1. Introduction
The pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to sweep the world and has resulted in extremely high morbidity (over 4.15 billion cases) and significant mortality (over 6.49 million deaths) [
1]. Intensive efforts have been made in developing vaccines that have been shown to provide significant protection against COVID-19 in patients with symptomatic to severe illness [
2]. However, the development of vaccines coincided with the emergence of variants of concern (VOC), including B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), B.1.617.1 (Kappa), B.1.617.2 (Delta), B.1.1.529 (Omicron), C.37 (Lambda), and B.1.621 (Mu) [
3]. Some of these variants have been reported to reduce the efficiency of currently licensed vaccines [
4,
5,
6], which could accelerate the spread of SARS-CoV-2. Noteworthily, new VOCs are arising, and transmissibility may increase with some VOCs [
7,
8]. Therefore, it is imperative to develop more effective and broad-spectrum vaccines to ensure the efficient control of such a public health crisis.
To date, diverse SARS-CoV-2 vaccine candidates have been developed by using multiple platforms, such as viral vectors, protein subunits, inactivated virus, live-attenuated virus, mRNA, and DNA [
9]. Of these, the mRNA technology has gained increasing interest due to various advantages, such as acceptable safety, higher efficacy, and most rapid development. The SARS-CoV-2 spike (S) protein has been thought to be one of the most essential targets for mRNA vaccine design [
10]. The S protein is a trimeric transmembrane protein on the viral envelope, which comprises the S1 and S2 subunits required for host receptor binding and fusion, respectively [
11]. Earlier studies on SARS-CoV and MERS-CoV demonstrated that the immune responses induced by the S protein played a significant role in antiviral immune response [
12,
13]. Strong binding of SARS-CoV-2 receptor-binding domain (RBD) to the ACE2 receptor resulted in a higher infection rate compared with SARS-CoV and MERS-CoV [
10]. Furthermore, collective studies suggested that mutations in the S protein was associated with increased replication and transmission of SARS-CoV-2 [
13]. Therefore, developing mRNA vaccines against the SARS-CoV-2 S protein could have great potential to reduce viral infection and combat its rapid spreading in the human population. Since December 2020, several mRNA vaccines encoding the SARS-CoV-2 S protein have been validated for use by the WHO, including BNT162b2 (Pfizer), mRNA-1273 (Moderna), and NVX-CoV2373 (Novavax). There are also several mRNA vaccine candidates encoding full-length S protein or the RBD under phase I/II/III clinical trials, such as CVnCoV, ARCoV, and BNT162 [
14].
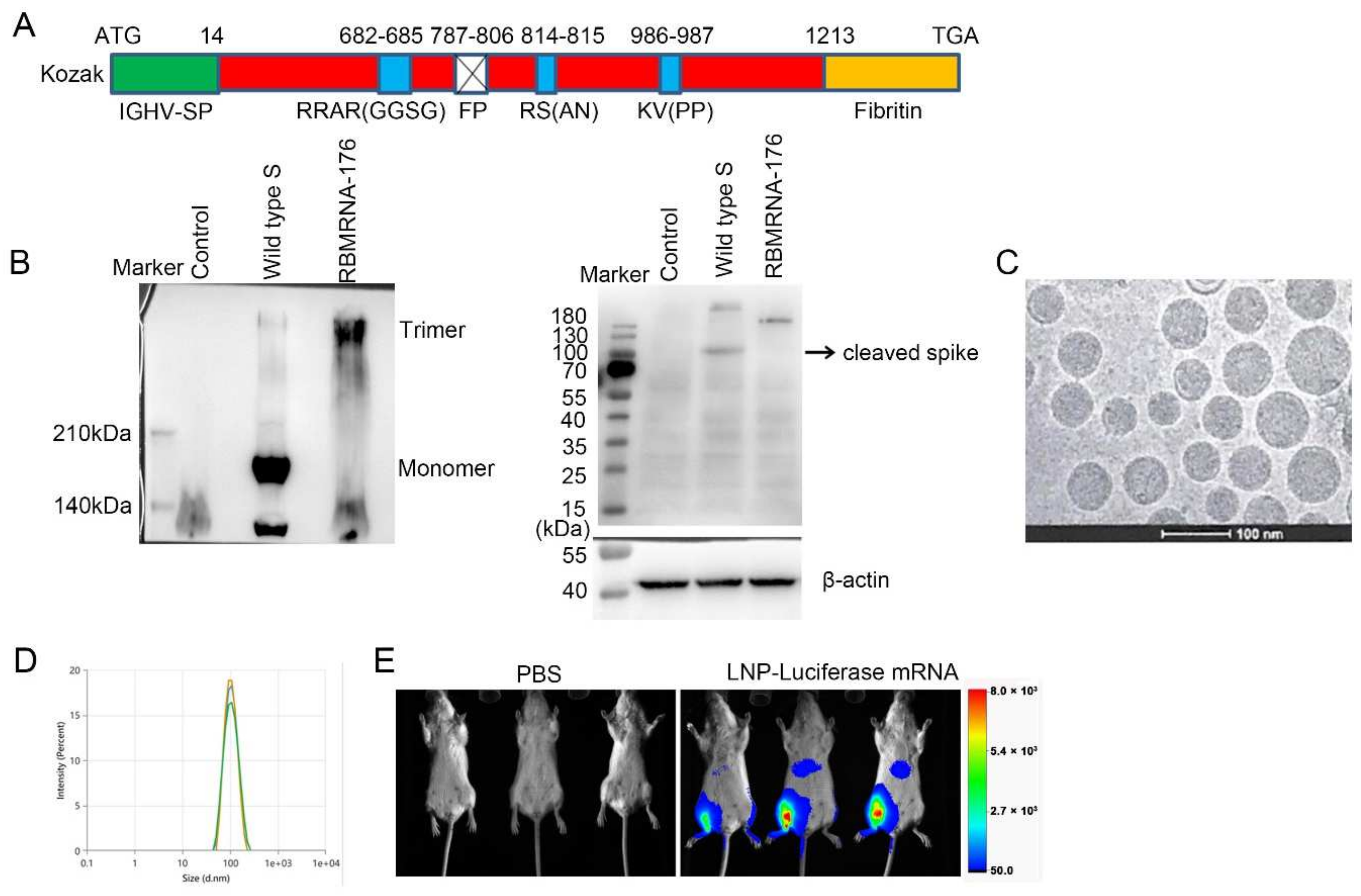
