Dynamics of B-Cell Responses after SARS-CoV-2 Vaccination in Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Reagents
2.3. Distribution of B-Cell Subpopulations and Cytokine and Chemokine Receptor Expression by Circulating PBs
2.4. Cell Preparation and Cell Cultures
2.5. Anti-SARS-CoV-2 Ab Detection in Sera and in Culture Supernatants
2.6. Data Analysis
3. Results
3.1. Total PB and MBC Cells Were Augmented in Blood after SARS-CoV-2 Immunisation
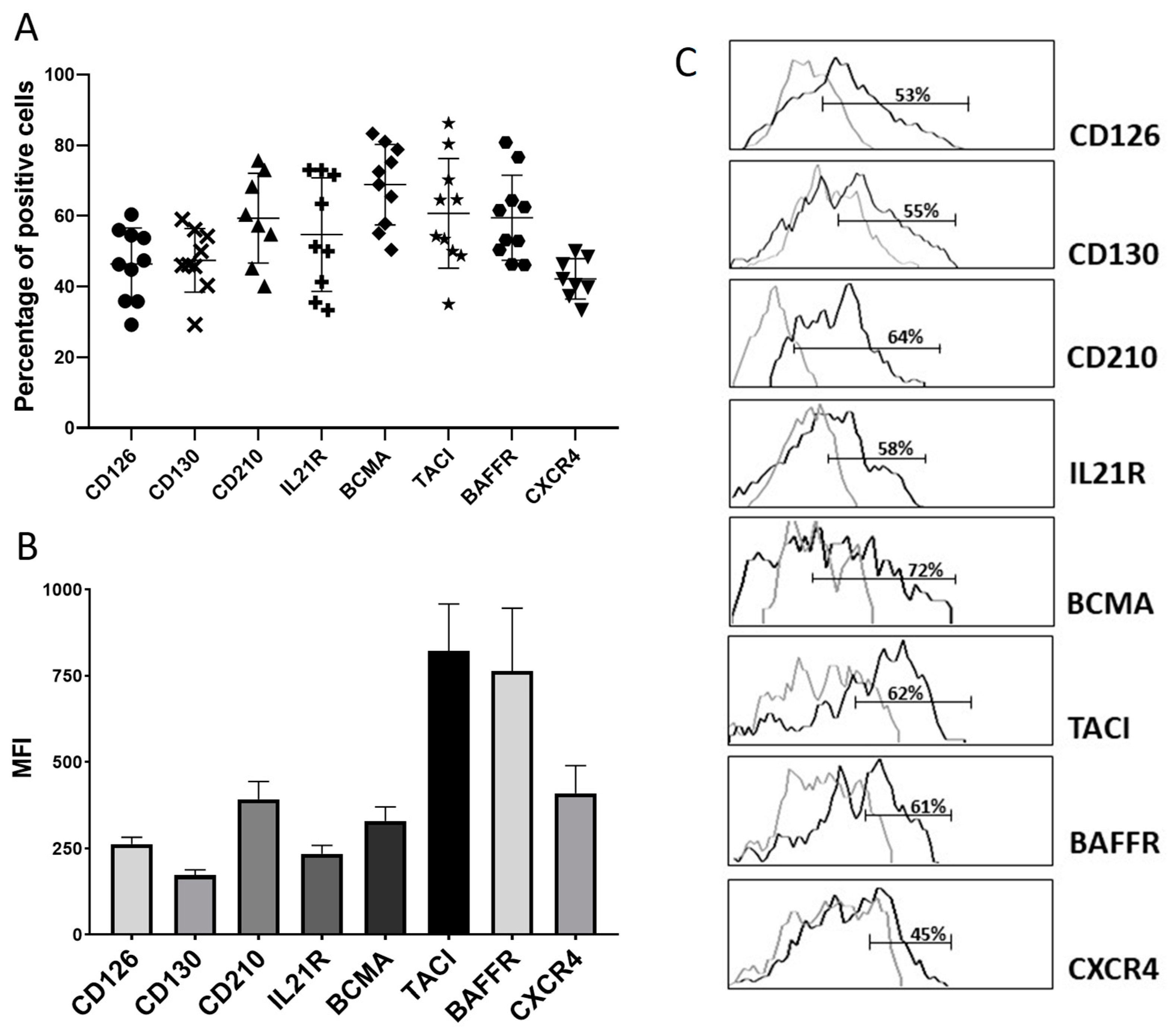
3.2. Blood PB Generated after Immunisation Express Receptors for Cytokines and Chemokines Implicated in the Maturation of PC
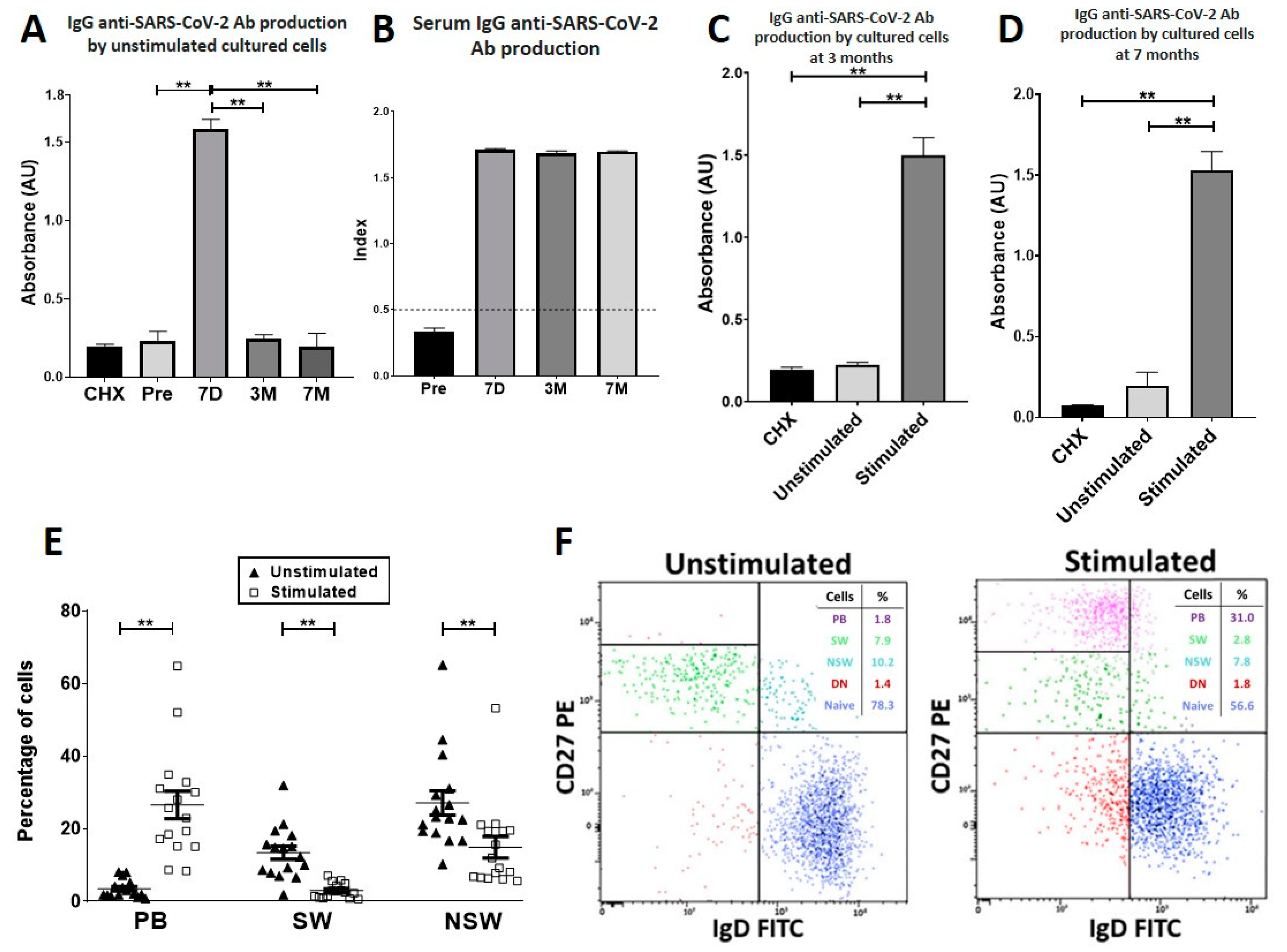
3.3. Seven Days after Vaccination, Specific PB-Producing Anti-SARS-CoV-2 Ab Circulate
3.4. At Three and Seven Months after Vaccination, Specific PBs Disappeared from Blood, but Specific MBCs Were Able to Differentiate into Circulating Anti-SARS-CoV-2-Producing Antibody-Secreting Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Torres-Estrella, C.U.; Reyes-Montes, M.D.R.; Duarte-Escalante, E.; Sierra Martínez, M.; Frías-De-León, M.G.; Acosta-Altamirano, G. Vaccines Against COVID-19: A Review. Vaccines 2022, 10, 414. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Rodda, L.B.; Netland, J.; Shehata, L.; Pruner, K.B.; Morawski, P.A.; Thouvenel, C.D.; Takehara, K.K.; Eggenberger, J.; Hemann, E.A.; Waterman, H.R.; et al. Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell. 2021, 184, 169–183.e17. [Google Scholar] [CrossRef]
- Kim, W.; Zhou, J.Q.; Horvath, S.C.; Schmitz, A.J.; Sturtz, A.J.; Lei, T.; Liu, Z.; Kalaidina, E.; Thapa, M.; Alsoussi, W.B.; et al. Germinal centre-driven maturation of B cell response to mRNA vaccination. Nature 2022, 604, 141–145. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef]
- Wang, Z.; Schmidt, F.; Weisblum, Y.; Muecksch, F.; Barnes, C.O.; Finkin, S.; Schaefer-Babajew, D.; Cipolla, M.; Gaebler, C.; Lieberman, J.A.; et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 2021, 592, 616–622. [Google Scholar] [CrossRef]
