Evaluation of Immunogenicity to Three Doses of the SARS-CoV-2 BNT162b2 mRNA Vaccine in Lung Transplant Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants and Surveillance
2.2. Determination of SARS-CoV-2 Anti-S and Anti-N
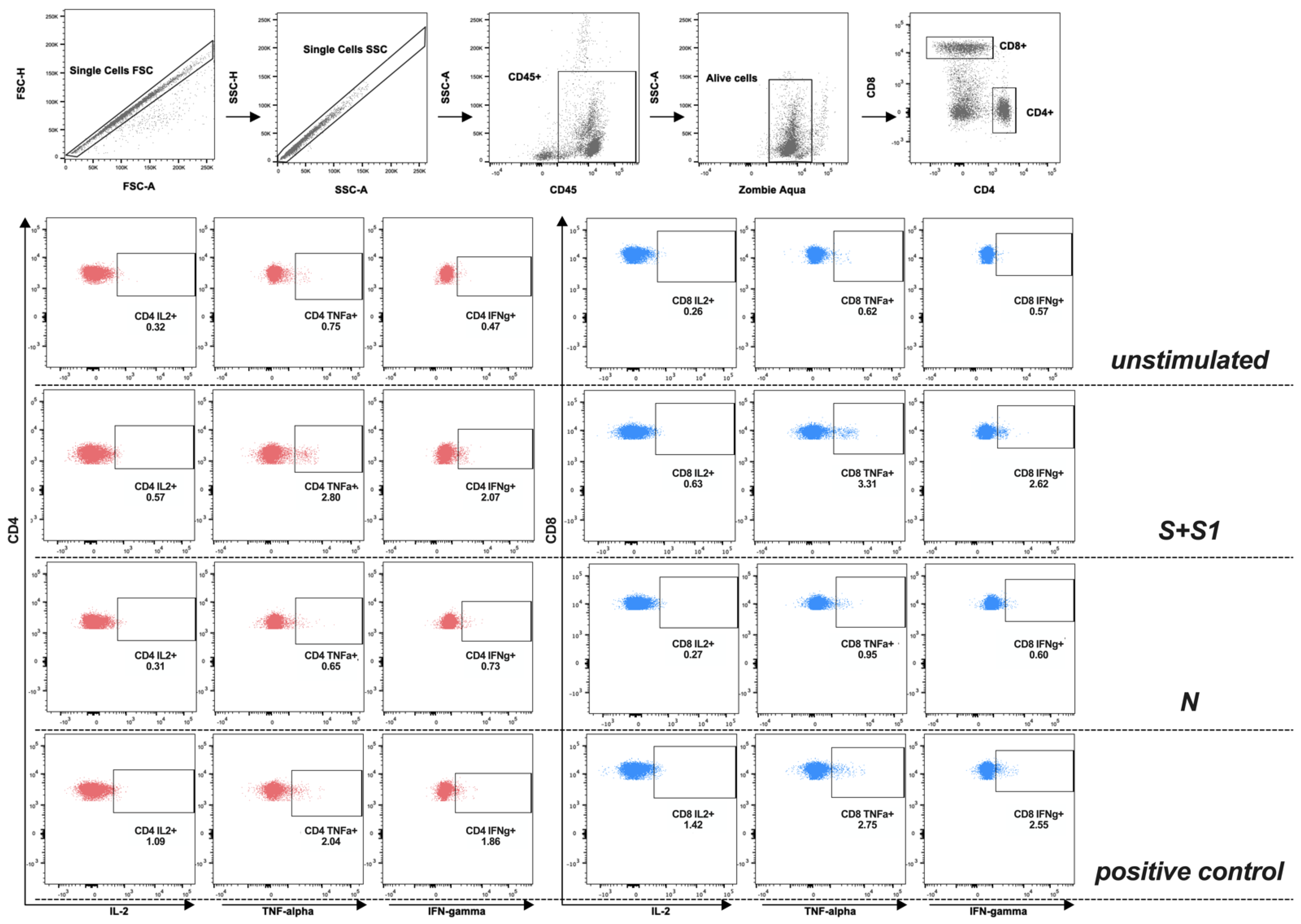
2.3. Stimulation of T-Cell Using SARS-CoV-2 Peptides Libraries
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Population, Clinical Presentation, and Laboratory Findings
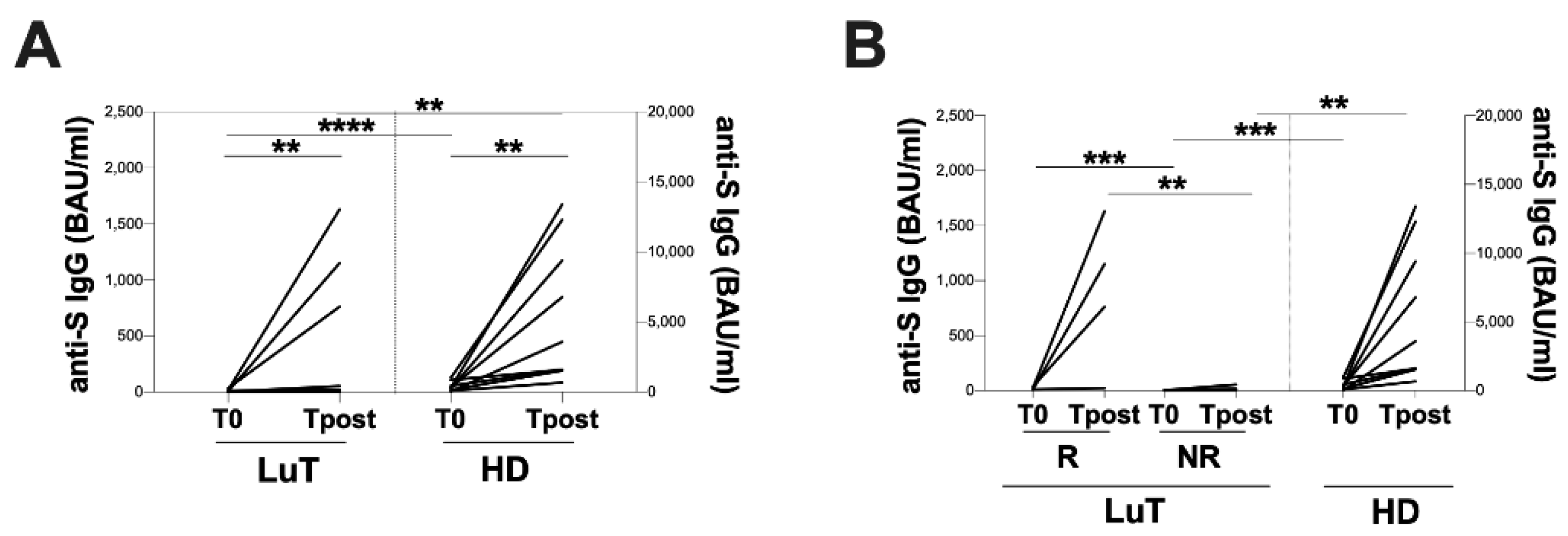
3.2. Vaccination-Induced Humoral Response
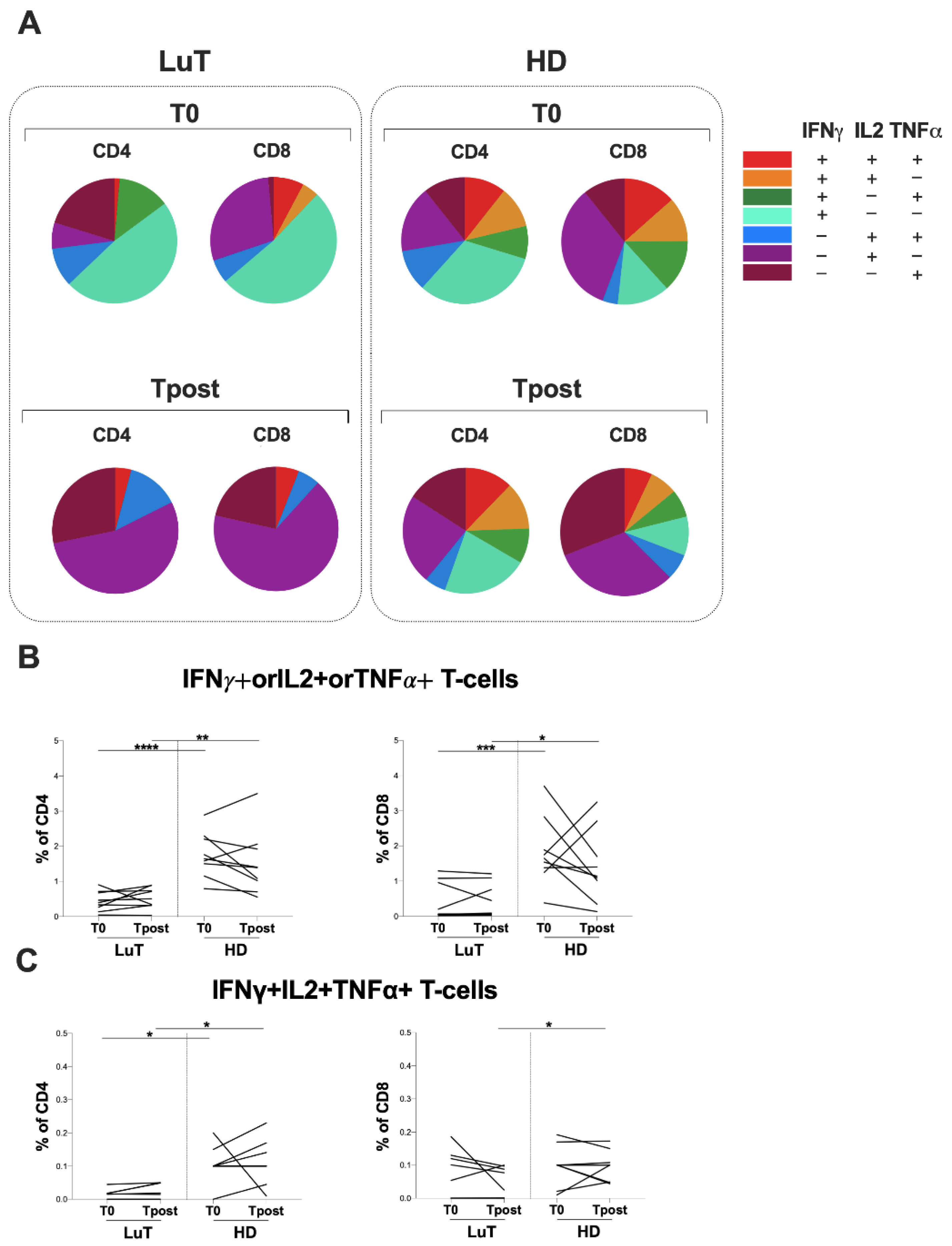
3.3. Evaluation of T-Cell Response against S and N Peptide Libraries
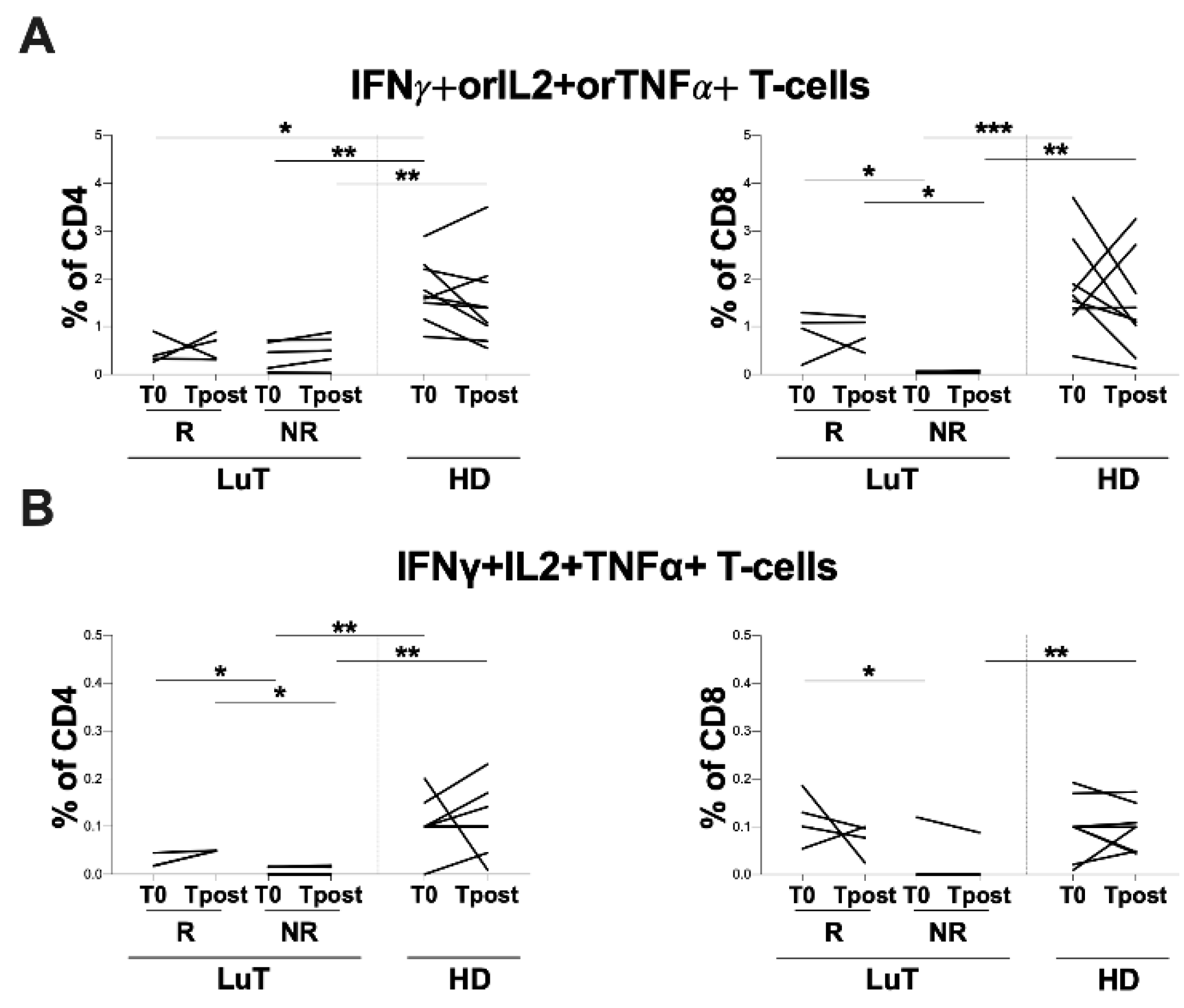
3.4. Evaluation of Spike-Specific T-Cell Response Elicited by Vaccination
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scharringa, S.; Hoffman, T.; van Kessel, D.A.; Rijkers, G.T. Vaccination and Their Importance for Lung Transplant Recipients in a COVID-19 World. Expert Rev. Clin. Pharmacol. 2021, 14, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Haidar, G.; Agha, M.; Bilderback, A.; Lukanski, A.; Linstrum, K.; Troyan, R.; Rothenberger, S.; McMahon, D.K.; Crandall, M.D.; Sobolewksi, M.D.; et al. Prospective Evaluation of Coronavirus Disease 2019 (COVID-19) Vaccine Responses Across a Broad Spectrum of Immunocompromising Conditions: The COVID-19 Vaccination in the Immunocompromised Study (COVICS.). Clin. Infect. Dis. 2022, 75, e630–e644. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.N.; Scott, J.H.; Criner, G.J.; Cordova, F.C.; Mamary, A.J.; Marchetti, N.; Shenoy, K.V.; Galli, J.A.; Mulhall, P.D.; Brown, J.C.; et al. COVID-19 in Lung Transplant Recipients. Transpl. Infect. Dis. 2020, 22, e13364. [Google Scholar] [CrossRef] [PubMed]