In the present study, we report the preclinical development of RBMRNA-176, an LNP-formulated pseudouridine (Ψ) nucleoside-modified mRNA vaccine that encodes the S ectodomain trimer with a two-proline mutation (K986P and V987P). This mutation strategy could stabilize the S protein in its prefusion conformation and prevent protease cleavage at S1/S2 and S2’ cleave sites. The wild-type signal peptide of the S protein was reported to be swapped with a signal peptide from human immunoglobulin heavy chain variable region (IGHV) for improved S post-translational translocation [
15,
16]. The fusion peptide was deleted to further reduce the cell fusion. The Ψ-modification could resist ribonuclease (RNase) degradation [
17], reduce activation of TLRs [
18] and PKR [
19], and, together with optimized non-coding sequence elements, enhance in vitro translational efficacy. The pre-fusion format of the S timer is the predominate form on the virion surface and is desirable for vaccine development due to the preservation of most or all neutralization-sensitive epitopes [
20,
21,
22,
23]. We then determined the immunogenicity of RBMRNA-176 and its ability to protect mice and nonhuman primates from SARS-CoV-2 infection. Our findings indicate the potential of an effective mRNA vaccine encoding viral S protein against COVID-19.
2. Materials and Methods
2.1. Cells and Virus
African green monkey kidney epithelial (Vero E6) cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM, GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) at 37 °C. The SARS-CoV-2 virus (GISAID accession no. EPI_ISI_402124) used in the studies of mice and nonhuman primates were grown in Vero E6 cells, and viral titer was determined by plaque assay or 50% tissue culture infective dose (TCID50). TCID50 was calculated by the Reed–Muench method. All experiments using an infectious virus were performed in a biosafety level-3 (BLS-3) laboratory.
2.2. mRNA Synthesis and LNP Formulation
The RBMRNA-176 mRNA was synthesized in vitro by T7 polymerase-mediated transcription from a linearized DNA template, where the UTP was substituted with pseudoUTP. Capped mRNAs were generated by supplementing the transcription reactions with 10 mmol RIBO Cap1. mRNA was purified by reversed-phase high-performance liquid chromatography (RP-HPLC) as previously reported [
24]. Lipid nanoparticles were prepared by microfluidic mixing using the previously described method [
25]. Briefly, four lipids were dissolved in ethanol at molar ratios of 50:38.5:10:1.5 (ionizable lipid: holesterol:DSPC:DMG-PEG2000). The lipid mixture was rapidly combined with a buffer of 50 mM sodium citrate (pH 4.0) containing mRNA at a volume ratio of aqueous:ethanol using a microfluidic mixer (PNI Nanosystems, Vancouver, BC, Canada). Formulations were dialyzed against PBS (pH 7.2) in the dialysis cassettes (Thermo Scientific, Rockford, IL, USA) for at least 18 h. Formulations were diluted with PBS (pH 7.2) to reach a required concentration, and then passed through a 0.22-μm filter and stored at 4 °C until use. Formulations were analyzed for particle size, mRNA encapsulation, residues, endotoxin, and bioburdens.