- Rodda, L.B.; Morawski, P.A.; Pruner, K.B.; Fahning, M.L.; Howard, C.A.; Franko, N.; Logue, J.; Eggenberger, J.; Stokes, C.; Golez, I.; et al. Imprinted SARS-CoV-2-specific memory lymphocytes define hybrid immunity. Cell 2022, 185, 1588–1601.e14. [Google Scholar] [CrossRef]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef]
- Lederer, K.; Castaño, D.; Atria, D.G.; Oguin, T.H.; Wang, S.; Manzoni, T.B.; Muramatsu, H.; Hogan, M.J.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 2020, 53, 1281–1295.e5. [Google Scholar] [CrossRef] [PubMed]
- Brewer, R.C.; Ramadoss, N.S.; Lahey, L.J.; Jahanbani, S.; Robinson, W.H.; Lanz, T.V. BNT162b2 vaccine induces divergent B cell responses to SARS-CoV-2 S1 and S2. Nat. Immunol. 2022, 23, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.S.; Kim, W.; Kalaidina, E.; Goss, C.W.; Rauseo, A.M.; Schmitz, A.J.; Hansen, L.; Haile, A.; Klebert, M.K.; Pusic, I.; et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature 2021, 595, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Radbruch, A.; Muehlinghaus, G.; Luger, E.O.; Inamine, A.; Smith, K.; Dörner, T.; Hiepe, F. Competence and competition: The challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 2006, 6, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Brieva, J.A.; Roldán, E.; Rodríguez, C.; Navas, G. Human tonsil, blood and bone marrow in vivo-induced B cells capable of spontaneous and high-rate immunoglobulin secretion in vitro: Differences in the requirements for factors and for adherent and bone marrow stromal cells, as well as distinctive adhesion molecule expression. Eur. J. Immunol. 1994, 24, 362–366. [Google Scholar] [CrossRef]
- Lightman, S.M.; Utley, A.; Lee, K.P. Survival of Long-Lived Plasma Cells (LLPC): Piecing Together the Puzzle. Front. Immunol. 2019, 10, 965. [Google Scholar] [CrossRef]