- Vietri, M.T.; Albanese, L.; Passariello, L.; D’Elia, G.; Caliendo, G.; Molinari, A.M.; Angelillo, I.F. Evaluation of Neutralizing Antibodies after Vaccine BNT162b2: Preliminary Data. J. Clin. Virol. 2022, 146, 105057. [Google Scholar] [CrossRef] [PubMed]
- Cassaniti, I.; Gregorini, M.; Bergami, F.; Arena, F.; Sammartino, J.C.; Percivalle, E.; Soleymaninejadian, E.; Abelli, M.; Ticozzelli, E.; Nocco, A.; et al. Effect of a Third Dose of SARS-CoV-2 MRNA BNT162b2 Vaccine on Humoral and Cellular Responses and Serum Anti-HLA Antibodies in Kidney Transplant Recipients. Vaccines 2022, 10, 921. [Google Scholar] [CrossRef]
- Gatti, M.; Rinaldi, M.; Bussini, L.; Bonazzetti, C.; Pascale, R.; Pasquini, Z.; Faní, F.; Pinho Guedes, M.N.; Azzini, A.M.; Carrara, E.; et al. Clinical Outcome in Solid Organ Transplant Recipients Affected by COVID-19 Compared to General Population: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2022, 28, 1057–1065. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Rincon-Arevalo, H.; Stefanski, A.-L.; Potekhin, A.; Straub-Hohenbleicher, H.; Choi, M.; Bachmann, F.; Pross, V.; Hammett, C.; Schrezenmeier, H.; et al. B and T Cell Responses after a Third Dose of SARS-CoV-2 Vaccine in Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 32, 3027–3033. [Google Scholar] [CrossRef]