2.3. Cryo-EM Sample Preparation
3–5 μL of the sample was dispensed on a plasma-cleaned grid in the Vitrobot chamber at 95% relative humidity and allowed to rest for 30 s. The grid was blotted with filter paper and plunged into liquid ethane cooled by liquid nitrogen. The frozen grids were then checked for visible defects and assembled into cassettes. Cryo TEM acquisition was performed at 200 kV using Falcon III and K3 cameras with DED Talos F200C (Fisher Scientific, Waltham, MA, USA).
2.4. Particle Size
Particle size was measured in triplicate and reported as Z-average diameter using dynamic light scattering (DLS) on a ZETASIZER (Malvern Instruments Ltd., Westborough, MA, UK). RBMRNA-176 was diluted with PBS buffer and filtered through a 0.22-μm membrane-pore diameter, shortly before acquiring its size distribution. The sample parameters used the following settings: Material Name: liposome, Cell Name: DTS0012, Dispersant Name: PBS. The temperature was maintained at 25 °C during the measurements. DLS values are the average of 10 distinct scans for 10 acquisitions on a given sample. The ZS XPLORER Software (V1.3.1.7, Malvern Panalytical, Westborough, MA, USA) was used to analyze the light scattering data.
2.5. Cell Transfections
10 μg of flag-tagged RBMRNA-176 mRNA or flag-tagged wild-type S mRNA was lipoplexed with 15 µL riboFECTTM mRNA transfection Reagent (C11055, Ribobio, Guangzhou, China), brought to a volume of 1 mL with Opti-MEM Reduced Serum Medium (Thermo Fisher Scientific, Waltham, MA, USA). After incubation for 5 min at room temperature, 1 mL of the mixture was added to 5 × 106 HEK293T cells. The cells were cultured for 48 h at 37 °C after transfection.
2.6. Native Western Blot
Cells were collected and lysed in RIPA Lysis Buffer (R0030, Solarbio, Beijing, China) and centrifugated at 12,000× g. The supernatant was collected for native PAGE and immunoblotting. Native PAGE (6%) was performed according to the same procedure as SDS PAGE with the following modifications: the protein preparations were not boiled before electrophoresis, SDS and b-mercaptoethanol were omitted from the gels, and Tris-HCl glycine (375 mM, 0.1% SDS, pH 8.3) was used as the running buffer. After electrophoresis, the samples were transferred onto a nitrocellulose membrane using a Trans-Blot SD Semi-dry transfer cell (BioRad, Hercules, CA, USA) for 2 h at 25 V. Membranes were blocked with 5% BSA in PBS pH 7.0 and incubated overnight with anti-DDDDK Tag antibody (ab1162, 1:2500) at 4 °C. After that, the membranes were washed three times with PBS-T (0.05% Tween-20, Sigma, Shanghai, China) and incubated with HRP-conjugated Goat Anti-Rabbit (IgG) secondary antibody (ab6721, 1:4000, Abcam, Cambridge, UK) for 1 h at room temperature. Thereafter, the membranes were washed as described above, and developed with ECL reagent (P0018AS, Beyotime, Shanghai, China) using Tanon 5200 Chemiluminescent Imaging System (Tanon Science & Technology Co., Ltd., Shanghai, China).
2.7. Bioluminescence Imaging Studies
Luciferase mRNA was synthesized in vitro by T7 polymerase and encapsulated using the same procedure as RBMRNA-176. 6–8-week-old female BALB/c mice (n = 3) were intramuscularly injected with 5 μg LNP-encapsulated luciferase mRNA. 3 h after administration, animals were anesthetized with 2–3% isoflurane and injected intraperitoneally (i.p.) with luciferase substrate (0000402802, Promega, Madison, WI, USA), followed by a reaction for 5 min. Fluorescence signals were collected by In-Vivo Xtreme System (XTREME, Bruker BioSpin Corp., Billerica, MA, USA) for 120 s, and the fluorescence signals in regions of interest (ROIs) were quantified using Bruker MI SE (v7.1.2.20435, Billerica, MA, USA).
