BCG and SARS-CoV-2—What Have We Learned?
Abstract
:1. Introduction
2. BCG Vaccine and Its Potential
3. Coronaviruses and SARS-CoV-2
4. Does BCG Have the Potential to Reduce the Pandemic’s Impact?
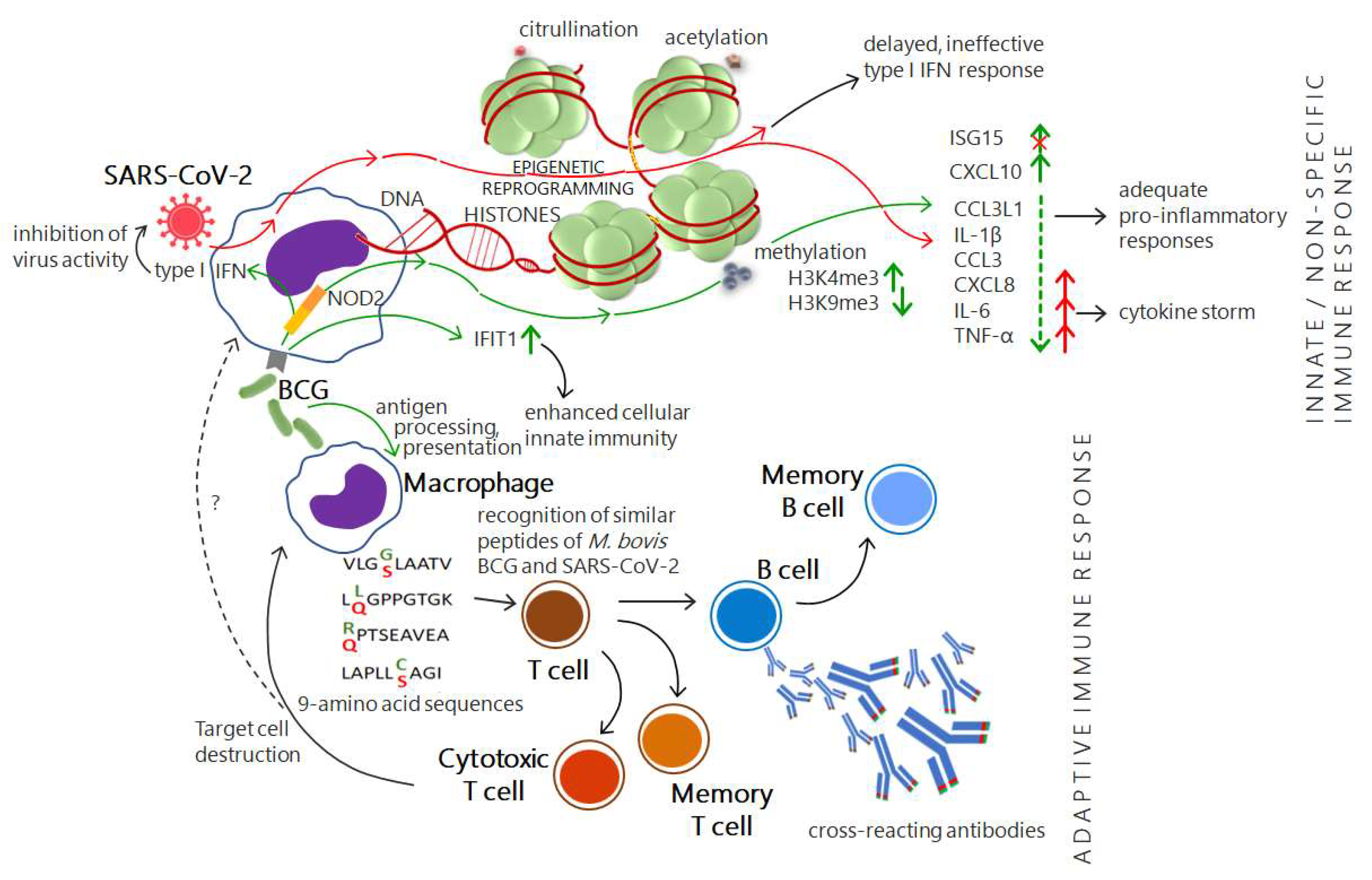
4.1. Cross-Protective Immunity Brings Hope
4.2. An Illusory Hope: BCG Does Not Change the COVID-19 Status
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Lancet. Predicting pandemics. Lancet 2016, 388, 2960. [Google Scholar] [CrossRef]
- Lin, P.Z.; Meissner, C.M. Persistent Pandemics. Econ. Hum. Biol. 2021, 43, 101044. [Google Scholar] [CrossRef] [PubMed]
- Morse, S.S.; Mazet, J.A.; Woolhouse, M.; Parrish, C.R.; Carroll, D.; Karesh, W.B.; Zambrana-Torrelio, C.; Lipkin, W.I.; Daszak, P. Prediction and prevention of the next pandemic zoonosis. Lancet 2012, 380, 1956–1965. [Google Scholar] [CrossRef]
- CDC. SARS (10 Years after). Available online: http://cdc.gov/dotw/sars/index.html (accessed on 30 June 2022).
- CDC. SARS Response Timeline. Available online: https://www.cdc.gov/about/history/sars/timeline.htm (accessed on 30 June 2022).
- ECDC. Factsheet about Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Available online: https://www.ecdc.europa.eu/en/middle-east-respiratory-syndrome-coronavirus/factsheet (accessed on 30 June 2022).