- Schramm, R.; Costard-Jäckle, A.; Rivinius, R.; Fischer, B.; Müller, B.; Boeken, U.; Haneya, A.; Provaznik, Z.; Knabbe, C.; Gummert, J. Poor Humoral and T-Cell Response to Two-Dose SARS-CoV-2 Messenger RNA Vaccine BNT162b2 in Cardiothoracic Transplant Recipients. Clin. Res. Cardiol. 2021, 110, 1142–1149. [Google Scholar] [CrossRef]
- Havlin, J.; Svorcova, M.; Dvorackova, E.; Lastovicka, J.; Lischke, R.; Kalina, T.; Hubacek, P. Immunogenicity of BNT162b2 MRNA COVID-19 Vaccine and SARS-CoV-2 Infection in Lung Transplant Recipients. J. Heart Lung Transplant. 2021, 40, 754–758. [Google Scholar] [CrossRef]
- Lozano-Ojalvo, D.; Camara, C.; Lopez-Granados, E.; Nozal, P.; Del Pino-Molina, L.; Bravo-Gallego, L.Y.; Paz-Artal, E.; Pion, M.; Correa-Rocha, R.; Ortiz, A.; et al. Differential Effects of the Second SARS-CoV-2 MRNA Vaccine Dose on T Cell Immunity in Naive and COVID-19 Recovered Individuals. Cell Rep. 2021, 36, 109570. [Google Scholar] [CrossRef]
- Mazzone, P.J.; Mossad, S.B.; Mawhorter, S.D.; Mehta, A.C.; Mauer, J.R. Cell-Mediated Immune Response to Influenza Vaccination in Lung Transplant Recipients. J. Heart Lung Transplant. 2004, 23, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Peled, Y.; Ram, E.; Lavee, J.; Segev, A.; Matezki, S.; Wieder-Finesod, A.; Halperin, R.; Mandelboim, M.; Indenbaum, V.; Levy, I.; et al. Third Dose of the BNT162b2 Vaccine in Heart Transplant Recipients: Immunogenicity and Clinical Experience. J. Heart Lung Transplant. 2022, 41, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 MRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef] [PubMed]