2.8. Vaccination and SARS-CoV-2 Challenge in Animals
In the studies of immunogenicity in the mouse model, BALB/c, C57BL/6J, and B6C3F1 mice experiments were performed in strict accordance with the requirements of Guangdong experimental animal management regulations and the Institutional Animal Care and Use Committee (IACUC Approval No.: IACUC-2020-014, 016, 017). The 6- to 8-week-old female mice were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. and housed in an SPF Laboratory Animal Facility under a 12 h light/dark cycle, with free access to food and water. For the assessment of humoral immune responses, BALB/c mice were vaccinated with three doses of RBMRNA-176 (1, 5, or 20 μg) at an interval of 21 days. At week 2 after the second dose, the serum (n = 6) was collected to determine specific IgG responses to SARS-CoV-2 wild-type and Omicron S protein. At this time point, serum from mice (n = 3) vaccinated with 20 μg RBMRNA-176 was also selected for determination of pseudovirus neutralization against S proteins of wild-type, Alpha, Beta, Gamma, and Kappa variants. In another experiment, mice were vaccinated with 4, 20 or 50 μg RBMRNA-176 at the same interval. At week 2 after the second shot, serum (n = 3) was collected and subjected to pseudovirus neutralization assay using SARS-CoV-2 Omicron S protein. At week 4, the serum (n = 4) was also used to determine pseudovirus neutralization against wild-type S protein. In addition, neutralization of SARS-CoV-2 live virus was also determined at week 24 by using serum from mice (n = 4) vaccinated with 5 μg RBMRNA-176. For the measurement of Th1 immune responses, 3 species of mice were administered intramuscularly a prime-boost immunization with RBMRNA-176 (1, 5, or 20 μg) at intervals of 21 days. For negative control, PBS was used following the same experimental scheme. On day 7 after a single dose immunization, the spleens (n = 3) were harvested to access T cell response by cytokine ELISPOT. At week 2 after the booster shot, the serum (n = 3) was collected to determine antibody titer by ELISA.
We further evaluated the immunogenicity of RBMRNA-176 in rhesus macaques. Nonhuman primate study was performed in strict accordance with the guidelines set by the Animal Care and Use Committees of the Wuhan Institute of Virology (WIV), Chinese Academy of Sciences (CAS) (Approval No.: WIVA42202002-01). Briefly, 3- to 5-year-old rhesus macaques purchased from Topgene Biotechnology Co., Ltd were randomized into low-dose (0.1 mg) and high-dose (0.3 mg) RBMRNA-176 groups (n = 2 males and n = 2 females per group) and vaccinated at a 21-day prime-boost interval. From day 7 to day 119 after the first shot, the serum was collected once every 7 days to measure anti-SARS-CoV-2 S protein IgG titer.
In SARS-CoV-2 challenge studies, the K18-hACE2 transgenic mice were used and the experiments were performed in accordance with protocols approved by the Animal Care and Use Committee of Guangzhou Medical University (Acceptance number: 2018-297). Briefly, male K18-hACE2 transgenic mice, 6- to 7-weeks-old, were vaccinated with 5 or 20 μg of RBMRNA-176 following a 21-day prime-boost interval regimen. On week 4 after the second shot, mice were intranasally challenged with 5 × 104 PFU of SARS-CoV-2. At 3 and 5 days post infection (dpi), the left lung and spleens were collected for histopathological examination, while the right lungs were collected to determine the viral titer using RT-qPCR assay. For macaques challenge studies, animals were divided into groups treated with placebo, low- and high-dose of RBMRNA-176 (n = 4 males and n = 4 females per group). On day 143–145 post-vaccination, macaques were intratracheally infected with 7 × 104 PFU of SARS-CoV-2 in 4 mL. Nasal swabs and throat swabs were collected once daily from 1 dpi to 7 dpi. At 7 dpi, macaques were sacrificed and the lungs from 4 animals per group were collected, homogenized, and subjected to RT-qPCR detection of viral S gene expression. The lungs from the remaining 4 animals were collected for histopathologic evaluation.