- Singh, A.K.; Netea, M.G.; Bishai, W.R. BCG turns 100: Its nontraditional uses against viruses, cancer, and immunologic diseases. J. Clin. Investig. 2021, 131, e148291. [Google Scholar] [CrossRef] [PubMed]
- Divangahi, M.; Aaby, P.; Khader, S.A.; Barreiro, L.B.; Bekkering, S.; Chavakis, T.; van Crevel, R.; Curtis, N.; DiNardo, A.R.; Dominguez-Andres, J.; et al. Trained immunity, tolerance, priming and differentiation: Distinct immunological processes. Nat. Immunol. 2021, 22, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Covián, C.; Fernández-Fierro, A.; Retamal-Díaz, A.; Díaz, F.E.; Vasquez, A.E.; Lay, M.K.; Riedel, C.A.; González, P.A.; Bueno, S.M.; Kalergis, A.M. BCG-induced cross-protection and development of trained immunity: Implication for vaccine design. Front. Immunol. 2019, 10, 2806. [Google Scholar] [CrossRef]
- Moorlag, S.; Arts, R.; van Crevel, R.; Netea, M. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019, 25, 1473–1478. [Google Scholar] [CrossRef]
- Luca, S.; Mihaescu, T. History of BCG vaccine. Maedica 2013, 8, 53–58. [Google Scholar]
- Krajewska-Wędzina, M.; Kozińska, M.; Radulski, Ł.; Lipiec, M.; Augustynowicz-Kopeć, E.; Weiner, M.; Szulowski, K. Molecular characterisation of the Mycobacterium bovis causing bovine tuberculosis outbreaks in Poland. J. Vet. Res. 2020, 64, 45–50. [Google Scholar] [CrossRef]
- Krajewska, M.; Lipiec, M.; Szulowski, K. Bovine tuberculosis in bison (Bison bonasus caucasicus) located in Poland. Post. Nauk Med. 2011, 10, 842–845. [Google Scholar]
- Salvador, L.; O’Brien, D.J.; Cosgrove, M.K.; Stuber, T.P.; Schooley, A.M.; Crispell, J.; Church, S.V.; Gröhn, Y.T.; Robbe-Austerman, S.; Kao, R.R. Disease management at the wildlife-livestock interface: Using whole-genome sequencing to study the role of elk in Mycobacterium bovis transmission in Michigan; USA. Mol. Ecol. 2019, 28, 2192–2220. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, M.; Sanchez-Tarjuelo, R.; Shor, B.; Nistal-Villan, E.; Ochando, J. The BCG vaccine for COVID-19: First verdict and future directions. Front. Immunol. 2021, 12, 632478. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Finn, A. (Eds.) Hot Topics in Infection and Immunity in Children II. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2005; Volume 568, pp. 117–134. [Google Scholar]
- Calmette, A.; Guerin, C.; Boquet, A.; Nègre, L. La Vaccination Préventive Contre la Tuberculose par le BCG; Masson et Cie: Paris, France, 1927. [Google Scholar]
- Calmette, A. L’ninfection Bacillaire et la Tuberculose Chez L’homme et Chez les Animaux; Masson et Cie: Paris, France, 1922; p. 644. [Google Scholar]
- Calmette, A.; Guerin, C.; Weill-Halle, B. Essai d’immunisation contre l’infection tuberculeuse. Bull. Acad. Med. 1924, 91, 787–797. [Google Scholar]
- Calmette, A.; Guerin, C.; Negre, L. Sur la vaccination préventive des enfants nouveau-nés contre la tuberculose par le BCG. Ann. Inst. Pasteur. 1927, 41, 201–232. [Google Scholar]
- Greenwood, M. Professor Calmette’s statistical study of bcg vaccination. BMJ 1928, 1, 793–795. [Google Scholar] [CrossRef]
- Petroff, S.A.; Branch, A.; Steenken, W. A study of Bacillus Calmette-Guerin (B.C.G.). Am. Rev. Tuberc. 1929, 19, 9–46. [Google Scholar]
- Andersen, P.; Doherty, T.M. The success and failure of BCG—Implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 2005, 3, 656–662. [Google Scholar] [CrossRef]
- McShane, H. Vaccine strategies against tuberculosis. Swiss Med. Wkly. 2009, 139, 156–160. [Google Scholar]
- Delogu, G.; Fadda, G. The quest for a new vaccine against tuberculosis. J. Infect. Dev. Ctries 2009, 3, 5–15. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E. Vaccination against tuberculosis: Revamping BCG by molecular genetics guided by immunology. Front. Immunol. 2020, 11, 316. [Google Scholar] [CrossRef]
- Davies, P.D. The role of DOTS in tuberculosis treatment and control. Am. J. Respir. Med. 2003, 2, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Al Abri, S.; Kasaeva, T.; Migliori, G.B.; Goletti, D.; Zenner, D.; Denholm, J.; Al Maani, A.; Cirillo, D.M.; Schön, T.; Lillebæk, T.; et al. Tools to implement the World Health Organization End TB Strategy: Addressing common challenges in high and low endemic countries. Int. J. Infect. Dis. 2020, 92S, S60–S68. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2021, ISBN 978-92-4-003702-1 (Electronic Version). Available online: https://www.who.int/publications/i/item/9789240037021 (accessed on 7 July 2022).