- Iannetta, M.; Landi, D.; Cola, G.; Campogiani, L.; Malagnino, V.; Teti, E.; Coppola, L.; Di Lorenzo, A.; Fraboni, D.; Buccisano, F.; et al. B- and T-Cell Responses After SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis Receiving Disease Modifying Therapies: Immunological Patterns and Clinical Implications. Front. Immunol. 2021, 12, 796482. [Google Scholar] [CrossRef] [PubMed]
- Sauzullo, I.; Mengoni, F.; Mascia, C.; Rossi, R.; Lichtner, M.; Vullo, V.; Mastroianni, C.M. Treatment of Latent Tuberculosis Infection Induces Changes in Multifunctional Mycobacterium Tuberculosis-Specific CD4+ T Cells. Med. Microbiol. Immunol. 2016, 205, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Rabinowich, L.; Grupper, A.; Baruch, R.; Ben-Yehoyada, M.; Halperin, T.; Turner, D.; Katchman, E.; Levi, S.; Houri, I.; Lubezky, N.; et al. Low Immunogenicity to SARS-CoV-2 Vaccination among Liver Transplant Recipients. J. Hepatol. 2021, 75, 435–438. [Google Scholar] [CrossRef]
- Collier, A.-R.Y.; Yu, J.; McMahan, K.; Liu, J.; Chandrashekar, A.; Maron, J.S.; Atyeo, C.; Martinez, D.R.; Ansel, J.L.; Aguayo, R.; et al. Differential Kinetics of Immune Responses Elicited by Covid-19 Vaccines. N. Engl. J. Med. 2021, 385, 2010–2012. [Google Scholar] [CrossRef]
- Tobudic, S.; Benazzo, A.; Koblischke, M.; Schneider, L.; Blüml, S.; Winkler, F.; Schmidt, H.; Vorlen, S.; Haslacher, H.; Perkmann, T.; et al. Immune Response after MRNA COVID-19 Vaccination in Lung Transplant Recipients: A 6-Month Follow-Up. Vaccines 2022, 10, 1130. [Google Scholar] [CrossRef]
- Picchianti-Diamanti, A.; Aiello, A.; Laganà, B.; Agrati, C.; Castilletti, C.; Meschi, S.; Farroni, C.; Lapa, D.; Najafi Fard, S.; Cuzzi, G.; et al. ImmunosuppressiveTherapies Differently Modulate Humoral- and T-Cell-Specific Responses to COVID-19 MRNA Vaccine in Rheumatoid Arthritis Patients. Front. Immunol. 2021, 12, 740249. [Google Scholar] [CrossRef]
