Relationship between Anti-Spike Antibodies and Risk of SARS-CoV-2 Infection in Infants Born to COVID-19 Vaccinated Mothers
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. ELISA
2.3. Clinical Outcomes
2.4. Analysis
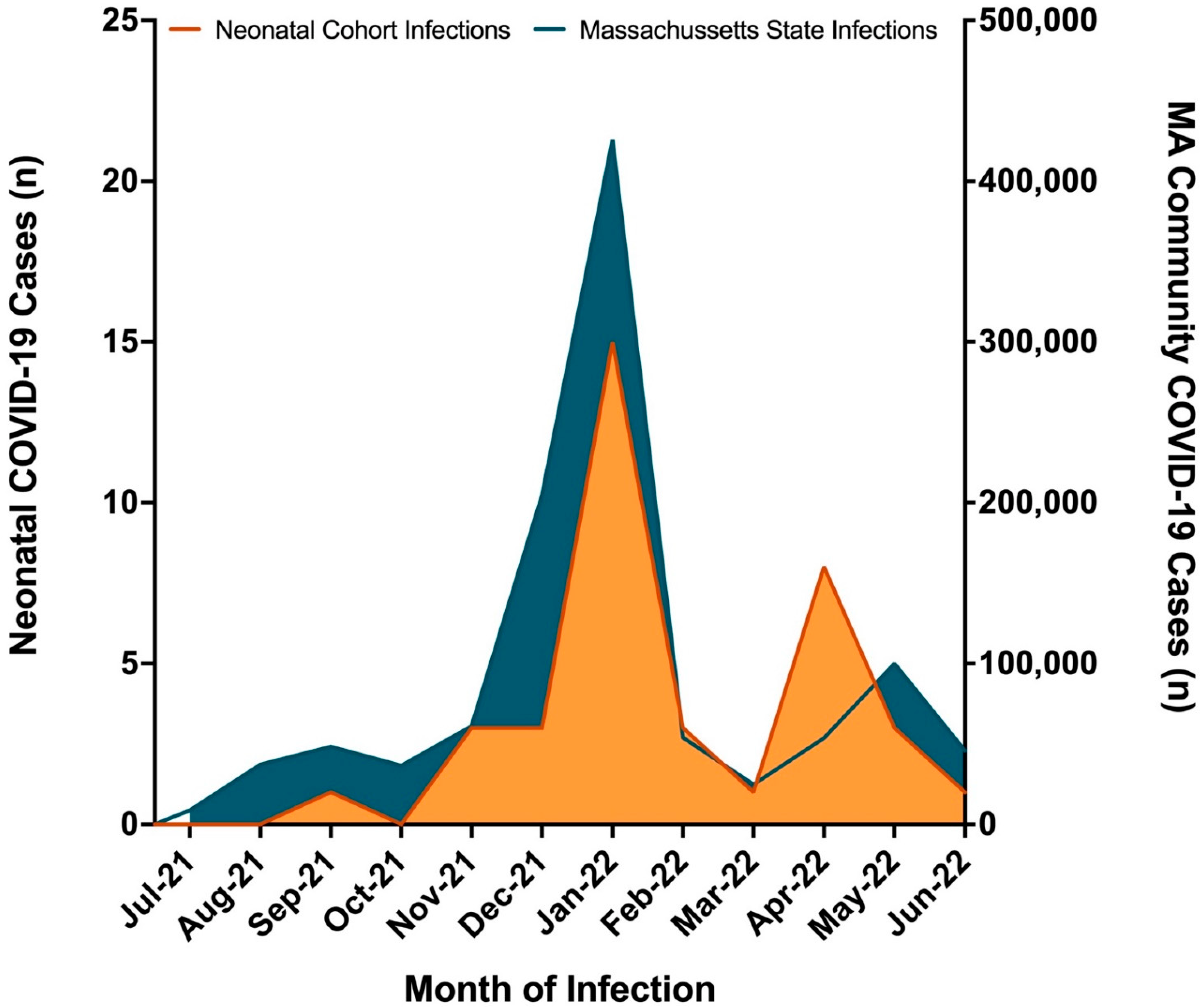
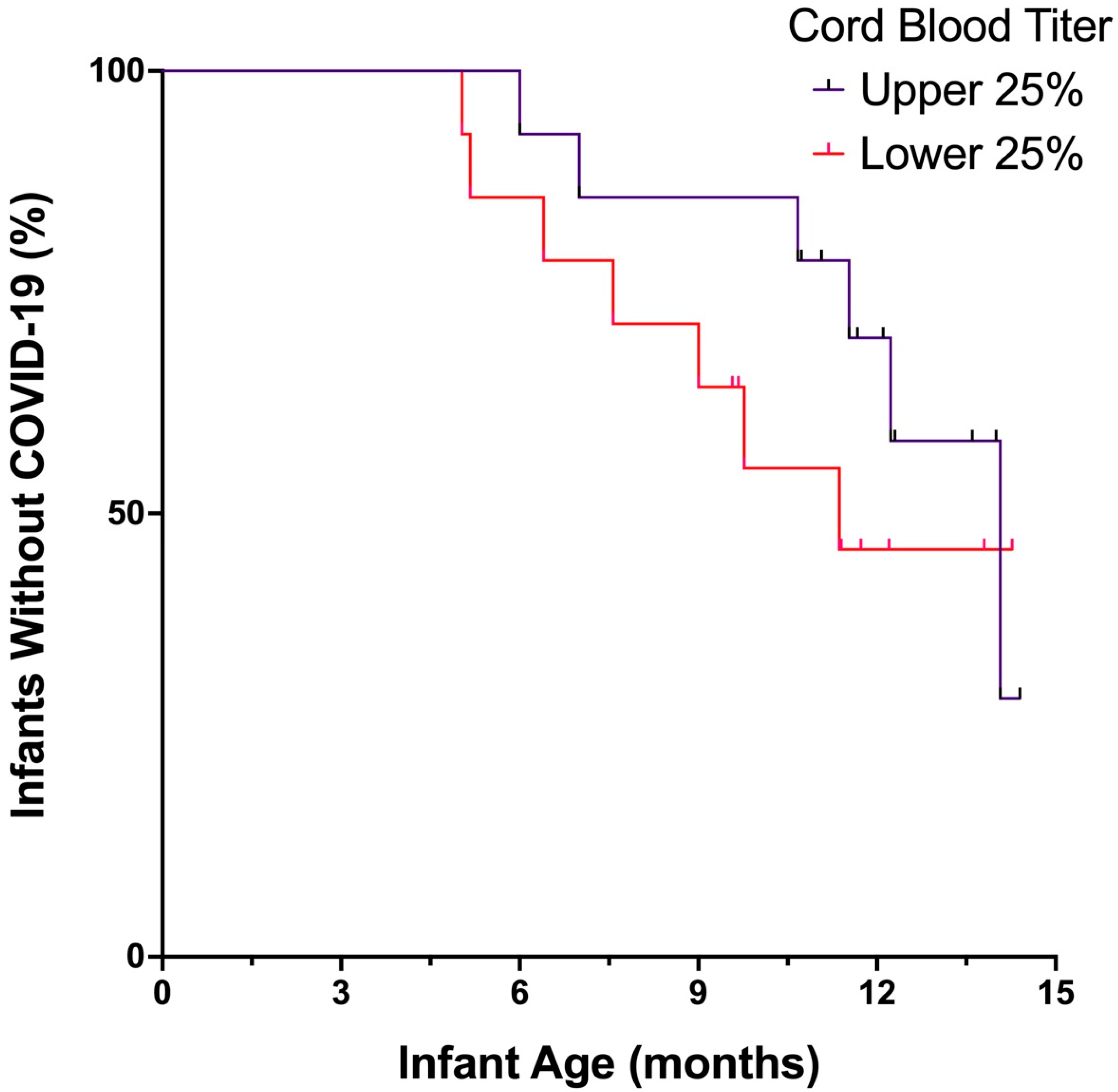
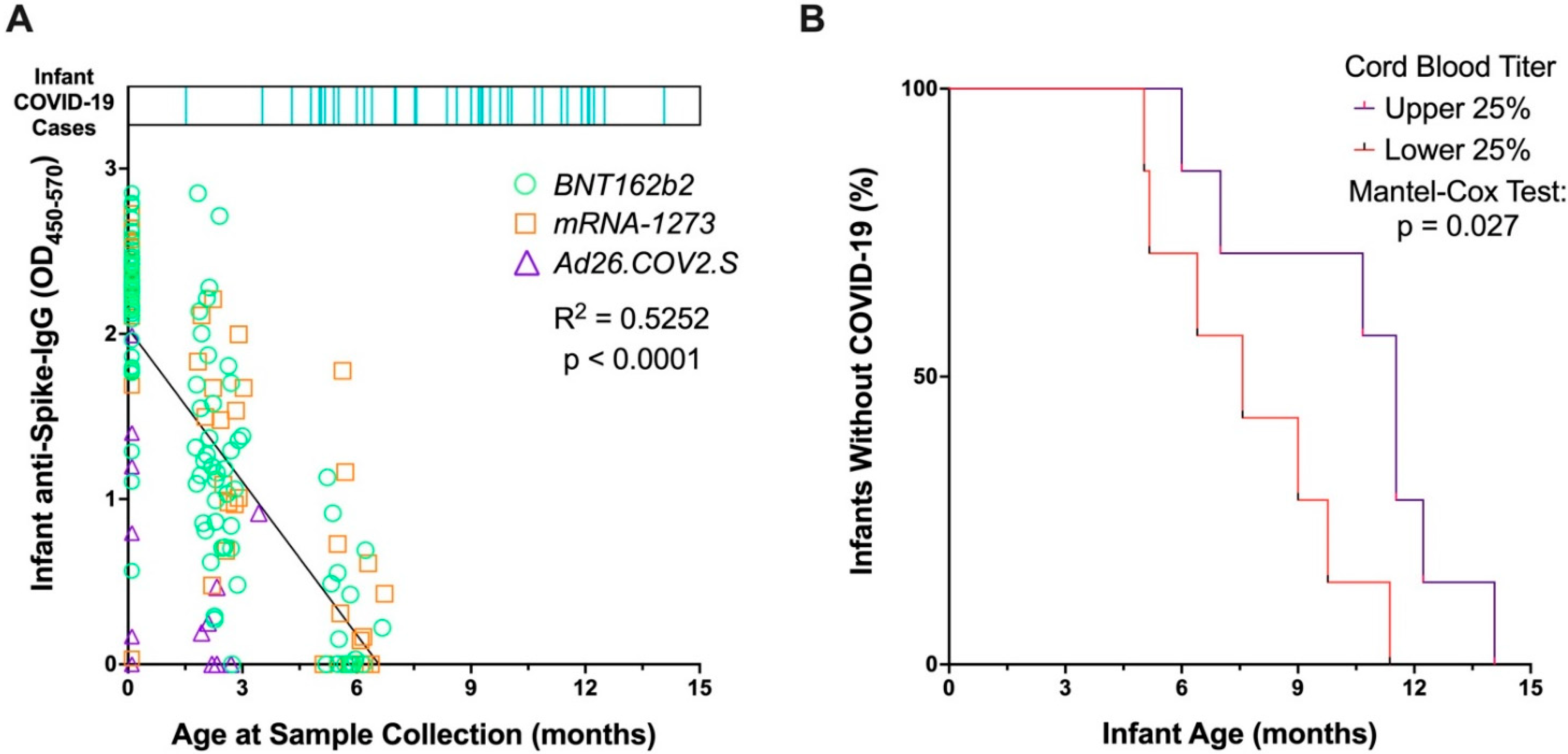
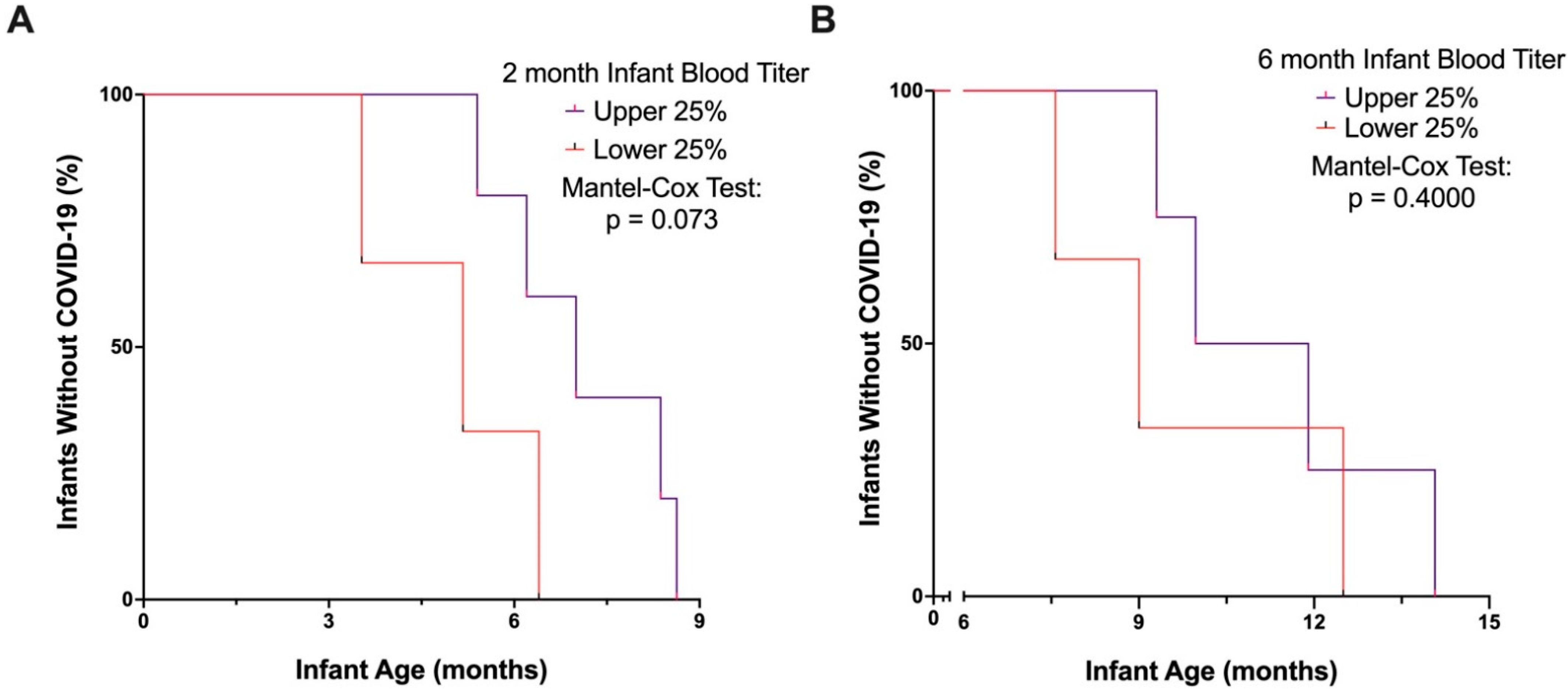
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Hobbs, C.V.; Woodworth, K.; Young, C.C.; Jackson, A.M.; Newhams, M.M.; Dapul, H.; Maamari, M.; Hall, M.W.; Maddux, A.B. Singh AR Frequency, Characteristics and Complications of COVID-19 in Hospitalized Infants. Pediatr. Infect. Dis. J. 2022, 41, e81–e86. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.; Quigley, M.A.; Placzek, A.; Knight, M.; Ladhani, S.; Draper, E.S.; Sharkey, D.; Doherty, C.; Mactier, H.; Kurinczuk, J.J. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: A prospective national cohort study using active surveillance. Lancet Child Adolesc. Health 2021, 5, 113–121. [Google Scholar] [CrossRef]