- Trial of BCG vaccines in south India for tuberculosis prevention: First report—Tuberculosis Prevention Trial. Bull. World Health Organ. 1979, 57, 819–827.
- Fine, P.E. Variation in protection by BCG: Implications of and for heterologous immunity. Lancet 1995, 346, 1339–1345. [Google Scholar] [CrossRef]
- Colditz, G.A.; Brewer, T.F.; Berkey, C.S.; Wilson, M.E.; Burdick, E.; Fineberg, H.V.; Mosteller, F. Efficacy of BCG vaccine in the prevention of tuberculosis: Meta-analysis of the published literature. JAMA 1994, 271, 698–702. [Google Scholar] [CrossRef]
- Walker, V.; Selby, G.; Wacogne, I. Does neonatal BCG vaccination protect against tuberculous meningitis? Arch. Dis. Child. 2006, 91, 789–791. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Diwan, V.K.; Wheeler, J.G. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: A meta-analysis. Int. J. Epidemiol. 1993, 22, 1154–1158. [Google Scholar] [CrossRef]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F.; et al. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef]
- Setia, M.S.; Steinmaus, C.; Ho, C.S.; Rutherford, G.W. The role of BCG in prevention of leprosy: A meta-analysis. Lancet Infect. Dis. 2006, 6, 162–170. [Google Scholar] [CrossRef]
- Cuevas, N.C.; Cardenas, V.M. Bacillus of Calmette and Guérin (BCG) and the risk of leprosy in Ciudad del Este; Paraguay; 2016–2017. Epidemiol. Health 2021, 43, e2021060. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Soysal, A.; Millington, K.A.; Bakir, M.; Dosanjh, D.; Aslan, Y.; Deeks, J.J.; Efe, S.; Staveley, I.; Ewer, K.; Lalvani, A. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: A prospective community-based study. Lancet 2005, 366, 1443–1451. [Google Scholar] [CrossRef]
- Näslund, C. Resultats des Experiences de Vaccination par le BCG Poursuivies dans le Norrbotten (Suède) (Septembre 1927–Décembre 1931); Vaccination Preventative de Tuberculose, Rapports et Documents; Institut Pasteur: Paris, France, 1932. [Google Scholar]
- Calmette, A. Vaccination préventive de la tuberculose de l’homme et des animaux par le B C G. Rapports et documents provenant des divers pays (la France exceptée) transmis à l’Institut Pasteur en 1932. JAMA 1932, 99, 940–941. [Google Scholar]
- Schaltz-Buchholzer, F.; Kjær Sørensen, M.; Benn, C.S.; Aaby, P. The introduction of BCG vaccination to neonates in Northern Sweden, 1927–1931: Re-analysis of historical data to understand the lower mortality among BCG-vaccinated children. Vaccine 2022, 40, 1516–1524. [Google Scholar] [CrossRef]
- Shann, F. The non-specific effects of vaccines. Arch. Dis. Child. 2010, 95, 662–667. [Google Scholar] [CrossRef]
- Aaby, P.; Roth, A.; Ravn, H.; Napirna, B.M.; Rodrigues, A.; Lisse, I.M.; Stensballe, L.; Diness, B.R.; Lausch, K.R.; Lund, N.; et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: Beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011, 204, 245–252. [Google Scholar] [CrossRef]
- Schaltz-Buchholzer, F.; Aaby, P.; Silva, I.; Monteiro, I.; Kollmann, T.R.; Amenyogbe, N.; Bjerregaard-Andersen, M.; Benn, C.S. Mortality risk among frail neonates might be associated with maternal BCG scar status: Observational study from Guinea-Bissau. J. Infect. Dis. 2022, jiac140, ahead of print. [Google Scholar] [CrossRef]
- Arts, R.; Moorlag, S.; Novakovic, B.; Li, Y.; Wang, S.Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.; et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 2018, 23, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Chumakov, K.; Avidan, M.S.; Benn, C.S.; Bertozzi, S.M.; Blatt, L.; Chang, A.Y.; Jamison, D.T.; Khader, S.A.; Kottilil, S.; Netea, M.G.; et al. Old vaccines for new infections: Exploiting innate immunity to control COVID-19 and prevent future pandemics. Proc. Natl. Acad. Sci. USA 2021, 118, e2101718118. [Google Scholar] [CrossRef]
- Moliva, J.I.; Turner, J.; Torrelles, J.B. Immune Responses to Bacillus Calmette-Guérin vaccination: Why do they fail to protect against Mycobacterium tuberculosis? Front. Immunol. 2017, 8, 407. [Google Scholar] [CrossRef]
- Li, H.; Javid, B. Antibodies and tuberculosis: Finally coming of age? Nat. Rev. Immunol. 2018, 18, 591–596. [Google Scholar] [CrossRef]
- Blok, B.A.; Arts, R.J.; van Crevel, R.; Benn, C.S.; Netea, M.G. Trained innate immunity as underlying mechanism for the long-term; nonspecific effects of vaccines. J. Leukoc. Biol. 2015, 98, 347–356. [Google Scholar] [CrossRef]
- Wannigama, D.L.; Jacquet, A. NOD2-dependent BCG-induced trained immunity: A way to regulate innate responses to SARS-CoV2? Int. J. Infect. Dis. 2020, 101, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Sato, R.; Ikeda, T.; Sakagami, T. BCG vaccine may generate cross-reactive T cells against SARS-CoV-2: In silico analyses and a hypothesis. Vaccine 2020, 38, 6352–6356. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Nasr, M.A.; Elshenawy, S.E.; Hussein, A.E.; El-Betar, A.H.; Mohamed, R.H.; El-Badri, N. BCG vaccination and the risk of COVID 19: A possible correlation. Virology 2022, 565, 73–81. [Google Scholar] [CrossRef]