- Agrati, C.; Di Cosimo, S.; Fenoglio, D.; Apolone, G.; Ciceri, F.; Ciliberto, G.; Baldanti, F.; Costantini, M.; Giannarelli, D.; Ippolito, G.; et al. COVID-19 Vaccination in Fragile Patients: Current Evidence and an Harmonized Transdisease Trial. Front. Immunol. 2021, 12, 704110. [Google Scholar] [CrossRef]
- Catry, E.; Favresse, J.; Gillot, C.; Bayart, J.-L.; Frérotte, D.; Dumonceaux, M.; Evrard, P.; Mullier, F.; Douxfils, J.; Carlier, F.M.; et al. Lung Transplant Recipients Immunogenicity after Heterologous ChAdOx1 NCoV-19—BNT162b2 MRNA Vaccination. Viruses 2022, 14, 1470. [Google Scholar] [CrossRef] [PubMed]
- Mrak, D.; Tobudic, S.; Koblischke, M.; Graninger, M.; Radner, H.; Sieghart, D.; Hofer, P.; Perkmann, T.; Haslacher, H.; Thalhammer, R.; et al. SARS-CoV-2 Vaccination in Rituximab-Treated Patients: B Cells Promote Humoral Immune Responses in the Presence of T-Cell-Mediated Immunity. Ann. Rheum. Dis. 2021, 80, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Cordero, E.; Manuel, O. Influenza Vaccination in Solid-Organ Transplant Recipients. Curr. Opin. Organ Transplant. 2012, 17, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Cucchiari, D.; Egri, N.; Bodro, M.; Herrera, S.; Del Risco-Zevallos, J.; Casals-Urquiza, J.; Cofan, F.; Moreno, A.; Rovira, J.; Banon-Maneus, E.; et al. Cellular and Humoral Response after MRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients. Am. J. Transplant. 2021, 21, 2727–2739. [Google Scholar] [CrossRef]