2.9. ELISA Assay for Total IgG and IgG Subclass
For determination of the mouse total IgG, IgG1, and IgG2c, 96-well plates (JET BIOFIL, Guangzhou, China) were coated with 2 μg/mL recombinant SARS-CoV-2 spike trimer protein (SPN-C52H4, ACROBiosystems, Beijing, China) or recombinant SARS-CoV-2 Omiron spike trimer protein (SPN-C52Hz, ACROBiosystems) diluted in carbonate buffer (0.05 M, pH 9.6) and incubated at 4 °C overnight. The plates were washed with PBS-T (0.05% Tween-20) 3 times and were blocked with 1% BSA in PBS for 1–2 h at 37 °C. Next, the plates were washed and heat-inactivated serum serially diluted in PBS-T. 100 μL/well diluted serum samples were added and incubated for 1 h at 37 °C. After washing plates three times with PBS-T, HRP-conjugate Goat anti-mouse IgG (H+L) (SA00001-1, 1:1000, Proteintech, Chicago, IL, USA), HRP-conjugated Goat anti-Mouse IgG1 (A90-105P, 1:5000, Bethyl Laboratories, Inc, Montgomery, TX, USA), HRP-conjugated Goat anti-Mouse IgG2a (1:5000, Bethyl Laboratories, A90-107P) or HRP-conjugated Goat anti-Mouse IgG2c (1:5000, Bethyl Laboratories, A90-136P) was added and incubated for 1 h at 37 °C. Plates were then washed and 100 μL of TMB peroxidase substrate (PA107-02, TIANGEN, Beijing, China) was added to each well for development. After 15 min, plate development was halted by the addition of 2 mol H2SO4 and the absorbance was read at 450 nm using TECAN Infinite M200 pro (Tecan, Männedorf, Switzerland). Endpoint titers were calculated as the highest serum dilution that exceeded the cut-off values (mean ± 3SD of negative controls at the lowest dilution).
For the detection of macaque IgG, 96-well microplates were coated with 4 μg/mL of SARS-CoV-2 S protein (DRA49, Novoprotein, Suzhou, China), then rinsed with PBS-T and blocked using 1% BSA blocking buffer. Serial diluted serum samples were then added to the coated plates and incubated at room temperature for 2 h. After washing with PBS-T, the HRP-conjugated mouse anti-monkey IgG (RS2140, 1:10,000, Immunoway, Plano, TX, USA) was added and incubated at room temperature for 1 h. Plates were washed again with PBS-T, which was followed by the addition of TMB peroxidase substrate (TIANGEN, PA107-02). After incubation for 30 min, the reaction was terminated with 2 mol H2SO4. Plates were read at OD 450 nm using a BIORAD iMark microplate absorbance reader. Endpoint titers were calculated as the highest serum dilution that OD value exceeded 0.01.
2.10. Mouse ELISPOT Assay
Mouse ELISPOT assays were performed with Mouse IL-4 precoated ELISPOT kit (2210005, Dakewe Biotech, Shenzhen, China), Mouse IL-5 ELISpotPLUS (HRP) (3391-4HPW-2, Mabtech, Nacka Strand, Sweden), Mouse IL-2 ELISpotPLUS (HRP) (Mabtech, 3441-4HPW-2), Mouse IFN-γ precoated ELISPOT kit (2210005, Dakewe Biotech, Shenzhen, China) according to the manufacturer’s instructions. Briefly, the plates were blocked using RPMI 1640 (GIBCO) containing 10% FBS and incubated for 10 min at room temperature. Spleen lymphocytes were isolated from BALB/c mice 7 days after the first vaccination and plated at 2 × 105 cells/well. Lymphocytes were stimulated with 1 μg SARS-CoV-2 Spike trimer protein (SPN-C52H4, ACROBiosystems, Beijing, China) and cultured for 20 h (3 °C, 5% CO2). The plates were washed 6 times with wash buffer and incubated for 1 h with biotinylated anti-mouse IL-4/IL-5/IL-2/IFN-γ antibody. The plates were washed 6 times and incubated for 1 h with Streptavidin-HRP. The final wash was followed by the addition of AEC substrate solution for 10 min. The chromagen was discarded and the plates were washed with water and dried in a dim place. Spot numbers were evaluated using ELISpotreader.