- Eggenhuizen, P.J.; Ng, B.H.; Chang, J.; Fell, A.L.; Cheong, R.M.Y.; Wong, W.Y.; Gan, P.Y.; Holdsworth, S.R.; Ooi, J.D. BCG vaccine derived peptides induce SARS-CoV-2 T cell cross-reactivity. Front. Immunol. 2021, 12, 692729. [Google Scholar] [CrossRef] [PubMed]
- Rahali, N.; Bahloul, C. Induction of cross-reacting antibodies against the COVID-19 by BCG vaccination in the mouse model. Curr. Microbiol. 2022, 79, 275. [Google Scholar] [CrossRef]
- Tarabini, R.F.; Rigo, M.M.; Faustino Fonseca, A.; Rubin, F.; Bellé, R.; Kavraki, L.E.; Ferreto, T.C.; Amaral Antunes, D.; de Souza, A.P.D. Large-scale structure-based screening of potential T cell cross-reactivities involving peptide-targets from BCG vaccine and SARS-CoV-2. Front. Immunol. 2022, 12, 812176. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef]
- Netea, M.G.; van Crevel, R. BCG-induced protection: Effects on innate immune memory. Sem. Immunol. 2014, 26, 512–517. [Google Scholar] [CrossRef]
- Lerm, M.; Netea, M.G. Trained immunity: A new avenue for tuberculosis vaccine development. J. Intern. Med. 2016, 279, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.; van der Meer, J.; Mhlanga, M.M.; Mulder, W.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.; Novakovic, B.; Ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.C.; Wang, S.Y.; et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016, 24, 807–819. [Google Scholar] [CrossRef]
- Bekkering, S.; Arts, R.; Novakovic, B.; Kourtzelis, I.; van der Heijden, C.; Li, Y.; Popa, C.D.; Ter Horst, R.; van Tuijl, J.; Netea-Maier, R.T.; et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell 2018, 172, 135–146.e9. [Google Scholar] [CrossRef]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonça, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.C.; et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 2018, 172, 176–190.e19. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423, Erratum in: J. Med. Virol. 2020, 92, 2249. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Kulcsar, K.; Misra, V.; Frieman, M.; Mossman, K. Bats and Coronaviruses. Viruses 2019, 11, 41. [Google Scholar] [CrossRef]
- Ogimi, C.; Kim, Y.J.; Martin, E.T.; Huh, H.J.; Chiu, C.H.; Englund, J.A. What’s new with the old coronaviruses? J. Pediatric Infect. Dis. Soc. 2020, 9, 210–217. [Google Scholar] [CrossRef]
- Traxler, S.; Schindler, M.; Bösmüller, H.; Klingel, K. Biologie und Pathologie von Coronaviren [Biology and pathology of coronaviruses]. Pathologe 2021, 42, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Masters, P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006, 66, 193–292. [Google Scholar]
- Amoah, I.D.; Kumari, S.; Bux, F. Coronaviruses in wastewater processes: Source, fate and potential risks. Environ. Int. 2020, 143, 105962. [Google Scholar] [CrossRef] [PubMed]
- Kesheh, M.M.; Hosseini, P.; Soltani, S.; Zandi, M. An overview on the seven pathogenic human coronaviruses. Rev. Med. Virol. 2022, 32, e2282. [Google Scholar] [CrossRef] [PubMed]
- Malik, Y.A. Properties of Coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020, 42, 3–11. [Google Scholar]
- Holmes, E.C.; Goldstein, S.A.; Rasmussen, A.L.; Robertson, D.L.; Crits-Christoph, A.; Wertheim, J.O.; Anthony, S.J.; Barclay, W.S.; Boni, M.F.; Doherty, P.C.; et al. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat Med. 2020, 26, 450–452. [Google Scholar] [CrossRef]
- Maxmen, A.; Mallapaty, S. The COVID lab-leak hypothesis: What scientists do and don’t know. Nature 2021, 594, 313–315. [Google Scholar] [CrossRef]
- Hartenian, E.; Nandakumar, D.; Lari, A.; Ly, M.; Tucker, J.M.; Glaunsinger, B.A. The molecular virology of coronaviruses. J. Biol. Chem. 2020, 295, 12910–12934. [Google Scholar] [CrossRef]
- Acharya, A.; Kevadiya, B.D.; Gendelman, H.E.; Byrareddy, S.N. SARS-CoV-2 infection leads to neurological dysfunction. J. Neuroimmune Pharmacol. 2020, 15, 167–173. [Google Scholar] [CrossRef]
- Araf, Y.; Faruqui, N.A.; Anwar, S.; Hosen, M.J. SARS-CoV-2: A new dimension to our understanding of coronaviruses. Int. Microbiol. 2021, 24, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Fotuhi, M.; Mian, A.; Meysami, S.; Raji, C.A. Neurobiology of COVID-19. J. Alzheimers Dis. 2020, 76, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Understanding the complexities of SARS-CoV2 infection and its immunology: A road to immune-based therapeutics. Int. Immunopharmacol. 2020, 88, 106980. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 20 September 2022).
