K.; Leier, H.C.; Guzman, G.; Lyski, Z.L.; Schoen, D.; Winders, B.; Lee, J.Y.; Lee, D.X.; Messer, W.B.; et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci. Immunol. 2022, 7, eabn8014. [Google Scholar] [CrossRef]
- Mozaffer, F.; Cherian, P.; Krishna, S.; Wahl, B.; Menon, G.I. Effect of hybrid immunity, school reopening, and the Omicron variant on trajectory of COVID-19 epidemic in India: A modelling study. medRxiv 2022. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: A retrospective, total population cohort study in Sweden. Lancet Infect. Dis. 2022, 22, 781–790. [Google Scholar] [CrossRef]
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ. Res. 2022, 209, 112911. [Google Scholar] [CrossRef]
- Busch, M.P.; Stramer, S.L.; Stone, M.; Yu, E.A.; Grebe, E.; Notari, E.; Saa, P.; Ferg, R.; Manrique, I.M.; Weil, N.; et al. Population-weighted seroprevalence from SARS-CoV-2 infection, vaccination, and hybrid immunity among US blood donations from January–December 2021. Clin. Infect. Dis. 2022.
- Bhiman, J.N.; Moore, P.L. Leveraging South African HIV research to define SARS-CoV-2 immunity triggered by sequential variants of concern. Immunol. Rev. 2022, 310, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.P.; Day, T.; Arino, J.; Colijn, C.; Dushoff, J.; Li, M.; Mechai, S.; Van Domselaar, G.; Wu, J.; Earn, D.J.; et al. The origins and potential future of SARS-CoV-2 variants of concern in the evolving COVID-19 pandemic. Curr. Biol. 2021, 31, R918–R929. [Google Scholar] [CrossRef] [PubMed]
- Ciunkiewicz, P.; Brooke, W.; Rogers, M.; Yanushkevich, S. Agent-based epidemiological modeling of COVID-19 in localized environments. Comput. Biol. Med. 2022, 144, 105396. [Google Scholar] [CrossRef] [PubMed]
- Usherwood, T.; LaJoie, Z.; Srivastava, V. A model and predictions for COVID-19 considering population behavior and vaccination. Sci. Rep. 2021, 11, 12051. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, B. Co-evolution of Vaccination Behavior and Perceived Vaccination Risk can lead to a Stag-Hunt like Game. arXiv 2022, arXiv:2207.05964. [Google Scholar]
- Piltch-Loeb, R.; Silver, D.R.; Kim, Y.; Norris, H.; McNeill, E.; Abramson, D.M. Determinants of the COVID-19 vaccine hesitancy spectrum. PLoS ONE 2022, 17, e0267734. [Google Scholar] [CrossRef]
- Moore, J.; Gilbert, K.; Lively, K.; Laurent, C.; Chawla, R.; Li, C.; Johnson, R.; Petcu, R.; Mehra, M.; Spooner, A.; et al. Correlates of COVID-19 Vaccine Hesitancy among a Community Sample of African Americans Living in the Southern United States. Vaccines 2021, 9, 879. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y. Multilevel determinants of COVID-19 vaccination hesitancy in the United States: A rapid systematic review. Prev. Med. Rep. 2021, 25, 101673. [Google Scholar] [CrossRef]
- Rane, M.S.; Kochhar, S.; Poehlein, E.; You, W.; Robertson, M.M.; Zimba, R.; A Westmoreland, D.; Romo, M.L.; Kulkarni, S.G.; Chang, M.; et al. Determinants and Trends of COVID-19 Vaccine Hesitancy and Vaccine Uptake in a National Cohort of US Adults: A Longitudinal Study. Am. J. Epidemiol. 2022, 191, 570–583. [Google Scholar] [CrossRef]
- Saluja, S.; Lam, C.N.; Wishart, D.; McMorris, A.; Cousineau, M.R.; Kaplan, C.M. Disparities in COVID-19 vaccine hesitancy among Los Angeles County adults after vaccine authorization. Prev. Med. Rep. 2021, 24, 101544. [Google Scholar] [CrossRef]
- Halbrook, M.; Gadoth, A.; Martin-Blais, R.; Gray, A.N.; Kashani, S.; Kazan, C.; Kane, B.; Tobin, N.H.; Ferbas, K.G.; Aldrovandi, G.M.; et al. Longitudinal Assessment of Coronavirus Disease 2019 Vaccine Acceptance and Uptake Among Frontline Medical Workers in Los Angeles, California. Clin. Infect. Dis. 2021, 74, 1166–1173. [Google Scholar] [CrossRef]
- Freund, A.J.; Giabbanelli, P.J. The Necessity and Difficulty of Navigating Uncertainty to Develop an Individual-Level Computational Model. In Proceedings of the International Conference on Computational Science, Krakow, Poland, 16–18 June 2021; pp. 407–421. [Google Scholar] [CrossRef]
- United States Census Bureau and Bureau of Labor Statistics. Current Population Survey (CPS) Annual Social and Economic (ASEC) Supplement. PINC-01. Selected Characteristics of People 15 Years and over, by Total Money Income, Work Experience, Race, Hispanic Origin, and Sex. Last Revised: 10/08/21. Available online: https://www.census.gov/data/tables/time-series/demo/income-poverty/cps-pinc/pinc-01.2019.html (accessed on 12 August 2022).