2.11. Flow Cytometric Analysis of T Cells and Intracellular Cytokine
BALB/c mice were vaccinated with two shots of 20 μg RBMRNA-176 at an interval of 21 days. At 7 days post-boost, splenocytes were isolated. A total of 1 × 106 splenocytes (100 μL) were seeded per well into 96-well plates and incubated with 1μg SARS-CoV-2 Spike trimer protein (SPN-C52H4, ACROBiosystems, Beijing, China) or 50 ng/mL phorbol myristate acetate (PMA) and 1 μg/mL ionomycin (Dakewe Biotech, 2030421) for 2 h at 37 °C. Next, Brefeldin A Solution (420601, BioLegend, San Diego, CA, USA) was added and incubated for 4 h at 37 °C. Unstimulated cells were used as a background control, and PMA/ionomycin stimulated cells were used as a positive control. After stimulation, splenocytes were washed twice with PBS and the Zombie Green™ Fixable Viability Kits (BioLegend, 423111) was used to determine the viability of cells prior to the fixation and permeabilization. Cells were then resuspended in FC buffer (PBS + 2% FBS). Cytokine production of T cells was evaluated by surface and intracellular cytokine staining using Cyto-Fast™ Fix/Perm Buffer Set (BioLegend, 426803). PerCP/Cyanine5.5 anti-mouse CD3 antibody (BioLegend, 100218), APC anti-mouse CD8a antibody (BioLegend, 100712), and APC anti-mouse CD4 antibody (BioLegend, 100412) were used for surface staining. PE anti-mouse/human IL-5 antibody (BioLegend, 504204), PE anti-mouse IL-2 antibody (BioLegend, 503808), PE anti-mouse IFN-γ antibody (BioLegend, 505808), and PE anti-mouse IL-4 antibody (BioLegend, 504104) were used for cytokine staining. Splenocytes were analyzed on Accuri C6 (BD Biosciences, San Jose, CA, USA). 1 × 105 events were collected per sample. Data were analyzed with FlowJo software. Cytokine expression in the unstimulated group was considered background and subtracted from the responses.
2.12. Lymphocyte Population
RBMRNA176-induced effects on the proliferation and dynamics of immune cell populations were evaluated using Accuri C6 (BD Biosciences). Briefly, 1 × 106 splenocytes (100 μL) were seeded per well into 96-well plates. Following two washes with PBS, the viability of cells was determined using Zombie Green™ Fixable Viability Kits (BioLegend, 423111) prior to the fixation and permeabilization. Next, splenocytes were permeabilized using Cyto-Fast™ Fix/Perm Buffer Set (BioLegend, 426803) and stained with PerCP/Cyanine5.5 anti-mouse CD3 (BioLegend, 100218), APC anti-mouse CD8a (BioLegend,100712), APC anti-mouse CD4 (BioLegend, 100412), PE anti-mouse CD62L (BioLegend, 161203), and APC anti-mouse/human CD44 (BioLegend, 103011). Data were analyzed with FlowJo software (v10, BD Biosciences, San Jose, CA, USA).
2.13. Pseudoviruses Neutralization Assay
The gene sequence of SARS-CoV-2 wild-type S protein (Genebank accession NO. 43740568) or variants, including Alpha (Genebank accession NO. ON442267.1), Beta (Genebank accession NO. ON322586.1), Gamma (Genebank accession NO. ON471228.1), Kappa (Genebank accession NO. OU322235.1), BA.1 (Genebank accession NO. OM287553.1) and BA. 2 (Genebank accession NO. OM617939.1) were codon-optimized for expression in human cells. A SARS-CoV-2 pseudoviruses neutralization assay was performed using a HIV-1 lentiviral packaging system following a similar approach that was reported previously [
26]. Plasmids expressing S protein and plasmids encoding luciferase-expressing lentivirus (pLV-Luc, pH 1) were co-transfected into HEK 293T cells using Lipofectamine 3000 Transfection Reagent (Thermo Fisher). Cell suspensions enriched with the pseudotype virus were harvested after 48 h and TCID
50 was measured. Pseudoviruses were stored at −80 °C.
Serial 3-fold diluted inactivated serum, starting at 1:10, were incubated with 300 TCID50 of the pseudovirus for 1 h at 37 °C in 96-well plates. DMEM was used as negative control and lentiviral SARS-CoV-2 pseudoviruses were used as positive control. After incubation, 4 × 104 293T-ACE2-p2A-mTagBFP2 cells (a cell line stably expressing ACE-2) were added and incubated for 48 h. Following this, cells were lysed, and luciferase signal was measured using GloMax® 96 Microplate Luminometer (Promega). The inhibition curve was determined by non-linear regression, i.e., log (inhibitor) vs. normalized response (variable slope) using GraphPad Prism 8.0 (GraphPad Software, Inc., San Diego, CA, USA) and IC50 titers were calculated using the Reed–Muench method.