- Mortaz, E.; Tabarsi, P.; Varahram, M.; Folkerts, G.; Adcock, I.M. The Immune Response and Immunopathology of COVID-19. Front. Immunol. 2020, 11, 2037. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Sapra, L.; Saini, C.; Azam, Z.; Mishra, P.K.; Verma, B.; Mishra, G.C.; Srivastava, R.K. COVID-19: Immunology; immunopathogenesis and potential therapies. Int. Rev. Immunol. 2022, 41, 171–206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, J.; Zhou, C.; Chen, B.; Fang, H.; Chen, S.; Zhang, X.; Wang, L.; Zhang, L. A review of SARS-CoV2: Compared with SARS-CoV and MERS-CoV. Front. Med. 2021, 8, 628370. [Google Scholar] [CrossRef]
- Salian, V.S.; Wright, J.A.; Vedell, P.T.; Nair, S.; Li, C.; Kandimalla, M.; Tang, X.; Carmona Porquera, E.M.; Kalari, K.R.; Kandimalla, K.K. COVID-19 transmission, current treatment, and future therapeutic strategies. Mol. Pharm. 2021, 18, 754–771. [Google Scholar] [CrossRef]
- Gordon, C.; Porteous, D.; Unsworth, J. COVID-19 vaccines and vaccine administration. Br. J. Nurs. 2021, 30, 344–349. [Google Scholar] [CrossRef]
- Hossain, M.K.; Hassanzadeganroudsari, M.; Feehan, J.; Apostolopoulos, V. The race for a COVID-19 vaccine: Where are we up to? Expert Rev. Vaccines 2022, 21, 355–376. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vora, L.K.; Apostolopoulos, V. Inhalable vaccines: Can they help control pandemics? Vaccines 2022, 10, 1309. [Google Scholar] [CrossRef]
- Chavda, V.P.; Kapadia, C.; Soni, S.; Prajapati, R.; Chauhan, S.C.; Yallapu, M.M.; Apostolopoulos, V. A global picture: Therapeutic perspectives for COVID-19. Immunotherapy 2022, 14, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Redelman-Sidi, G. Could BCG be used to protect against COVID-19? Nat. Rev. Urol. 2020, 17, 316–317. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Ahmed, S.S.; Curtis, N.; Kollmann, T.R.; Levy, O.; Netea, M.G.; Pollard, A.J.; van Crevel, R.; Wilson, C.B. Harnessing the beneficial heterologous effects of vaccination. Nat. Rev. Immunol. 2016, 16, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.; Jacobs, C.; van Loenhout, J.; Xavier, R.J.; Aaby, P.; van der Meer, J.W.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate. Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Counoupas, C.; Johansen, M.D.; Stella, A.O.; Nguyen, D.H.; Ferguson, A.L.; Aggarwal, A.; Bhattacharyya, N.D.; Grey, A.; Hutchings, O.; Patel, K.; et al. A single dose, BCG-adjuvanted COVID-19 vaccine provides sterilising immunity against SARS-CoV-2 infection. NPJ Vaccines 2021, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Pittet, L.F.; Curtis, N. Does bacillus Calmette-Guérin vaccine prevent herpes simplex virus recurrences? A systematic review. Rev. Med. Virol. 2021, 31, 1–9. [Google Scholar] [CrossRef]
- Walk, J.; de Bree, L.C.J.; Graumans, W.; Stoter, R.; van Gemert, G.J.; van de Vegte-Bolmer, M.; Teelen, K.; Hermsen, C.C.; Arts, R.J.W.; Behet, M.C.; et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat. Commun. 2019, 10, 874. [Google Scholar] [CrossRef]
- Kurthkoti, K.; Das, G. Mechanism of Heterologous Resistance of BCG to COVID-19. OSF Prepr. 2020. [Google Scholar] [CrossRef]
- Sultana, J.; Mazzaglia, G.; Luxi, N.; Cancellieri, A.; Capuano, A.; Ferrajolo, C.; de Waure, C.; Ferlazzo, G.; Trifirò, G. Potential effects of vaccinations on the prevention of COVID-19: Rationale, clinical evidence, risks, and public health considerations. Expert Rev. Vaccines 2020, 19, 919–936. [Google Scholar] [CrossRef]
- Su, W.; Wang, H.; Sun, C.; Li, N.; Guo, X.; Song, Q.; Liang, Q.; Liang, M.; Ding, X.; Sun, Y. The association between previous influenza vaccination and COVID-19 infection risk and severity: A systematic review and meta-analysis. Am. J. Prev. Med. 2022, 63, 121–130. [Google Scholar] [CrossRef]
- Pavan Kumar, N.; Padmapriyadarsini, C.; Rajamanickam, A.; Marinaik, S.B.; Nancy, A.; Padmanaban, S.; Akbar, N.; Murhekar, M.; Babu, S. Effect of BCG vaccination on proinflammatory responses in elderly individuals. Sci. Adv. 2021, 7, eabg7181. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, Z.; Sarfraz, A.; Pandav, K.; Singh Makkar, S.; Hasan Siddiqui, S.; Patel, G.; Platero-Portillo, T.; Singh, B.M.; Maideen, M.I.H.; Sarvepalli, D.; et al. Variances in BCG protection against COVID-19 mortality: A global assessment. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 24, 100249. [Google Scholar] [CrossRef]