- Hallett, A.M.; Greenberg, R.S.; Boyarsky, B.J.; Shah, P.D.; Ou, M.T.; Teles, A.T.; Krach, M.R.; López, J.I.; Werbel, W.A.; Avery, R.K.; et al. SARS-CoV-2 Messenger RNA Vaccine Antibody Response and Reactogenicity in Heart and Lung Transplant Recipients. J. Heart Lung Transplant. Off. 2021, 40, 1579–1588. [Google Scholar] [CrossRef]
- Messika, J.; Eloy, P.; Roux, A.; Hirschi, S.; Nieves, A.; Le Pavec, J.; Sénéchal, A.; Saint Raymond, C.; Carlier, N.; Demant, X.; et al. COVID-19 in Lung Transplant Recipients. Transplantation 2021, 105, 177–186. [Google Scholar] [CrossRef]
- Agrati, C.; Castilletti, C.; Goletti, D.; Meschi, S.; Sacchi, A.; Matusali, G.; Bordoni, V.; Petrone, L.; Lapa, D.; Notari, S.; et al. Coordinate Induction of Humoral and Spike Specific T-Cell Response in a Cohort of Italian Health Care Workers Receiving BNT162b2 MRNA Vaccine. Microorganisms 2021, 9, 1315. [Google Scholar] [CrossRef]
- Callaghan, C.J.; Mumford, L.; Curtis, R.M.K.; Williams, S.V.; Whitaker, H.; Andrews, N.; Lopez Bernal, J.; Ushiro-Lumb, I.; Pettigrew, G.J.; Thorburn, D.; et al. Real-World Effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S Vaccines Against SARS-CoV-2 in Solid Organ and Islet Transplant Recipients. Transplantation 2022, 106, 436–446. [Google Scholar] [CrossRef]
- Puius, Y.A.; Bartash, R.M.; Zingman, B.S. Maintaining Mask Momentum in Transplant Recipients. Transpl. Infect. Dis. 2021, 23, e13697. [Google Scholar] [CrossRef]