- Petrakis, V.; Panagopoulos, P.; Papazoglou, D.; Papanas, N. Diabetes Mellitus and Hypertension as Major Risk Factors of Mortality from Covid-19 Pneumonia. Exp. Clin. Endocrinol. Diabetes 2020, 130, 205–206. [Google Scholar] [CrossRef]
- Merga, B.T.; Ayana, G.M.; Raru, T.B.; Alemu, A.; Negash, B.; Bekana, M.; Birhanu, A.; Dessie, Y. Association of Pre-Existing Comorbidities with Disease Severity Among COVID-19 Patients in Eastern Ethiopia. Infect. Drug Resist. 2022, 15, 2825. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, E.; Linschoten, M.; Asselbergs, F.W.; Lacy, P.S.; Jedrzejewski, D.; Williams, B. The impact of pre-existing hypertension and its treatment on outcomes in patients admitted to hospital with COVID-19. Hypertens. Res. 2022, 45, 834–845. [Google Scholar] [CrossRef] [PubMed]
- United States Census Bureau. Educational Attainment in the United States: 2019. Table 1. Educational Attainment of the Population 18 Years and over, by Age, Sex, Race, and Hispanic Origin: 2019. Last Revised: 10/08/21. Available online: https://www.census.gov/data/tables/2019/demo/educational-attainment/cps-detailed-tables.html (accessed on 12 August 2022).
- United States Census Bureau. Annual Estimates of the Resident Population for Selected Age Groups by Sex for the United States: April 1, 2010 to July 1, 2019. Available online: https://www2.census.gov/programs-surveys/popest/tables/2010-2019/national/asrh/nc-est2019-asr5h.xlsx (accessed on 12 August 2022).
- Igielnick, R.; Keeter, S.; Hartig, H. Behind Biden’s 2020 Victory: An examination of the 2020 Electorate, Based on Validated Voters. Pew Research Center: Report. 30 June 2021. Available online: https://www.pewresearch.org/politics/2021/06/30/behind-bidens-2020-victory/ (accessed on 12 August 2022).
- United States Census Bureau. Voting and Registration in the Election of November 2020. Last Revised: 10/08/21. Available online: https://www.census.gov/data/tables/time-series/demo/voting-and-registration/p20-585.html (accessed on 16 August 2022).
- Centers for Disease Prevention and Control. National Diabetes Statistics Report. 2020. Available online: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf (accessed on 12 October 2022).
- National Center for Health Statistics. Table 022: Hypertension among Adults Aged 20 and over, by Selected Characteristics: United States, Selected Years 1988–1994 through 2015–2018. Available online: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Publications/Health_US/hus19tables/table022.xlsx (accessed on 12 August 2022).
- Stierman, B.; Afful, J.; Carroll, M.D.; Chen, T.C.; Davy, O.; Fink, S.; Fryar, C.D.; Gu, Q.; Hales, C.M.; Hughes, J.P.; et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports 158. 2021. Available online: https://www.cdc.gov/nchs/data/nhsr/nhsr158-508.pdf (accessed on 16 August 2022).