- Charoenlap, S.; Piromsopa, K.; Charoenlap, C. Potential role of Bacillus Calmette-Guérin (BCG) vaccination in COVID-19 pandemic mortality: Epidemiological and Immunological aspects. Asian Pac. J. Allergy Immunol. 2020, 38, 150–161. [Google Scholar] [PubMed]
- Amirlak, L.; Haddad, R.; Hardy, J.D.; Khaled, N.S.; Chung, M.H.; Amirlak, B. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum. Vaccin Immunother. 2021, 17, 3913–3915. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.N.; Ebinger, J.E.; Wu, M.; Sun, N.; Braun, J.; Sobhani, K.; Van Eyk, J.E.; Cheng, S.; Arditi, M. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J. Clin. Investig. 2021, 131, e145157. [Google Scholar] [CrossRef]
- Pathak, S.; Jolly, M.K.; Nandi, D. Countries with high deaths due to flu and tuberculosis demonstrate lower COVID-19 mortality: Roles of vaccinations. Hum. Vaccin Immunother. 2021, 17, 2851–2862. [Google Scholar] [CrossRef]
- Hamiel, U.; Kozer, E.; Youngster, I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. JAMA 2020, 323, 2340–2341. [Google Scholar] [CrossRef]
- Upton, C.M.; van Wijk, R.C.; Mockeliunas, L.; Simonsson, U.S.H.; McHarry, K.; van den Hoogen, G.; Muller, C.; von Delft, A.; van der Westhuizen, H.M.; van Crevel, R.; et al. BCG CORONA Consortium. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: A double-blind, randomised, controlled, phase 3 trial. EClinicalMedicine 2022, 48, 101414. [Google Scholar] [CrossRef]
- Czajka, H.; Zapolnik, P.; Krzych, Ł.; Kmiecik, W.; Stopyra, L.; Nowakowska, A.; Jackowska, T.; Darmochwał-Kolarz, D.; Szymański, H.; Radziewicz-Winnicki, I.; et al. A multi-center, randomised, double-blind, placebo-controlled phase iii clinical trial evaluating the impact of BCG re-vaccination on the incidence and severity of SARS-CoV-2 infections among symptomatic healthcare professionals during the COVID-19 pandemic in Poland-first results. Vaccines 2022, 10, 314. [Google Scholar]
- Bates, M.N.; Herron, T.J.; Lwi, S.J.; Baldo, J.V. BCG vaccination at birth and COVID-19: A case-control study among U.S. military Veterans. Hum. Vaccin Immunother. 2022, 18, 1981084. [Google Scholar] [CrossRef]
- Madsen, A.M.R.; Schaltz-Buchholzer, F.; Benfield, T.; Bjerregaard-Andersen, M.; Dalgaard, L.S.; Dam, C.; Ditlev, S.B.; Faizi, G.; Johansen, I.S.; Kofoed, P.E.; et al. Using BCG vaccine to enhance non-specific protection of health care workers during the COVID-19 pandemic: A structured summary of a study protocol for a randomised controlled trial in Denmark. Trials 2020, 21, 799. [Google Scholar] [CrossRef] [PubMed]
- BRACE Trial. Available online: https://www.mcri.edu.au/brace (accessed on 4 July 2022).
- Senoo, Y.; Suzuki, Y.; Tsuda, K.; Tanimoto, T.; Takahashi, K. Association between COVID-19 morbidity and mortality rates and BCG vaccination policies in OECD countries. J. Infect. Prev. 2021, 22, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, P.R.; Mishra, B.; Behera, B. BCG vaccination induced protection from COVID 19. Indian J. Tuberc. 2021, 68, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Pandita, A.; Bhat, A.; Koul, A.; Singh, S.K. BCG Vaccination program mitigates COVID19 related mortality: A reality check. Curr. Pharm. Biotechnol. 2021, 22, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.E.; Molina-Cruz, A.; Barillas-Mury, C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc. Natl. Acad. Sci. USA. 2020, 117, 17720–17726. [Google Scholar] [CrossRef]
- Kumar, A.; Misra, S.; Verma, V.; Vishwakarma, R.K.; Kamal, V.K.; Nath, M.; Prakash, K.; Upadhyay, A.D.; Sahu, J.K. Global impact of environmental temperature and BCG vaccination coverage on the transmissibility and fatality rate of COVID-19. PLoS ONE 2020, 15, e0240710. [Google Scholar] [CrossRef]
- Ozdemir, C.; Kucuksezer, U.C.; Tamay, Z.U. Is BCG vaccination affecting the spread and severity of COVID-19? Allergy 2020, 75, 1824–1827. [Google Scholar] [CrossRef]
- Miller, A.; Reandelar, M.J.; Fasciglione, K.; Roumenova, V.; Li, Y.; Otazu, G.H. Correlation between universal BCG vaccination policy and reduced mortality for COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Hensel, J.; McAndrews, K.M.; McGrail, D.J.; Dowlatshahi, D.P.; LeBleu, V.S.; Kalluri, R. Exercising caution in correlating COVID-19 incidence and mortality rates with BCG vaccination policies due to variable rates of SARS CoV-2 testing. medRxiv 2020. [Google Scholar] [CrossRef]