- Kumar, D.; Hu, Q.; Samson, R.; Ferreira, V.H.; Hall, V.G.; Ierullo, M.; Majchrzak-Kita, B.; Hardy, W.; Gingras, A.; Humar, A. Neutralization against Omicron Variant in Transplant Recipients after Three Doses of MRNA Vaccine. Am. J. Transplant. 2022, 22, 2089–2093. [Google Scholar] [CrossRef]
- Yetmar, Z.A.; Beam, E.; O’Horo, J.C.; Ganesh, R.; Bierle, D.M.; Brumble, L.; Seville, M.T.; Razonable, R.R. Monoclonal Antibody Therapy for COVID-19 in Solid Organ Transplant Recipients. Open Forum Infect Dis 2021, 8, ofab255. [Google Scholar] [CrossRef] [PubMed]
- Sarrell, B.A.; Bloch, K.; El Chediak, A.; Kumm, K.; Tracy, K.; Forbes, R.C.; Langone, A.; Thomas, L.; Schlendorf, K.; Trindade, A.J.; et al. Monoclonal Antibody Treatment for COVID-19 in Solid Organ Transplant Recipients. Transpl. Infect. Dis. 2022, 24, e13759. [Google Scholar] [CrossRef] [PubMed]
- Yetmar, Z.A.; Bhaimia, E.; Razonable, R.R.; Bierle, D.M.; Ganesh, R.; Razonable, R.R. Breakthrough COVID-19 after SARS-CoV-2 Vaccination in Solid Organ Transplant Recipients: An Analysis of Symptomatic Cases and Monoclonal Antibody Therapy. Transpl. Infect. Dis. 2022, 24, e13779. [Google Scholar] [CrossRef] [PubMed]
| Variable | |
| Age, median (IQR) years | 56.0 (46.0–61.5) |
| Male/Female | 7/2 |
| Months since transplant, median (IQR) | 24.0 (24.0–102.0) |
| Immunosuppression treatment | |
| Steroids | 9/9 |
| Antimetabolites | 6/9 |
| Calcineurin inhibitors | 9/9 |
| Laboratory Data | |
| WBC (109/L) | 8.2 (5.4–10.0) |
| Neutrophils (109/L) | 5.1 (3.6–6.7) |
| Lymphocytes (109/L) | 1.4 (1.3–2.6) 243 (145–265) |
| PLT (109/L) | |
| Creatinine (mg/dL) | 1.3 (1.2–2.1) |
| Azotemia (mg/dL) | 54 (7.7–85.1) |
| Comorbidities | |
| Diabetes | 4/9 |
| Arterial hypertension | 5/9 |