- Albrecht, M.; Arck, P.C. Vertically Transferred Immunity in Neonates: Mothers, Mechanisms and Mediators. Front. Immunol. 2020, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Mithal, L.B.; Otero, S.; Shanes, E.D.; Goldstein, J.A.; Miller, E.S. Cord blood antibodies following maternal coronavirus disease 2019 vaccination during pregnancy. Am. J. Obstet. Gynecol. 2021, 225, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Atyeo, C.G.; Shook, L.L.; Brigida, S.; De Guzman, R.M.; Demidkin, S.; Muir, C.; Akinwunmi, B.; Baez, A.M.; Sheehan, M.L.; McSweeney, E.; et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. Nat. Commun. 2022, 13, 3571. [Google Scholar] [CrossRef] [PubMed]
- Halasa, N.B.; Olson, S.M.; Staat, M.A.; Newhams, M.M.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Cameron, M.A.; Pannaraj, P.S.; Bline, K.E. Effectiveness of Maternal Vaccination with mRNA COVID-19 Vaccine During Pregnancy Against COVID-19-Associated Hospitalization in Infants Aged <6 Months-17 States, July 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 264–270. [Google Scholar]
- Halasa, N.B.; Olson, S.M.; Staat, M.A.; Newhams, M.M.; Price, A.M.; Pannaraj, P.S.; Boom, J.A.; Sahni, L.C.; Chiotos, K.; Cameron, M.A.; et al. Maternal Vaccination and Risk of Hospitalization for COVID-19 among Infants. N. Engl. J. Med. 2022, 387, 109–119. [Google Scholar] [CrossRef]
- Shook, L.L.; Atyeo, C.G.; Yonker, L.M.; Fasano, A.; Gray, K.J.; Alter, G.; Edlow, A.G. Durability of Anti-Spike Antibodies in Infants After Maternal COVID-19 Vaccination or Natural Infection. JAMA 2022, 327, 1087–1089. [Google Scholar] [CrossRef]
- Zohar, T.; Loos, C.; Fischinger, S.; Atyeo, C.; Wang, C.; Slein, M.D.; Burke, J.; Yu, J.; Feldman, J.; Hauser, B.M.; et al. Compromised Humoral Functional Evolution Tracks with SARS-CoV-2 Mortality. Cell 2020, 183, 1508–1519.e12. [Google Scholar] [CrossRef]
- Harvey, R.A.; Rassen, J.A.; Kabelac, C.A.; Turenne, W.; Leonard, S.; Klesh, R.; Meyer, W.A., 3rd; Kaufman, H.W.; Anderson, S.; Cohen, O.; et al. Association of SARS-CoV-2 Seropositive Antibody Test with Risk of Future Infection. JAMA Intern. Med. 2021, 181, 672–679. [Google Scholar] [CrossRef]