- Gursel, M.; Gursel, I. Is global BCG vaccination-induced trained immunity relevant to the progression of SARS-CoV-2 pandemic? Allergy 2020, 75, 1815–1819. [Google Scholar] [CrossRef]
- Klinger, D.; Blass, I.; Rappoport, N.; Linial, M. Significantly improved COVID-19 outcomes in countries with higher BCG vaccination coverage: A multivariable analysis. Vaccines 2020, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, N.; Montazeri, H. On BCG vaccine protection from COVID-19: A review. SN Compr. Clin. Med. 2021, 3, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khera, D.; Chugh, A.; Khasbage, S.; Khera, P.S.; Chugh, V.K. BCG vaccination impact on mortality and recovery rates in COVID-19: A meta-analysis. Monaldi Arch. Chest Dis. 2021, 91. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Tanaka, M. Impact of Routine Infant BCG Vaccination on COVID-19. J. Infect. 2020, 81, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Hauer, J.; Fischer, U.; Auer, F.; Borkhardt, A. Regional BCG vaccination policy in former East-and West Germany may impact on both severity of SARS-CoV-2 and incidence of childhood leukemia. Leukemia 2020, 34, 2217–2219. [Google Scholar] [CrossRef]
- Samrah, S.M.; Al-Mistarehi, A.W.; Ibnian, A.M.; Raffee, L.A.; Momany, S.M.; Al-Ali, M.; Hayajneh, W.A.; Yusef, D.H.; Awad, S.M.; Khassawneh, B.Y. COVID-19 outbreak in Jordan: Epidemiological features, clinical characteristics, and laboratory findings. Ann. Med. Surg. 2020, 57, 103–108. [Google Scholar] [CrossRef]
- Lawton, G. Trials of BCG vaccine will test for COVID-19 protection. New Sci. 2020, 246, 9. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. BCG Vaccine for Health Care Workers as Defense against COVID 19 (BADAS). Available online: https://clinicaltrials.gov/ct2/show/NCT04348370 (accessed on 4 July 2022).
- Allam, M.F.; Amin, G.E.E. BCG Vaccine does not Protect Against COVID-19. Open Respir. Med. J. 2020, 14, 45–46. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Y. Evaluating childhood vaccination coverage of NIP vaccines: Coverage survey versus Zhejiang provincial immunization information system. Int. J. Environ. Res. Public Health 2017, 14, 758. [Google Scholar] [CrossRef]
- Miyasaka, M. Is BCG vaccination causally related to reduced COVID-19 mortality? EMBO Mol. Med. 2020, 12, e12661. [Google Scholar] [CrossRef]
- Wassenaar, T.M.; Buzard, G.S.; Newman, D.J. BCG vaccination early in life does not improve COVID-19 outcome of elderly populations, based on nationally reported data. Lett. Appl. Microbiol. 2020, 71, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Hensel, J.; McAndrews, K.M.; McGrail, D.J.; Dowlatshahi, D.P.; LeBleu, V.S.; Kalluri, R. Protection against SARS-CoV-2 by BCG vaccination is not supported by epidemiological analyses. Sci. Rep. 2020, 10, 18377. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Kawaguchi, K.; Matsuura, H. Does TB vaccination reduce COVID-19 infection? No evidence from a regression discontinuity analysis. medRxiv 2020. [Google Scholar] [CrossRef]
- Lindestam Arlehamn, C.S.; Sette, A.; Peters, B. Lack of evidence for BCG vaccine protection from severe COVID-19. Proc. Natl. Acad. Sci. USA. 2020, 117, 25203–25204. [Google Scholar] [CrossRef] [PubMed]
- de Chaisemartin, C.; de Chaisemartin, L. Bacille Calmette-Guérin vaccination in infancy does not protect against coronavirus disease 2019 (COVID-19): Evidence from a natural experiment in Sweden. Clin. Infect. Dis. 2021, 72, e501–e505. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=COVID&term=bcg&cntry=&state=&city=&dist=&Search=Search (accessed on 8 July 2022).
- Junqueira-Kipnis, A.P.; Dos Anjos, L.; Barbosa, L.; da Costa, A.C.; Borges, K.; Cardoso, A.; Ribeiro, K.M.; Rosa, S.; Souza, C.C.; das Neves, R.C.; et al. BCG revaccination of health workers in Brazil to improve innate immune responses against COVID-19: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 881. [Google Scholar] [CrossRef]
- EU Clinical Trials Register. Available online: www.clinicaltrialsregister.eu/ctr-search/trial/2020-001678-31/FR (accessed on 4 July 2022).
- ClinicalTrials.gov. Performance Evaluation of BCG Vaccination in Healthcare Personnel to Reduce the Severity of COVID-19 Infection. Available online: https://clinicaltrials.gov/ct2/show/NCT04362124 (accessed on 4 July 2022).
- ClinicalTrials.gov. Application of BCG Vaccine for Immune-Prophylaxis among Egyptian Healthcare Workers during the Pandemic of COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04350931 (accessed on 4 July 2022).
