The Antibody Response to the BNT162b2 mRNA COVID-19 Booster in Healthcare Workers: Association between the IgG Antibody Titers and Anthropometric and Body Composition Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Study Design
2.3. Anti-SARS-CoV-2 IgG Measurement
2.4. Body Mass Index and Body Composition Measurement
2.5. Statistical Analysis
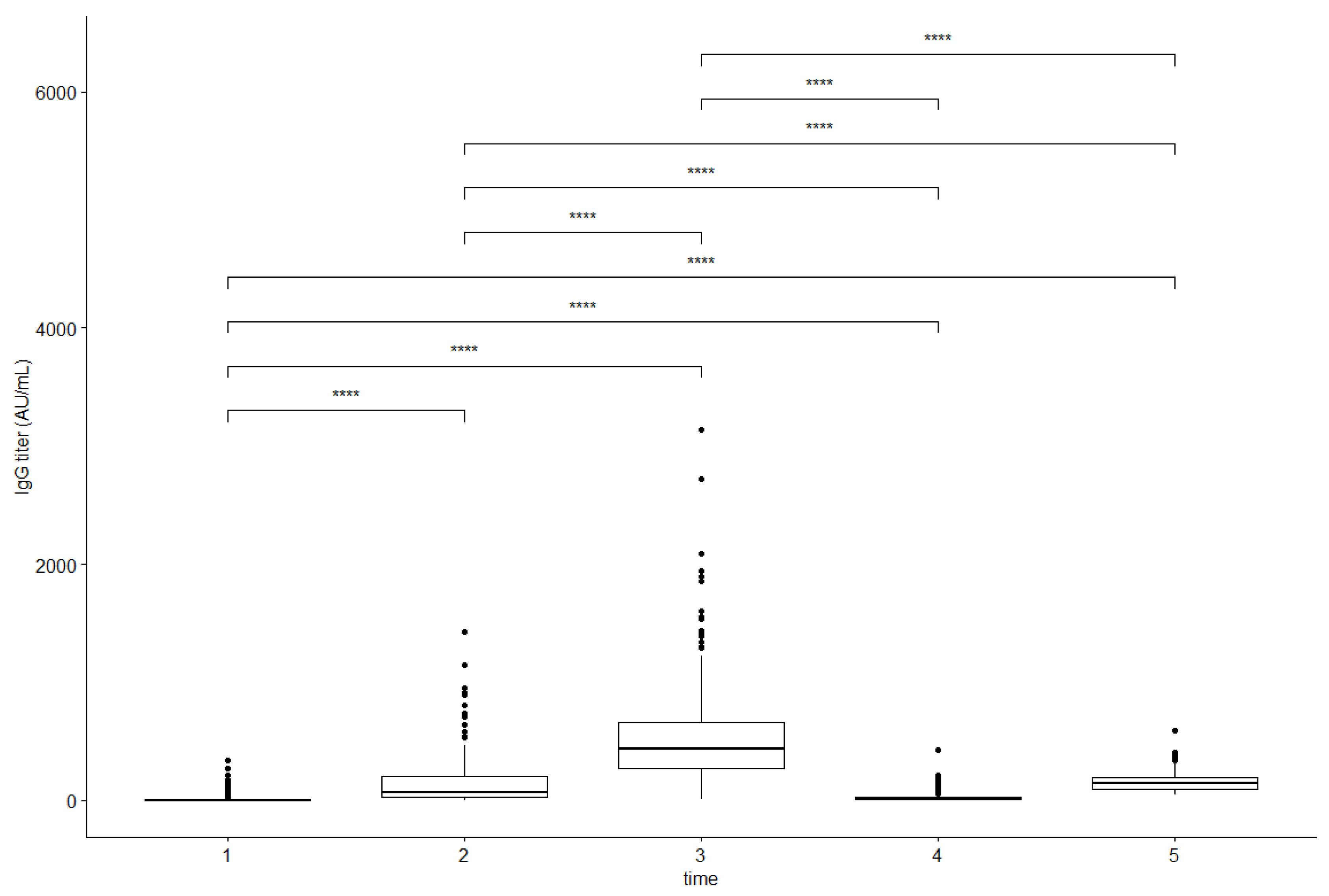
3. Results
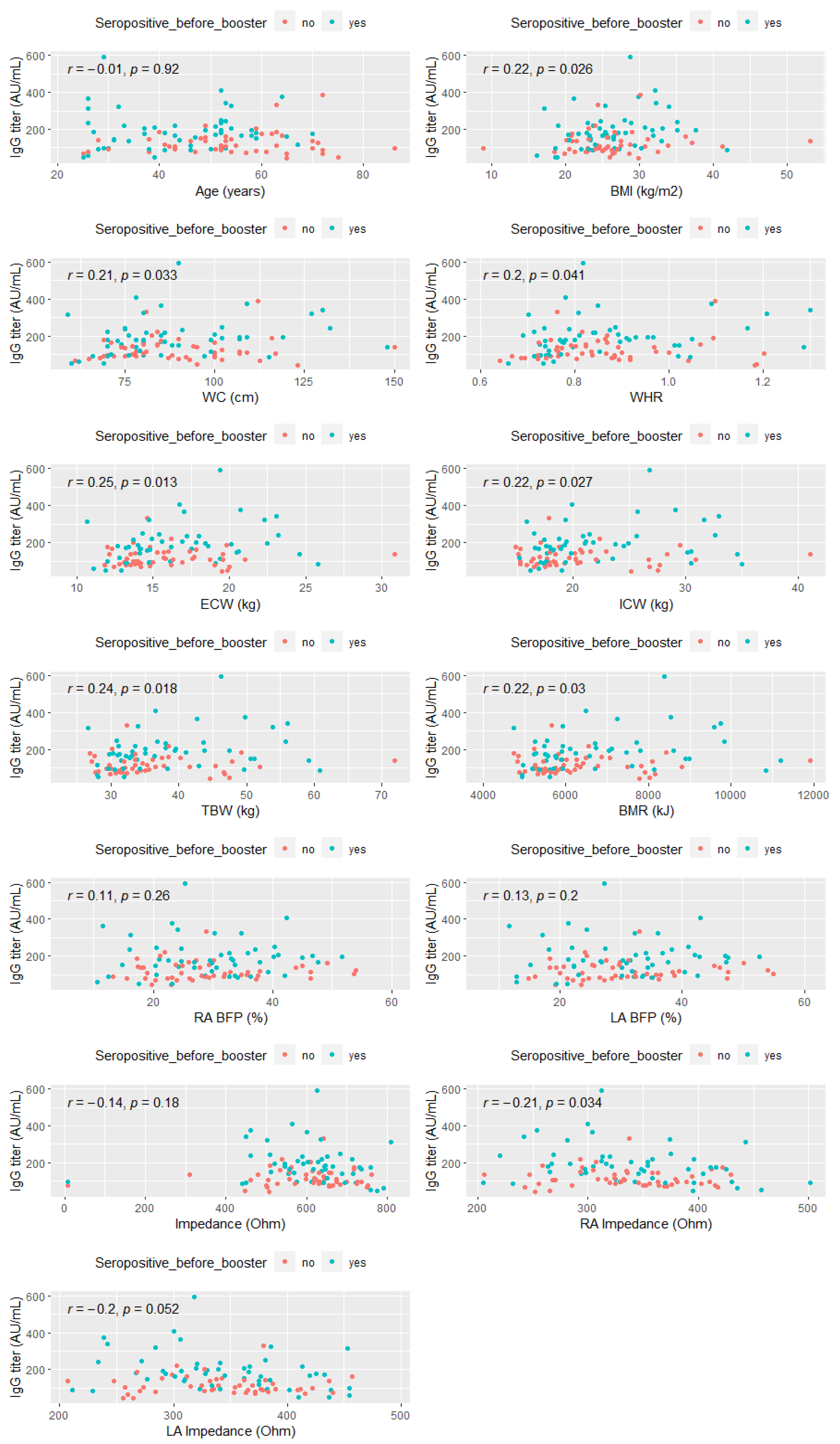
- The correlation between post-booster anti-SARS-CoV-2 IgG antibody titer and basic anthropometric measurements and derived ratios, and body composition parameters
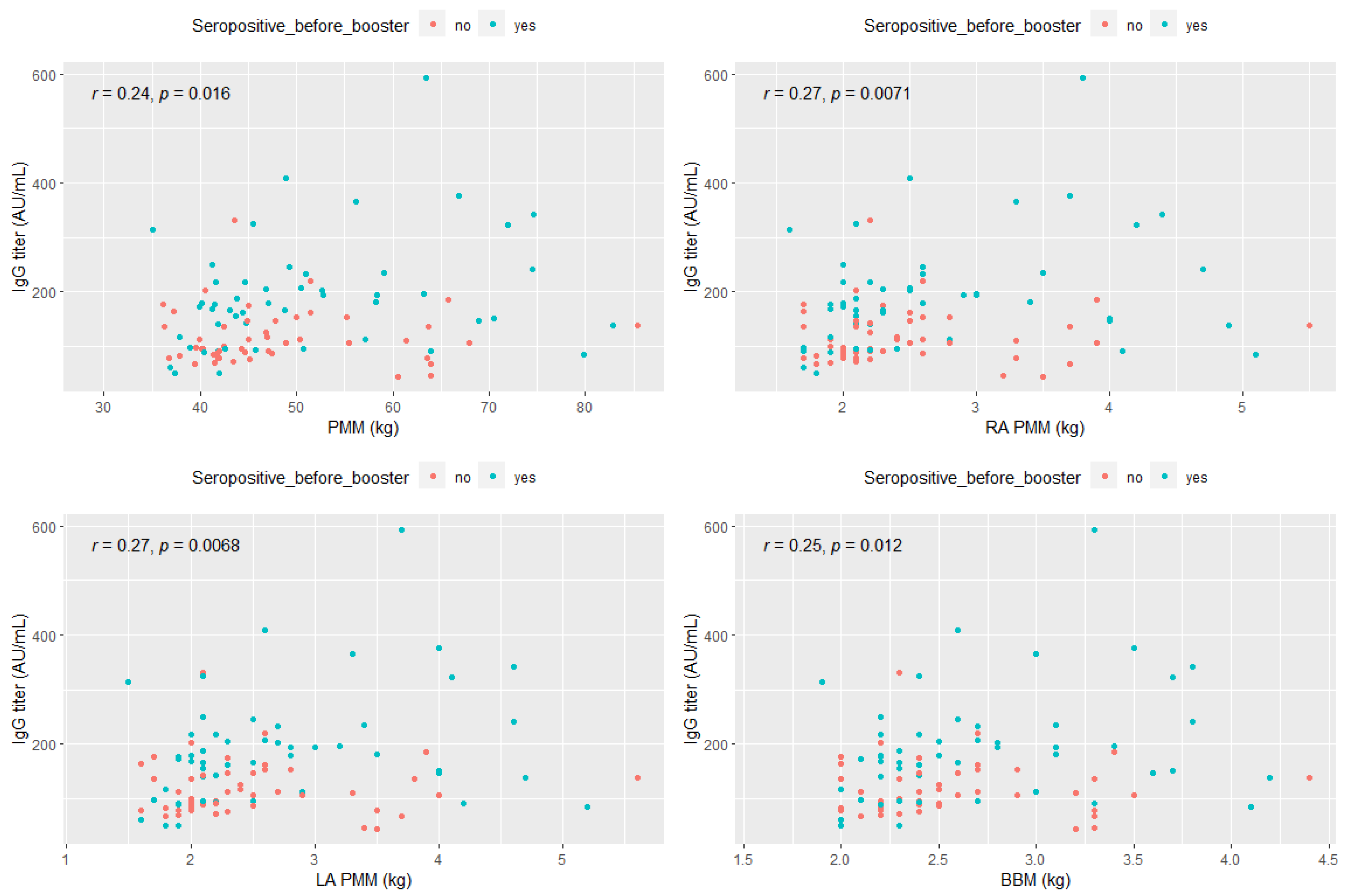