- Finch, E.; Lowe, R.; Fischinger, S.; de St Aubin, M.; Siddiqui, S.M.; Dayal, D.; Loesche, M.A.; Rhee, J.; Beger, S.; Hu, Y.; et al. SARS-CoV-2 antibodies protect against reinfection for at least 6 months in a multicentre seroepidemiological workplace cohort. PLoS Biol. 2022, 20, e3001531. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, L.D.; Newhams, M.M.; Olson, S.M.; Halasa, N.B.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Kamidani, S.; Tarquinio, K.M.; Maddux, A.B.; et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA Vaccination Against Multisystem Inflammatory Syndrome in Children Among Persons Aged 12–18 Years-United States, July–December 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Creech, C.B.; Anderson, E.; Berthaud, V.; Yildirim, I.; Atz, A.M.; Baez, I.M.; Finkelstein, D.; Pickrell, P.; Kirstein, J.; Yut, C.; et al. Evaluation of mRNA-1273 COVID-19 Vaccine in Children 6 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 2011–2023. [Google Scholar] [CrossRef] [PubMed]
- Marks, K.J.; Whitaker, M.; Agathis, N.T.; Anglin, O.; Milucky, J.; Patel, K.; Pham, H.; Kirley, P.D.; Kawasaki, B.; Meek, J.; et al. Hospitalization of Infants and Children Aged 0–4 Years with Laboratory-Confirmed COVID-19-COVID-NET, 14 States, March 2020–February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Recher, M.; Hubert, H.; Javouhey, E.; Fléchelles, O.; Leteurtre, S.; Angoulvant, F. Multisystem Inflammatory Syndrome in Children by COVID-19 Vaccination Status of Adolescents in France. JAMA 2022, 327, 281–283. [Google Scholar] [CrossRef]
- Lima, R.; Gootkind, E.F.; De la Flor, D.; Yockey, L.J.; Bordt, E.A.; D’Avino, P.; Ning, S.; Heath, K.; Harding, K.; Zois, J.; et al. Establishment of a pediatric COVID-19 biorepository: Unique considerations and opportunities for studying the impact of the COVID-19 pandemic on children. BMC. Med. Res. Methodol. 2020, 20, 228. [Google Scholar] [CrossRef]
- Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 8 August 2022).