2.14. Live Virus Neutralization Assay
Serum samples collected from immunized mice were inactivated at 56 °C for 30 min and serially diluted with DMEM medium (GIBCO) in two-fold steps. The diluted serums were mixed with 100 TCID50 SARS-CoV-2 live virus in 96-well plates at a ratio of 1:1 (v/v) and incubated at 37 °C for 1 h. Next, virus/serum mixtures were added to Vero-E6 cells monolayers in quadruplicate in 96-well plates and the plates were incubated for 3–5 days at 37 °C in a 5% CO2 incubator. The cytopathic effect (CPE) of each well was recorded under microscopes, and the 50% neutralization titer (NT50) was calculated.
2.15. RT-qPCR Assay
The total RNA from the supernatants of nasal swab, throat swab, and lung homogenization was extracted by using QIAamp Viral RNA mini-Kit (Qiagen, Hilden, Germany), and reverse transcription was performed by using the PrimeScript RT Master Mix kit (Takara Bio, Shiga, Japan). RT-PCR was then carried out with primers specific to the SARS-CoV-2 Orf1ab-N gene by using the SARS-CoV-2 Orf1ab-N Probe qRT-PCR Kit (15-81930, Tiandz, Inc., Beijing, China). RT-PCR was performed at the reaction conditions of 50 °C for 30 min, 94 °C for 10 min, followed by 40 cycles of 94 °C for 15 s, and 60 °C for 1 min. The resulting data were further analyzed by using the detection platform StepOne Real-Time PCR System (Applied Biosystems Co., Ltd., Foster City, CA, USA). Standard curves were generated by using serial ten-fold dilutions of control substance (from 1 × 108 copies/μL − 1 × 103 copies/μL). The viral load was expressed as copies/mL.
2.16. Histopathology
Mice lungs and spleens, as well as macaque lungs, were fixed in 4% paraformaldehyde and cut into 4 µm-thick paraffin sections, which was further stained with hematoxylin-eosin (HE) and captured by light microscopy (ECLIPSE NI-U, Nikon, Tokyo, Japan). Semiquantitative assessment of SARS-CoV-2-induced inflammation in 6 lobes of lungs from macaques was performed regarding the extent of pink-stained exudation in alveolar septum, inflammatory cell infiltration in perivascular and alveolar space, and the presence of interstitial pneumonia. The histopathology was scored as: 0, normal; 1, slight; 2, mild; 3, moderate; 4, severe.
2.17. Statistical Analysis
Statistical analysis of the virologic and immunologic data was performed using GraphPad Prism 8 software (GraphPad Software, Inc.). Values of p < 0.05 were considered as significant. The used statistical tests were the two-way ANOVA test with Tukey’s multiple comparisons test and non-linear regression model.
4. Discussion
In the present study, we designed a prefusion SARS-CoV-2 S RNA vaccine, RBMRNA-176, that expressed S ectodomain and stabilized the prefusion S trimer by introducing two-proline mutation (K986P and V987P). The two-proline mutation plays a key role in inducing neutralizing antibodies and has been adopted in the development of other licensed mRNA vaccines, such as Moderna mRNA-1273, Pfizer BNT162b and Novavax NVX-CoV2373 [
11,
27,
28]. In addition, studies showed that the Ψ nucleoside-modification resulted in a 10-fold increase in translation, and this processing did not activate pathogen-associated molecular pattern sensing machinery, thereby reducing redundant inflammation and, hence, vaccine-related adverse effects [
29,
30]. Such modification could improve the translatability and safety of RBMRNA-176 vaccine. A previous study by Y.-M. Kwon et al. reported that RSV F glycoprotein with fusion peptide deletion and cleavage site mutation induced higher levels of IgG antibodies and RSV-neutralizing activity titers, as well as efficient lung virus clearance [
31]. In contrast, recent studies showed that the fusion peptide was a candidate epitope site for pan-coronavirus-neutralizing antibodies [
32,
33]. It has also been suggested that mAbs with broader epitope coverage outside of the RBD exhibited higher protection from MERS-CoV and SARS-CoV infection [
34,
35]. Therefore, further efforts should be made to develop a fusion peptide-based vaccine with increased virus-neutralizing activity compared to RBMRNA-176.