- The correlation between post-booster anti-SARS-CoV-2 IgG antibody titer and selected parameters associated with the musculoskeletal system in the study group
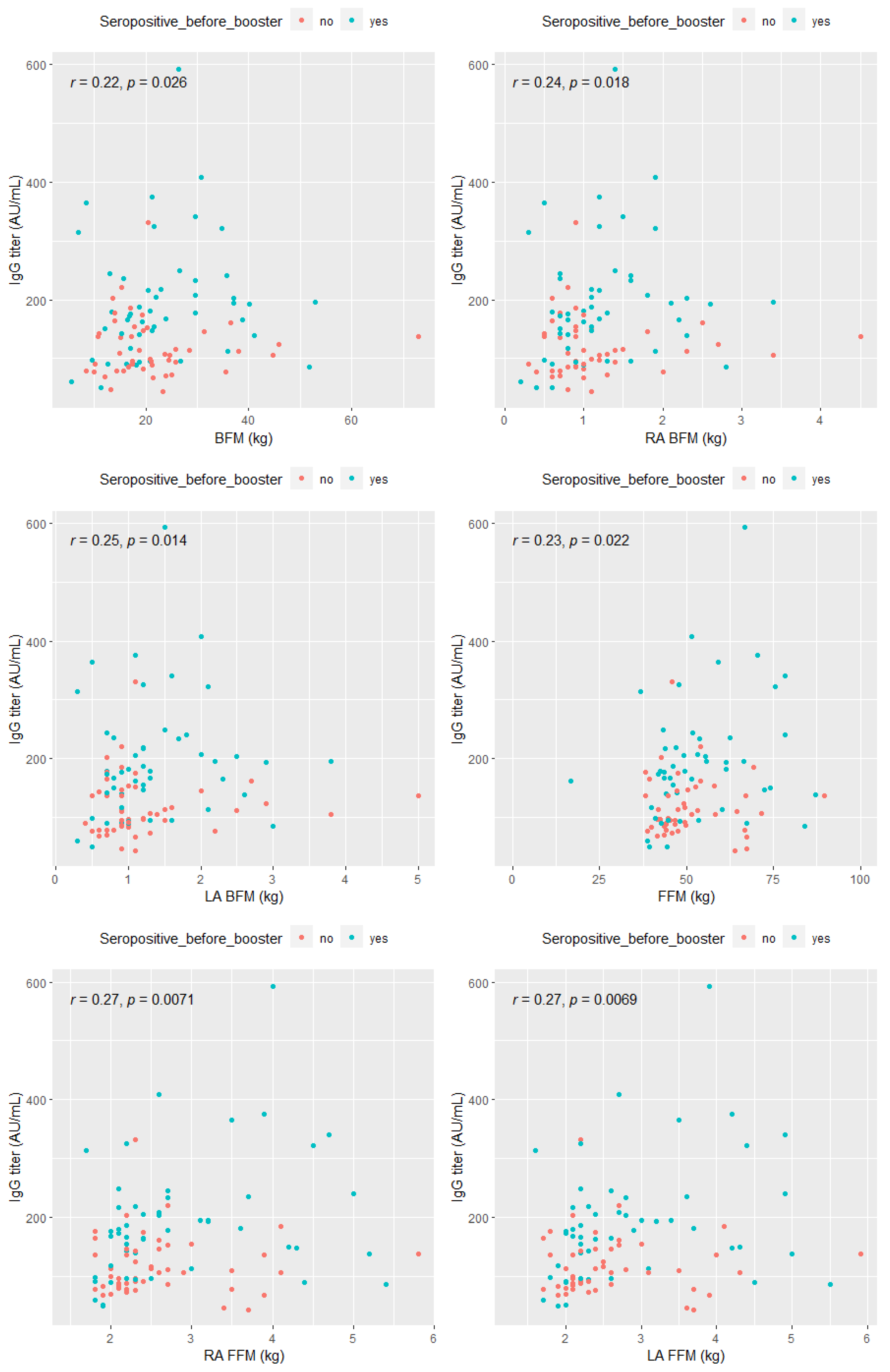
- The correlation between post-booster anti-SARS-CoV-2 IgG antibody titer and adipose tissue levels in the study group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Randolph, H.; Barriero, L. Herd Immunity: Understanding COVID-19. Immunity 2020, 52, 737–741. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Strategy to Achieve Global COVID-19 Vaccination by Mid-2022. Available online: https://cdn.who.int/media/docs/default-source/immunization/covid-19/strategy-to-achieve-global-covid-19-vaccination-by-mid-2022.pdf (accessed on 12 August 2022).