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 225, 303.e1–303.e17. [Google Scholar] [CrossRef]
- Atyeo, C.; Pullen, K.M.; Bordt, E.A.; Fischinger, S.; Burke, J.; Michell, A.; Slein, M.D.; Loos, C.; Shook, L.L.; Boatin, A.A.; et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell 2021, 184, 628–642.e10. [Google Scholar] [CrossRef]
- Bordt, E.A.; Shook, L.L.; Atyeo, C.; Pullen, K.M.; De Guzman, R.M.; Meinsohn, M.C.; Chauvin, M.; Fischinger, S.; Yockey, L.J.; Jameset, K.; et al. Sexually dimorphic placental responses to maternal SARS-CoV-2 infection. bioRxiv 2021, 13, eabi7428. [Google Scholar]
- Atyeo, C.; Shook, L.L.; Nziza, N.; Deriso, E.A.; Muir, M.C.; Baez, M.A.M.; Lima, M.R.S.; Demidkin, S.; Brigida, M.S.; De Guzman, R.M.; et al. COVID-19 booster dose induces robust antibody response in pregnant, lactating, and nonpregnant women. Am. J. Obstet. Gynecol. 2022. [Google Scholar] [CrossRef]
- Juan, J.; Gil, M.M.; Rong, Z.; Zhang, Y.; Yang, H.; Poon, L.C. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: Systematic review. Ultrasound Obs. Gynecol. 2020, 56, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Stafford, I.A.; Parchem, J.G.; Sibai, B.M. The coronavirus disease 2019 vaccine in pregnancy: Risks, benefits, and recommendations. Am. J. Obstet. Gynecol. 2021, 224, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Biron-Shental, T.; Makov-Assif, M.; Key, C.; Kohane, I.S.; Hernán, M.A.; Lipsitch, M.; Hernandez-Diaz, S.; Reis, B.Y.; et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat. Med. 2021, 27, 1693–1695. [Google Scholar] [CrossRef] [PubMed]
- Egloff, C.; Couffignal, C.; Cordier, A.G.; Deruelle, P.; Sibiude, J.; Anselem, O.; Benachi, A.; Luton, D.; Mandelbrot, L.; Vauloup-Fellous, C.; et al. Pregnant women’s perceptions of the COVID-19 vaccine: A French survey. PLoS ONE 2022, 17, e0263512. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, O.; Siddiquea, B.N.; Shetty, A.; Afroz, A.; Billah, B. COVID-19 vaccine hesitancy among pregnant women: A systematic review and meta-analysis. BMJ Open 2022, 12, e061477. [Google Scholar] [CrossRef]
- Available online: https://www.mass.gov/info-details/archive-of-covid-19-cases-in-massachusetts#june-2022 (accessed on 8 August 2022).