- Curtis, N.; Sparrow, A.; Ghebreyesus, T.A.; Netea, M.G. Considering BCG vaccination to reduce the impact of COVID-19. Lancet 2020, 395, 1545–1546. [Google Scholar] [CrossRef]
| Topic/Title | Study Design | Objective /Outcome | References |
|---|---|---|---|
| Variances in BCG protection against COVID-19 mortality: a global assessment. | epidemiological study, an a-priori methodology used to identify the top ten and bottom ten countries according to the death rate (the relative number of COVID-19 deaths within a population per unit of time). For every country until 31 March 2020, which had at least 100 confirmed cases, a tabulated list was arranged with the total deaths within that population from a day at which at least 100 confirmed cases were recorded. | Global assessment of countries according to COVID-19 death rate per population and BCG vaccination policies. COVID-19 mortality may be associated with BCG vaccination as countries like South Korea, Japan, being ones with an active BCG vaccination policy, have a lower COVID-19 mortality rate than countries with no active BCG vaccination policy such as the USA and Italy. | [101] |
| BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers | longitudinal, retrospective observational study (healthcare workers, USA) | Multivariate analysis to determine whether a history of BCG vaccination was associated with decreased rates of SARS-CoV-2 infection and seroconversion. A history of BCG vaccination, but not meningococcal, pneumococcal, or influenza vaccination, was associated with decreased SARS-CoV-2 IgG seroconversion. | [104] |
| Countries with high deaths due to flu and tuberculosis demonstrate lower COVID-19 mortality: roles of vaccinations | epidemiological, multifactor study; Data analysis from the set of 21 (17 May 2020), 51 (1 October 2020) and 83 (31 December 2020) countries regarding TB exposure and BCG policies with respect to COVID-19 incidences and deaths | Countries with high BCG coverage have lower deaths due to COVID-19. COVID-19 deaths are much lower in countries with high flu and TB deaths. Conversely, countries with low flu and TB deaths, in the absence of BCG vaccinations, demonstrate high COVID-19 deaths. | [105] |
| SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults | cohort study, participants born from 1979 to 1981 (national immunization program ended in 1982) and from 1983 to 1985 (Israel) | The comparison of rates of coronavirus PCR test positivity among persons with symptoms suspicious for COVID-19 who did and did not receive BCG vaccination as part of routine childhood immunization in the early 1980s. No evidence to support the hypothesis that BCG vaccination in childhood has a protective effect against COVID-19 in adulthood. | [106] |
| Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: A doubleblind, randomised, controlled, phase 3 trial | multicentre, randomised, double-blind, placebo-controlled trial (healthcare workers, South Africa) | BCG does not protect healthcare workers from SARS-CoV-2 infection or related severe COVID-19 disease and hospitalisation. | [107] |
| Phase III clinical trial evaluating the Impact of BCG re-vaccination on the incidence and severity of SARS-CoV-2 Infections among symptomatic healthcare professionals during the COVID-19 pandemic | multicenter, randomised, double-blind, placebo-controlled (healthcare workers, Poland) | The assessment of re-vaccination against tuberculosis with the BCG-10 vaccine (Biomed Lublin S. A., Lublin, Poland) on an impact on SARS-CoV-2 virus infection and the course of COVID-19 disease (incidence, severity) in healthcare workers with a history of BCG vaccination. No significant correlation between the frequency of incidents suspected of COVID-19 and BCG-10 vaccination, the result of the tuberculin test and the number of scars. | [108] |
| BCG vaccination at birth and COVID-19: a case-control study among U.S. military Veteran | case-control study of COVID-19 infections with a retrospective cohort study of mortality nested within the infections (anonymized records of U.S. Military Veterans treated by the Department of Veterans Affairs, USA) | No evidence to support the hypothesis that infant BCG vaccination protects against infection or death from COVID-19. | [109] |
| Using BCG vaccine to enhance non-specific protection of health care workers during the COVID-19 pandemic. | multicentre, placebo-controlled, single-blinded, randomised controlled clinical trial (healthcare workers, Danmark) | The reduction of absenteeism due to illness among healthcare workers during the COVID-19 pandemic. The reduction in the number of healthcare workers that are infected with SARS-CoV-2, and the reduction in the number of hospital admissions among healthcare workers during the COVID-19 pandemic. (in progress) | [110] |
| The BCG vaccination to Reduce the impact of COVID-19 in Australian healthcare workers following Coronavirus Exposure (BRACE) | multicentre, open label randomized controlled clinical trial (healthcare workers, Australia) | Does BCG vaccine reduce the incidence of symptomatic and severe COVID-19, as well as other respiratory illnesses and allergic diseases? (in progress) | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulesza, J.; Kulesza, E.; Koziński, P.; Karpik, W.; Broncel, M.; Fol, M. BCG and SARS-CoV-2—What Have We Learned? Vaccines 2022, 10, 1641. https://doi.org/10.3390/vaccines10101641
Kulesza J, Kulesza E, Koziński P, Karpik W, Broncel M, Fol M. BCG and SARS-CoV-2—What Have We Learned? Vaccines. 2022; 10(10):1641. https://doi.org/10.3390/vaccines10101641
Chicago/Turabian StyleKulesza, Jakub, Ewelina Kulesza, Piotr Koziński, Wojciech Karpik, Marlena Broncel, and Marek Fol. 2022. "BCG and SARS-CoV-2—What Have We Learned?" Vaccines 10, no. 10: 1641. https://doi.org/10.3390/vaccines10101641
APA StyleKulesza, J., Kulesza, E., Koziński, P., Karpik, W., Broncel, M., & Fol, M. (2022). BCG and SARS-CoV-2—What Have We Learned? Vaccines, 10(10), 1641. https://doi.org/10.3390/vaccines10101641