- Our World in Data Website. Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 9 August 2022).
- Bianchi, F.P.; Germinario, C.A.; Migliore, G.; Vimercati, L.; Martinelli, A.; Lobifaro, A.; Tafuri, S.; Stefanizzi, P. Control Room Working Group, BNT162b2 mRNA COVID-19 Vaccine Effectiveness in the Prevention of SARS-CoV-2 Infection: A Preliminary Report. J. Infect. Dis. 2021, 224, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Keehner, J.; Horton, L.E.; Pfeffer, M.A.; Longhurst, C.A.; Schooley, R.T.; Currier, J.S.; Abeles, S.R.; Torriani, F.J. SARS-CoV-2 infection after vaccination in health care workers in California. N. Engl. J. Med. 2021, 384, 1774–1775. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C.; Henry, B.M. Updated picture of SARS-CoV-2 variants and mutations. Diagnosis 2021, 9, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Korth, J.; Wilde, B.; Dolff, S.; Anastasiou, O.E.; Krawczyk, A.; Jahn, M.; Cordes, S.; Ross, B.; Esser, S.; Lindemann, M.; et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J. Clin. Virol. 2020, 128, 104437. [Google Scholar] [CrossRef] [PubMed]
- Antibody Testing Is Not Currently Recommended to Assess Immunity after COVID-19 Vaccination: FDA Safety Communication. Available online: https://www.fda.gov/medical-devices/safety-communications/antibody-testing-not-currently-recommended-assess-immunity-after-covid-19-vaccination-fda-safety (accessed on 9 September 2022).