- Kriss, J.L.; Hung, M.C.; Srivastav, A.; Black, C.L.; Lindley, M.C.; Lee, J.T.; Koppaka, R.; Tsai, Y.; Lu, P.J.; Yankey, D.; et al. COVID-19 Vaccination Coverage, by Race and Ethnicity—National Immunization Survey Adult COVID Module, United States, December 2020–November 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 757. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.M.; Higiro, J.D. Managing Pending Events in Sequential and Parallel Simulations Using Three-tier Heap and Two-tier Ladder Queue. ACM Trans. Model. Comput. Simul. 2019, 29, 1–28. [Google Scholar] [CrossRef]
- Dignum, V.; Dignum, F. Agents are dead. Long live agents! In Proceedings of the 19th International Conference on Autonomous Agents and MultiAgent Systems, Auckland, New Zealand, 9–13 May 2020; pp. 1701–1705. [Google Scholar]
- Giubilini, A.; Savulescu, J. Vaccination, Risks, and Freedom: The Seat Belt Analogy. Public Health Ethic 2019, 12, 237–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, D. The Side Effects of Not Being Vaccinated: Individual Risk and Vaccine Hesitancy Nationalism. J. Bioethical Inq. 2021, 19, 7–10. [Google Scholar] [CrossRef]
- Burden, D.; Savin-Baden, M. Virtual Humans: Today and Tomorrow; Chapman and Hall/CRC: Boca Raton, FL, USA, 2019. [Google Scholar]
- Nyblade, B.; O’Mahony, A.; Sieck, K. Building on Social Science: Theoretic Foundations for Modelers. Soc.-Behav. Model. Complex Syst. 2019, 63–99. [Google Scholar] [CrossRef]
- Grantham, E.O.; Giabbanelli, P.J. Creating perceptual uncertainty in agent-based models with social interactions. In Proceedings of the 2020 Spring Simulation Conference (SpringSim), Fairfax, VA, USA, 18–21 May 2020; pp. 1–12. [Google Scholar]
- Sun, R. Cognitive social simulation for policy making. Policy Insights Behav. Brain Sci. 2018, 5, 240–246. [Google Scholar] [CrossRef]
- Espinoza, B.; Swarup, S.; Barrett, C.L.; Marathe, M. Heterogeneous adaptive behavioral responses may increase epidemic burden. Sci. Rep. 2022, 12, 11276. [Google Scholar] [CrossRef] [PubMed]
- Morin, O.; Jacquet, P.O.; Vaesen, K.; Acerbi, A. Social information use and social information waste. Philos. Trans. R. Soc. B 2021, 376, 20200052. [Google Scholar] [CrossRef]
- Negahban, A.; Giabbanelli, P.J. Hybrid Agent-Based Simulation of Adoption Behavior and Social Interactions: Alternatives, Opportunities, and Pitfalls. IEEE Trans. Comput. Soc. Syst. 2021, 9, 770–780. [Google Scholar] [CrossRef]
- Castro e Silva, A.; Bernardes, A.T.; Barbosa, E.A.; Chagas, I.A.; Dáttilo, W.; Reis, A.B.; Ribeiro, S.P. Successive Pandemic Waves with Different Virulent Strains and the Effects of Vaccination for SARS-CoV-2. Vaccines 2022, 10, 343. [Google Scholar] [CrossRef]
- U.S. Department of Health & Human Services (HHS). Biden-Harris Administration Secures 66 Million Doses of Moderna’s Variant-Specific COVID-19 Vaccine Booster for Potential Use in Fall and Winter 2022. Released 08/29/22. Available online: https://www.hhs.gov/about/news/2022/07/29/biden-harris-administration-secures-66-million-doses-modernas-variant-specific-covid-19-vaccine-booster-for-potential-use-in-fall-winter-2022.html (accessed on 8 August 2022).
- Lee, B.Y. Is Omicron BA.5 ‘Worst Version’ of Covid-19 Coronavirus Seen? Forbes. 07/03/22. Available online: https://www.forbes.com/sites/brucelee/2022/07/03/is-omicron-ba5-worst-version-of-covid-19-coronavirus-seen (accessed on 9 August 2022).