| Dyslipidemia | 1/9 |
| Cardiopathy | 2/9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guardiani, M.; Zingaropoli, M.A.; Cogliati Dezza, F.; Centofanti, A.; Carillo, C.; Tortellini, E.; Dominelli, F.; Napoli, A.; Del Borgo, C.; Gaeta, A.; et al. Evaluation of Immunogenicity to Three Doses of the SARS-CoV-2 BNT162b2 mRNA Vaccine in Lung Transplant Patients. Vaccines 2022, 10, 1642. https://doi.org/10.3390/vaccines10101642
Guardiani M, Zingaropoli MA, Cogliati Dezza F, Centofanti A, Carillo C, Tortellini E, Dominelli F, Napoli A, Del Borgo C, Gaeta A, et al. Evaluation of Immunogenicity to Three Doses of the SARS-CoV-2 BNT162b2 mRNA Vaccine in Lung Transplant Patients. Vaccines. 2022; 10(10):1642. https://doi.org/10.3390/vaccines10101642
Chicago/Turabian StyleGuardiani, Mariasilvia, Maria Antonella Zingaropoli, Francesco Cogliati Dezza, Anastasia Centofanti, Carolina Carillo, Eeva Tortellini, Federica Dominelli, Anna Napoli, Cosmo Del Borgo, Aurelia Gaeta, and et al. 2022. "Evaluation of Immunogenicity to Three Doses of the SARS-CoV-2 BNT162b2 mRNA Vaccine in Lung Transplant Patients" Vaccines 10, no. 10: 1642. https://doi.org/10.3390/vaccines10101642
APA StyleGuardiani, M., Zingaropoli, M. A., Cogliati Dezza, F., Centofanti, A., Carillo, C., Tortellini, E., Dominelli, F., Napoli, A., Del Borgo, C., Gaeta, A., Venuta, F., Vullo, V., Lichtner, M., Ciardi, M. R., Mastroianni, C. M., & Russo, G. (2022). Evaluation of Immunogenicity to Three Doses of the SARS-CoV-2 BNT162b2 mRNA Vaccine in Lung Transplant Patients. Vaccines, 10(10), 1642. https://doi.org/10.3390/vaccines10101642