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.; Simmons, R.; Wellington, E.; Cole, M.J.; Saei, A.; Oguti, B.; et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN). Lancet 2021, 397, 1459–1469. [Google Scholar] [CrossRef]
- Lumley, S.F.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; Warren, F.; et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N. Engl. J. Med. 2020, 384, 533–540. [Google Scholar] [CrossRef]
- Harvey, R.A.; Rassen, J.A.; Kabelac, C.A.; Turenne, W.; Leonard, S.; Klesh, R.; Meyer, W.A.; Kaufman, H.W.; Anderson, S.; Cohen, O.; et al. Association of SARS-CoV-2 Seropositive Antibody Test With Risk of Future Infection. JAMA Intern. Med. 2021, 181, 672–679. [Google Scholar] [CrossRef]
- Stephens, D.S.; McElrath, M.J. COVID-19 and the Path to Immunity. JAMA 2020, 324, 1279–1281. [Google Scholar] [CrossRef]
- Jagannathan, P.; Wang, T.T. Immunity after SARS-CoV-2 infections. Nat. Immunol. 2021, 22, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Seraceni, S.; Zocca, E.; Cervone, T.E.; Tomassetti, F.; Polidori, I.; Valisi, M.; Broccolo, F.; Calugi, G.; Bernardini, S.; Pieri, M. T-Cell Assay after COVID-19 Vaccination Could Be a Useful Tool? A Pilot Study on Interferon-Gamma Release Assay in Healthcare Workers. Diseases 2022, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Golec, M.; Fronczek, M.; Zembala-John, J.; Chrapiec, M.; Konka, A.; Wystyrk, K.; Botor, H.; Brzoza, Z.; Kasperczyk, S.; Bułdak, R.J. Early and Longitudinal Humoral Response to the SARS-CoV-2 mRNA BNT162b2 Vaccine in Healthcare Workers: Significance of BMI, Adipose Tissue and Muscle Mass on Long-Lasting Post-Vaccinal Immunity. Viruses 2022, 14, 868. [Google Scholar] [CrossRef]
- WHO, Interim Recommendations for Use of the Pfizer–BioNTech COVID-19 Vaccine, BNT162b2, under Emergency Use Listing. 2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/338484/WHO-2019-nCoV-vaccines-SAGE_recommendation-BNT162b2-2021.1-eng.pdf (accessed on 23 June 2022).
- Serwis Rzeczypospolitej Polskiej, Narodowy Program Szczepień Przeciw COVID-19. Available online: https://www.gov.pl/web/szczepimysie/narodowy-program-szczepien-przeciw-covid-19 (accessed on 30 June 2022).
- Serwis Szczepienia Info. Szczepionka Przeciw COVID-19. Available online: https://szczepienia.pzh.gov.pl/szczepionki/covid-19-2/?strona=6#kto-i-kiedy-powinien-zostac-zaszczepiony-przeciw-covid-19 (accessed on 2 August 2022).
- SARS-CoV-2 w Polsce. 2022. Available online: https://sarswpolsce.com (accessed on 13 March 2022).
- WHO Website: Body Mass Index—BMI. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 14 March 2022).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 12 February 2022).
- Perry, J.; Osman, S.; Wright, J.; Richard-Greenblatt, M.; Buchan, S.A.; Sadarangani, M.; Bolotin, S. Does a humoral correlate of protection exist for SARS-CoV-2? A systematic review. PLoS ONE 2022, 17, e0266852. [Google Scholar] [CrossRef]
- Wei, J.; Matthews, P.C.; Stoesser, N.; Maddox, T.; Lorenzi, L.; Studley, R.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. Anti-spike antibody response to natural SARS-CoV-2 infection in the general population. Nat. Commun. 2021, 12, 6250. [Google Scholar] [CrossRef]
- Kung, Y.-A.; Huang, C.-G.; Huang, S.-Y.; Liu, K.-T.; Huang, P.-N.; Yu, K.-Y.; Yang, S.-L.; Chen, C.-P.; Cheng, C.-Y.; Lin, Y.-T.; et al. Antibody titers measured by commercial assays are correlated with neutralizing antibody titers calibrated by international standards. medRxiv 2021. medRxiv 2021.07.16.21260618. Available online: https://www.medrxiv.org/content/10.1101/2021.07.16.21260618v1 (accessed on 2 August 2022).