We showed that RBMRNA-176 vaccination resulted in Th1-skewed response as determined by the production of Th-1 cytokines IFNγ and IL-2 by total CD4 T cells, which was similar to previous studies using Pfizer BNT162b2 and Moderna mRNA-1273 vaccines [
28,
36]. The Th1-skewed response is critical for viral control and is important for the development of SARS-CoV-2 vaccines [
37]. In contrast, Th2 polarization may be related to vaccine-associated enhanced respiratory disease (VAERD) [
37]. Thus, the Th1 bias induced by RBMRNA-176 vaccination may reduce the risk of unfavorable effects triggered by Th2 functional polarization. In addition, RBMRNA-176 elicited a significantly stronger IgG2a response than IgG1, and the ratio of IgG2a/IgG1 has been thought to reflect the balance of Th1- and Th2-response. It has also been shown that higher IgG2a titer contributes to macrophage-induced cell-mediated cytotoxicity (ADCC) effect and opsonophagocytosis, hence facilitating virus clearance [
38,
39]. The IgG response elicited by RBMRNA-176 vaccination appeared comparable to that induced by Pfizer BNT162b2 in mice, as determined by pseudovirus neutralization assays [
28]. The dynamic IgG response was also comparable across nonhuman primates vaccinated with RBMRNA-176 or Pfizer BNT162b2 at a dosage of 100 μg [
28]. Following vaccination with RBMRNA-176, the mice had increased IgG and mAb titers and a robust CD4 T cell response, but a negligible-to-undetectable CD8 T cell response in mice. Increased CD4 T cell response could possibly result in high IgG and mAb titer, whereas CD8 T cells have not been shown to be required for mAb titer or effective protection against SARS-CoV-2 infection via vaccination [
37,
40]. Therefore, the pronounced humoral immune response evoked by RBMRNA-176 is most likely attributable to CD4 T cell activation rather than CD8 T cells, which is consistent with the overall effect of mRNA-1273 and BNT162b2 against SARS-CoV-2 [
41]. However, the limitations of our work on RBMRNA-176 immunogenicity are that we did not determine cellular immune responses in macaques, evaluate the neutralization of SARS-CoV-2 wild type and variants with macaque serum, or compare the immunogenicity of wild-type S protein with RBMRNA-176.
According to our findings, RBMRNA-176 induces high levels of neutralizing antibodies against the wild-type virus and a range of VOCs, including Alpha, Beta, Gamma, and Kappa variants, although this vaccine exhibits slightly reduced (0.85- to 3.33-fold) neutralizing potency to VOCs compared to the wild type. Although the Omicron variant could escape from antibody neutralization induced by current vaccines, T cell epitopes were confirmed conserved in this variant and memory T cells may partially respond to its S protein [
42]. An mRNA vaccine also provided a long-term immune protection against SARS-CoV-2 in animal models [
8]. However, Omicron was found to be the most resistant VOC to neutralization based on the results obtained from mRNA-vaccinated individuals [
43]. Shan-Lu Liu et al. also demonstrated that immunity waned by 6 months after vaccination in human population and that minimal neutralizing antibody responses to the Omicron variant were observed at this time point [
44]. In addition, recent studies have shown that COVID-19 vaccines based on previous strains provided less protection against the Omicron variants, including BA.4 and BA.5, due to the escape from antibody response [
45,
46,
47]. L452R, F486V and F486V mutations contributed to BA.4/5 evasion of neutralizing antibodies [
45,
47]. K417N and E484A were two mutations in the RBD of Omicron that were thought to be the driving factors in vaccine breakthroughs [
46]. We have determined the cross-neutralization activity induced by RBMRNA-176 vaccination against BA.1 and BA.2 pseudoviruses and found a 15- and 12-fold reduction, respectively, as compared to the wild-type virus. These results indicate that Omicron BA.1 and BA.2 have substantial but incomplete escape of RBMRNA-176-induced neutralization; nevertheless, RBMRNA-176 induced-neutralization against these variants needs to be confirmed using live viruses.
The K18-hACE2 mice and nonhuman primates allowed us to study the protective effect of vaccines on respiratory disease resembling severe COVID-19. We showed that RBMRNA-176 vaccination could significantly neutralize an infectious virus in the lungs of mice as well as in the respiratory tract and lungs of macaques. Following the same vaccination strategy as RBMRNA-176, 5 μg BNT162b2 reduced viral titer to undetectable levels in all mice at 5 dpi [
48]; however, one of four mice immunized with RBMRNA-176 at this dose still showed virus growth at this time point. Similarly, two shots of Novavax NVX-CoV2373, Moderna mRNA-1273 or Pfizer BNT162b2 appeared to be more effective than RBMRNA-176 in neutralizing the virus in the lungs of mice and nonhuman primates [
11,
28,
49]. Furthermore, the in vivo protection of RBMRNA-176 vaccination against SARS-CoV-2 variant infection warrants further investigation.