| Maternal Cohort Characteristics | Vaccinated (n = 105) | ||
|---|---|---|---|
| Maternal Age at Sample Collection, mean (SD), years | 34.6 (3.3) | ||
| Gestational Age at Vaccination, mean (SD), weeks | 25.8 (7.4) | ||
| Maternal Vaccine Platform, number (%) | |||
| BNT162b2 | 62 (59.0) | ||
| mRNA-1273 | 34 (32.4) | ||
| Ad26.COV2.S | 9 (8.6) | ||
| SARS-CoV-2 Infection During Pregnancy, number (%) | 6 (5.7) | ||
| Gestational Age at SARS-CoV-2 Infection, mean (SD), weeks | 17.5 (13.6) | ||
| Hispanic, number (%) | 6 (5.7) | ||
| Race, number (%) | |||
| Asian | 7 (6.7) | ||
| Black | 3 (2.9) | ||
| Other | 3 (2.9) | ||
| White | 90 (85.7) | ||
| Unknown | 2 (1.9) | ||
| Neonatal Cohort Characteristics | SARS-CoV-2 Infected (n = 38) | Uninfected (n = 69) | p |
| Male sex, number (%) | 20 (52.6) | 33 (47.8) | 0.634 |
| Gestational Age at Delivery, mean (SD), weeks | 39.1 (1.0) | 39.0 (1.6) | 0.983 |
| Birthweight, mean (SD), g | 3422 (477) | 3237 (550) | 0.053 |
| Breastfed #, number (%) | 24 (63.2) | 42 (60.9) | 0.839 |
| Clinical Characteristics of Infants with COVID-19 | Infants (n = 38) |
|---|---|
| Age at SARS-CoV-2 Infection, average (SD), months | 8.5 (3.0) |
| COVID-19-Related Hospitalizations, n (%) | 0 |
| Symptoms Reported, n (%) | |
| Congestion/rhinorrhea | 33 (86.8) |
| Cough | 25 (65.8) |
| Fever | 22 (57.9) |
| Increased fussiness | 7 (18.4) |
| Parent perception of fatigue | 6 (15.8) |
| Parent perception of reduced appetite | 3 (7.9) |
| Parent perception of difficulty swallowing | 2 (5.3) |
| Parent perception of labored breathing | 2 (5.3) |
| Parent perception of difficulty sleeping | 2 (5.3) |
| Vomiting | 2 (5.3) |
| Diarrhea | 2 (5.3) |
| Rash | 2 (5.3) |
| At-Home Treatments Reported, n (%) | |
| Antipyretics | 18 (47.4) |
| Oral Steroids for wheezing | 2 (5.3) |
| None | 18 (47.4) |
| Testing Modality, number (%) | |
| PCR | 21 (55.3) |
| Rapid Antigen | 15 (39.5) |
| Never tested, but exposed and symptomatic | 2 (5.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burns, M.D.; Muir, C.; Atyeo, C.; Davis, J.P.; Demidkin, S.; Akinwunmi, B.; Fasano, A.; Gray, K.J.; Alter, G.; Shook, L.L.; et al. Relationship between Anti-Spike Antibodies and Risk of SARS-CoV-2 Infection in Infants Born to COVID-19 Vaccinated Mothers. Vaccines 2022, 10, 1696. https://doi.org/10.3390/vaccines10101696
Burns MD, Muir C, Atyeo C, Davis JP, Demidkin S, Akinwunmi B, Fasano A, Gray KJ, Alter G, Shook LL, et al. Relationship between Anti-Spike Antibodies and Risk of SARS-CoV-2 Infection in Infants Born to COVID-19 Vaccinated Mothers. Vaccines. 2022; 10(10):1696. https://doi.org/10.3390/vaccines10101696
Chicago/Turabian StyleBurns, Madeleine D., Cordelia Muir, Caroline Atyeo, Jameson P. Davis, Stepan Demidkin, Babatunde Akinwunmi, Alessio Fasano, Kathryn J. Gray, Galit Alter, Lydia L. Shook, and et al. 2022. "Relationship between Anti-Spike Antibodies and Risk of SARS-CoV-2 Infection in Infants Born to COVID-19 Vaccinated Mothers" Vaccines 10, no. 10: 1696. https://doi.org/10.3390/vaccines10101696
APA StyleBurns, M. D., Muir, C., Atyeo, C., Davis, J. P., Demidkin, S., Akinwunmi, B., Fasano, A., Gray, K. J., Alter, G., Shook, L. L., Edlow, A. G., & Yonker, L. M. (2022). Relationship between Anti-Spike Antibodies and Risk of SARS-CoV-2 Infection in Infants Born to COVID-19 Vaccinated Mothers. Vaccines, 10(10), 1696. https://doi.org/10.3390/vaccines10101696