- Gniadek, T.J.; Thiede, J.M.; Matchett, W.E.; Gress, A.R.; Pape, K.A.; Fiege, J.K.; Jenkins, M.K.; Menachery, V.D.; Langlois, R.A.; Bold, T.D. SARS-CoV-2 neutralization and serology testing of COVID-19 convalescent plasma from donors with nonsevere disease. Transfusion 2021, 61, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Clementi, N.; Criscuolo, E.; Ambrosi, A.; Corea, F.; Di Resta, C.; Tomaiuolo, R.; Mancini, N.; Locatelli, M.; Plebani, M.; et al. Antibody Titer Kinetics and SARS-CoV-2 Infections Six Months after Administration with the BNT162b2 Vaccine. Vaccines 2021, 9, 1357. [Google Scholar] [CrossRef]
- Favresse, J.; Bayart, J.-L.; Mullier, F.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Roy, T.; Wieers, G.; Laurent, C.; Dogné, J.-M.; et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg. Microbes Infect. 2021, 10, 1495–1498. [Google Scholar] [CrossRef]
- Brisotto, G.; Muraro, E.; Montico, M.; Corso, C.; Evangelista, C.; Casarotto, M.; Caffau, C.; Vettori, R.; Cozzi, M.R.; Zanussi, S.; et al. IgG antibodies against SARS-CoV-2 decay but persist 4 months after vaccination in a cohort of healthcare workers. Clin. Chim. Acta 2021, 523, 476–482. [Google Scholar] [CrossRef]
- Announcement No. 14 of the Minister of Health. Available online: https://www.gov.pl/web/zdrowie/komunikat-nr-14-ministra-zdrowia-w-sprawie-szczepien-przeciw-covid-19-dawka-przypominajaca-oraz-dawka-dodatkowa-uzupelniajaca-schemat-podstawowy (accessed on 2 May 2022).
- Skorupa, M.; Szczepanek, J.; Goroncy, A.; Jarkiewicz-Tretyn, J.; Ptaszynska, B.; Rajewski, P.; Koper, W.; Pałgan, K.; Tretyn, A. The Dynamics of Changes in the Concentration of IgG against the S1 Subunit in Polish Healthcare Workers in the Period from 1 to 12 Months after Injection, Including Four COVID-19 Vaccines. Vaccines 2022, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Hammerman, A.; Sergienko, R.; Friger, M.; Peretz, A.; Netzer, D.; Yaron, S. BNT162b2 Vaccine Booster and Mortality Due to Covid-19. N. Engl. J. Med. 2021, 385, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, C.; Takov, K.; Burnap, S.A.; Singh, B.; Ali, H.; Theofilatos, K.; Reed, E.; Hasman, M.; Nabeebaccus, A.; Fish, M.; et al. SARS-CoV-2 RNAemia and proteomic trajectories inform prognostication in COVID-19 patients admitted to intensive care. Nat. Commun. 2021, 12, 3406. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes/Metab. Res. Rev. 2022, 38, e3465. [Google Scholar] [CrossRef]
- Parthymou, A.; Habeos, E.E.; Habeos, G.I.; Deligakis, A.; Livieratos, E.; Marangos, M.; Chartoumpekis, D.V. Factors associated with anti-SARS-CoV-2 antibody titres 3 months post-vaccination with the second dose of BNT162b2 vaccine: A longitudinal observational cohort study in western Greece. BMJ Open 2022, 12, e057084. [Google Scholar] [CrossRef] [PubMed]
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaum, V.; Mandelboim, M.; Doolman, R.; Amit, S.; et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021, 9, 999–1009. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Impact of Adiposity and Fat Distribution, Rather Than Obesity, on Antibodies as an Illustration of Weight-Loss-Independent Exercise Benefits. Medicines 2021, 8, 57. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Dehghan, M.; Merchant, A.T. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr. J. 2008, 7, 26. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, K.H.; Noh, G.T.; Lee, R.A.; Chung, S.S. Body composition index obtained by using a bioelectrical impedance analysis device can be a predictor of prolonged operative time in patients undergoing minimally invasive colorectal surgery. Ann. Coloproctology 2022, in press. [Google Scholar] [CrossRef]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.; O’Neill, L.A. The interleukin-1 receptor/Toll-like receptor superfamily: Signal generators for pro-inflammatory interleukins and microbial products. J. Leukoc. Biol. 2000, 67, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Nikolajczyk, B.S.; Jagannathan-Bogdan, M.; Shin, H.; Gyurko, R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011, 12, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Diaz, A.; Romero, M.; Ferracci, F.; Blomberg, B.B. Micro-RNAs miR-155 and miR-16 decrease AID and E47 in B cells from elderly individuals. J. Immunol. 2015, 195, 2134–2140. [Google Scholar] [CrossRef]
- La Cava, A.; Matarese, G. The weight of leptin in immunity. Nat. Rev. Immunol. 2004, 4, 371–379. [Google Scholar] [CrossRef]
- Wang, H.; Lafdil, F.; Kong, X.; Gao, B. Signal transducer and activator of transcription 3 in liver diseases: A novel therapeutic target. Int. J. Biol. Sci. 2011, 7, 536–550. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef]
- Senn, J.J. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J. Biol. Chem. 2006, 281, 26865–26875. [Google Scholar] [CrossRef]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. 2014, 7, 241–253. [Google Scholar] [CrossRef]
- Frasca, D.; Romero, M.; Landin, A.M.; Diaz, A.; Riley, R.L.; Blomberg, B.B. Protein phosphatase 2A (PP2A) is increased in old murine B cells and mediates p38 MAPK/tristetraprolin dephosphorylation and E47 mRNA instability. Mech. Ageing Dev. 2010, 131, 306–314. [Google Scholar] [CrossRef]
- Alemayehu, B.; Buysman, E.; Parry, D.; Becker, L.; Nathan, F. Economic burden and healthcare utilization associated with castration-resistant prostate cancer in a commercial and Medicare Advantage US patient population. J. Med. Econ. 2010, 13, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cowling, B.J.; Wu, P.; Chan, W.M.; Lee, S.Y.; Lau, E.H.; Schooling, C.M. Adiposity and influenza-associated respiratory mortality: A cohort study. Clin. Infect. Dis. 2015, 60, e49–e57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Weir, S.; Noah, T.L.; Beck, M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Hollstein, M.M.; Münsterkötter, L.; Schön, M.P.; Bergmann, A.; Husar, T.M.; Abratis, A.; Eidizadeh, A.; Dierks, S.; Schaffrinski, M.; Zachmann, K.; et al. Long-term effects of homologous and heterologous SARS-CoV-2 vaccination on humoral and cellular immune responses. Allergy 2022, 77, 2560–2564. [Google Scholar] [CrossRef]

| Parameters | n (%) 103 (100.00) | |
|---|---|---|
| Women | 79 (76.70) | |
| Men | 24 (23.30) | |
| Medical staff 1 | 81 (78.64) | |
| Auxiliary medical staff 2 | 14 (13.59) | |
| Administrative staff 3 | 8 (7.77) | |
| Chronic diseases 4 | 32 (31.07) | |
| Obesity (BMI ≥ 30.00) | 22 (21.36) | |
| Mean ± SD | Median (Q1; Q3) | |
| Age (years) | 48.98 ± 13.76 | 51.00 (39.00; 59.00) |
| Metabolic age (years) | 44.37 ± 16.52 | 44.00 (33.00; 57.00) |
| BMI (kg/m2) | 26.32 ± 5.90 | 25.56 (23.04; 29.05) |
| WC (cm) | 88.55 ± 18.49 | 85.00 (75.00; 100.00) |
| HC (cm) | 103.05 ± 11.66 | 102.00 (97.00; 110.00) |
| WHR | 0.86 ± 0.14 | 0.82 (0.75; 0.92) |
| ECW (%) | 16.18 ± 3.48 | 15.00 (13.70; 18.40) |
| ICW (%) | 21.08 ± 5.30 | 19.20 (17.50; 23.50) |