- Zhang, W.; Liu, S.; Osgood, N.; Zhu, H.; Qian, Y.; Jia, P. Using simulation modelling and systems science to help contain COVID-19: A systematic review. Syst. Res. Behav. Sci. 2022. [Google Scholar] [CrossRef]
- Sachs, J.D.; Karim, S.S.A.; Aknin, L.; Allen, J.; Brosbøl, K.; Colombo, F.; Barron, G.C.; Espinosa, M.F.; Gaspar, V.; Gaviria, A.; et al. The Lancet Commission on lessons for the future from the COVID-19 pandemic. Lancet 2022. [Google Scholar] [CrossRef]
- Mansouri, M.A.; Garcia, L.; Kee, F.; Bradley, D.T. Systems-oriented modelling methods in preventing and controlling emerging infectious diseases in the context of healthcare policy. Systems, 2022; in print. [Google Scholar]
- Squazzoni, F.; Polhill, J.G.; Edmonds, B.; Ahrweiler, P.; Antosz, P.; Scholz, G.; Chappin, É.; Borit, M.; Verhagen, H.; Giardini, F.; et al. Computational Models That Matter During a Global Pandemic Outbreak: A Call to Action. J. Artif. Soc. Soc. Simul. 2020, 23. [Google Scholar] [CrossRef] [Green Version]
- Voinov, A.; Shugart, H.H. ‘Integronsters’, integral and integrated modeling. Environ. Model. Softw. 2013, 39, 149–158. [Google Scholar] [CrossRef]
- Shroeder, S.A.; Vendome, C.; Giabbanelli, P.J.; Montfort, A.M. Towards reusable building blocks to develop COVID-19 simulation models. In Proceedings of the 2022 Winter Simulation Conference, Singapore, 11–14 December 2022. [Google Scholar]
- Dou, K.; Yang, J.; Wang, L.-X.; Li, J.-B. Theory of planned behavior explains males’ and females’ intention to receive COVID-19 vaccines differently. Hum. Vaccines Immunother. 2022, 2086393. [Google Scholar] [CrossRef]
- Jelnov, A.; Jelnov, P. Vaccination policy and trust. Econ. Model. 2022, 108, 105773. [Google Scholar] [CrossRef]
- Lastrucci, V.; Collini, F.; Forni, S.; D’Arienzo, S.; Di Fabrizio, V.; Buscemi, P.; Lorini, C.; Gemmi, F.; Bonaccorsi, G. The indirect impact of COVID-19 pandemic on the utilization of the emergency medical services during the first pandemic wave: A system-wide study of Tuscany Region, Italy. PLoS ONE 2022, 17, e0264806. [Google Scholar] [CrossRef]
- Bazaid, A.S.; Barnawi, H.; Qanash, H.; Alsaif, G.; Aldarhami, A.; Gattan, H.; Alharbi, B.; Alrashidi, A.; Abu Al-Soud, W.; Moussa, S.; et al. Bacterial Coinfection and Antibiotic Resistance Profiles among Hospitalised COVID-19 Patients. Microorganisms 2022, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, S.; Wei, X.; Dong, Q.; Xu, N.; Li, H.; Zhao, J.; Sun, Q. Global prevalence, treatment and outcome of tuberculosis and COVID-19 coinfection: A systematic review and meta-analysis (from November 2019 to March 2021). BMJ Open 2022, 12, e059396. [Google Scholar] [CrossRef] [PubMed]
- Ojo, M.M.; Benson, T.O.; Peter, O.J.; Goufo, E.F.D. Nonlinear optimal control strategies for a mathematical model of COVID-19 and influenza co-infection. Phys. A Stat. Mech. Its Appl. 2022, 607, 128173. [Google Scholar] [CrossRef] [PubMed]

| Handling of Immunity | ||||
|---|---|---|---|---|
| Ref | Variants | Natural | Vaccine | Hybrid |
| [35] | Delta variant only | Lasts 180 days | Impact on 5 parameters (e.g., death, infection, hospitalization, transmission, asymptomatic) defined via piecewise linear functions. Peak reached 2 weeks after one shot, remains constant for 8 months, then linear decay for 6 months. The level of immunity for each linear segment depended on the vaccine used. Booster restores peak vaccination benefit in 1 day. | Exists but unspecified |