| BBM (kg) | 2.63 ± 0.56 | 2.40 (2.20; 3.00) |
| BFP (%) | 29.14 ± 7.95 | 28.30 (24.00; 35.50) |
| BFM (kg) | 22.49 ± 10.96 | 20.30 (15.40; 26.60) |
| FFM (kg) | 52.05 ± 12.39 | 47.50 (43.80; 59.20) |
| TBW (%) | 37.23 ± 8.62 | 34.00 (31.30; 41.60) |
| PMM (kg) | 49.70 ± 11.33 | 45.10 (41.60; 56.20) |
| Impedance (Ohm) | 602.65 ± 125.32 | 625.00 (546.00; 673.00) |
| BMR (kJ) | 6524.55 ± 1495.80 | 5924.00 (5481.00; 7456.00) |
| VAT (level) | 7.38 ± 5.04 | 7.00 (4.00; 10.00) |
| RA BFP (%) | 29.58 ± 9.82 | 28.90 (22.50; 35.80) |
| RA BFM (kg) | 1.20 ± 0.72 | 1.00 (0.70; 1.40) |
| RA FFM (kg) | 2.72 ± 0.90 | 2.30 (2.10; 3.10) |
| RA PMM (kg) | 2.58 ± 0.84 | 2.20 (2.00; 2.90) |
| RA Impedance (Ohm) | 336.19 ± 59.51 | 335.00 (295.00; 376.00) |
| LA BFP (%) | 30.67 ± 9.74 | 30.20 (23.50; 36.20) |
| LA BFM (kg) | 1.29 ± 0.80 | 1.00 (0.80; 1.50) |
| LA FFM (kg) | 2.74 ± 0.92 | 2.40 (2.10; 3.10) |
| LA PMM (kg) | 2.60 ± 0.86 | 2.30 (2.00; 2.90) |
| LA Impedance (Ohm) | 343.67 ± 60.09 | 341.00 (301.00; 382.00) |
| Vaccination Process | Anti-SARS-CoV-2 IgG Antibody Titer (AU/mL) | |||||
|---|---|---|---|---|---|---|
| Seronegative (<10.00 AU/mL) | Seropositive (≥10.00 AU/mL) | |||||
| n (%) | Mean ± SD | Median (Q1; Q3) | n (%) | Mean ± SD | Median (Q1; Q3) | |
| Before the firstdose 1 | 73 (70.87) | 0.88 ± 2.05 | 0.21 (0.14; 0.35) | 30 (29.13) | 41.50 ± 48.50 | 24.90 (12.89; 39.91) |
| Before the seconddose 2 | 13 (12.62) | 5.52 ± 3.06 | 5.44 (3.73; 8.25) | 90 (87.38) | 153.53 ± 183.15 | 75.28 (33.16; 243.15) |
| Early follow-up 3 | 2 (1.94) | 7.19 ± 0.68 | 7.19 | 101 (98.06) | 505.79 ± 367.16 | 425.17 (267.85; 620.74) |
| Late follow-up 4 before the booster dose | 51 (49.51) | 5.77 ± 2.12 | 5.84 (4.37; 7.27) | 52 (50.49) | 34.77 ± 32.71 | 22.15 (13.93; 42.86) |
| Early follow-up after thebooster 5 | 0 (0.00) | - | - | 103 (100.00) | 158.94 ± 90.34 | 142.80 (94.03; 193.16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golec, M.; Konka, A.; Fronczek, M.; Zembala-John, J.; Chrapiec, M.; Wystyrk, K.; Kasperczyk, S.; Brzoza, Z.; Bułdak, R.J. The Antibody Response to the BNT162b2 mRNA COVID-19 Booster in Healthcare Workers: Association between the IgG Antibody Titers and Anthropometric and Body Composition Parameters. Vaccines 2022, 10, 1638. https://doi.org/10.3390/vaccines10101638
Golec M, Konka A, Fronczek M, Zembala-John J, Chrapiec M, Wystyrk K, Kasperczyk S, Brzoza Z, Bułdak RJ. The Antibody Response to the BNT162b2 mRNA COVID-19 Booster in Healthcare Workers: Association between the IgG Antibody Titers and Anthropometric and Body Composition Parameters. Vaccines. 2022; 10(10):1638. https://doi.org/10.3390/vaccines10101638
Chicago/Turabian StyleGolec, Marlena, Adam Konka, Martyna Fronczek, Joanna Zembala-John, Martyna Chrapiec, Karolina Wystyrk, Sławomir Kasperczyk, Zenon Brzoza, and Rafał Jakub Bułdak. 2022. "The Antibody Response to the BNT162b2 mRNA COVID-19 Booster in Healthcare Workers: Association between the IgG Antibody Titers and Anthropometric and Body Composition Parameters" Vaccines 10, no. 10: 1638. https://doi.org/10.3390/vaccines10101638
APA StyleGolec, M., Konka, A., Fronczek, M., Zembala-John, J., Chrapiec, M., Wystyrk, K., Kasperczyk, S., Brzoza, Z., & Bułdak, R. J. (2022). The Antibody Response to the BNT162b2 mRNA COVID-19 Booster in Healthcare Workers: Association between the IgG Antibody Titers and Anthropometric and Body Composition Parameters. Vaccines, 10(10), 1638. https://doi.org/10.3390/vaccines10101638