| [36] | Emerge ahead of the winter, every 6 or 12 months | Initial peak at 95%, exponential decay to 20% in 600 days | Initial peak at 85%, exponential decay to 15%, half-life 105 days. Higher peak after a booster, but same decay. | None |
| [57] | Randomly appear 4/6/10 months after past variant | 2-part exponential decay, with half-life and duration parameters fit to data. Neutralization level depends on variant and vaccine. | None | |
| [58] | Omicron variant only | Gamma distribution set either to 9 months (shape 7 and scale 39.11) or one year (shape 3.7 and 98.65). | None | |
| [59] | Omicron variant only | Protection against same variant has exponential duration of mean 1/900, then no protection | After an average of 6 months since the second dose or a booster, individuals transition into a ‘waned vaccine effectiveness’ status. | Exists but unspecified |
| [55] | Unspecified (2021 data) | Delayed gamma-distributed temporary immunity with mean 350 (for vaccines) and 242 days (for recovery) | None | |
| [56] | Delta variant only | Full immunity for entire duration of simulation (March 2020–Nov. 2021) | 10% of individuals do not lose immunity, 10% have little protection, the remaining 80% get temporary protection by moving through a series of compartments instead of a single one (which would cause an exponential decay) hence using a Gamma distribution with peak efficacy of 92% and decay to 35% over 6 months | None |
| Determinant | Category | Get Vaccine | Effect Strength | References |
|---|---|---|---|---|
| Age | 18–29 | No | Strong | [77,78] |
| 30–49 | Yes | [79] | ||
| 50–64 | Yes | |||
| 65+ | Yes | |||
| Sex | Male | No | [77] | |
| Ethnicity | Non-Hispanic White | Yes | [79] | |
| Non-Hispanic Black | No | [77,80,81] | ||
| Hispanic | No | |||
| Asian/Pacific Islander | Yes | [80] | ||
| Other | No | [79,80] | ||
| Employment | Unemployed | Yes | Weak | [77] |
| Employed (full or part time) | No | Strong | ||
| Education level | Bachelor’s degree or more | Yes | [79,80,82] | |
| No college degree | No | [80,82] | ||
| Annual income | <25,000 | No | [81] | |
| ≥70,000 | Yes | [80] | ||
| Comorbidities | Yes | Yes | ||
| Political party | Republican | No | [79] | |
| Democrat | Yes | |||
| Prior COVID-19 infection | Yes | Yes | [77] | |
| Know someone who died | Yes | Yes | ||
| Religion | Catholic | No | Weak |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beerman, J.T.; Beaumont, G.G.; Giabbanelli, P.J. A Scoping Review of Three Dimensions for Long-Term COVID-19 Vaccination Models: Hybrid Immunity, Individual Drivers of Vaccinal Choice, and Human Errors. Vaccines 2022, 10, 1716. https://doi.org/10.3390/vaccines10101716
Beerman JT, Beaumont GG, Giabbanelli PJ. A Scoping Review of Three Dimensions for Long-Term COVID-19 Vaccination Models: Hybrid Immunity, Individual Drivers of Vaccinal Choice, and Human Errors. Vaccines. 2022; 10(10):1716. https://doi.org/10.3390/vaccines10101716
Chicago/Turabian StyleBeerman, Jack T., Gwendal G. Beaumont, and Philippe J. Giabbanelli. 2022. "A Scoping Review of Three Dimensions for Long-Term COVID-19 Vaccination Models: Hybrid Immunity, Individual Drivers of Vaccinal Choice, and Human Errors" Vaccines 10, no. 10: 1716. https://doi.org/10.3390/vaccines10101716
APA StyleBeerman, J. T., Beaumont, G. G., & Giabbanelli, P. J. (2022). A Scoping Review of Three Dimensions for Long-Term COVID-19 Vaccination Models: Hybrid Immunity, Individual Drivers of Vaccinal Choice, and Human Errors. Vaccines, 10(10), 1716. https://doi.org/10.3390/vaccines10101716