- González-García, I.; Ocaña, E.; Jiménez-Gómez, G.; Campos-Caro, A.; Brieva, J.A. Immunization-induced perturbation of human blood plasma cell pool: Progressive maturation, IL-6 responsiveness, and high PRDI-BF1/BLIMP1 expression are critical distinctions between antigen-specific and nonspecific plasma cells. J. Immunol. 2006, 176, 4042–4050. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bayona, B.; Ramos-Amaya, A.; López-Blanco, R.; Campos-Caro, A.; Brieva, J.A. STAT-3 activation by differential cytokines is critical for human in vivo-generated plasma cell survival and Ig secretion. J. Immunol. 2013, 191, 4996–5004. [Google Scholar] [CrossRef]
- Martínez, R.D.L.V.; Añez, G.; Varo, F.M.; Venegas, J.J.P.; Brieva, J.A.; Rodríguez, C.; Rodríguez-Bayona, B. Clinical relevance of circulating anti-ENA and anti-dsDNA secreting cells from SLE patients and their dependence on STAT-3 activation. Eur. J. Immunol. 2017, 47, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.D.L.V.; Rodríguez-Bayona, B.; Campos-Caro, A.; Añez, G.; Medina-Varo, F.; Rodríguez, C.; La Varga-Martínez, R. Autoreactive B-lymphocytes in SLE and RA patients: Isolation and characterisation using extractable nuclear and citrullinated antigens bound to immunobeads. Eur. J. Immunol. 2019, 49, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bayona, B.; Pérez-Venegas, J.J.; Rodríguez, C.; Brieva, J.A. CD95-Mediated control of anti-citrullinated protein/peptides antibodies (ACPA)-producing plasma cells occurring in rheumatoid arthritis inflamed joints. Rheumatology 2007, 46, 612–616. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weisel, F.J.; Zuccarino-Catania, G.V.; Chikina, M.; Shlomchik, M.J. A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity 2016, 44, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.; Barmania, F.; Mellet, J.; Peta, K.; Strydom, A.; Viljoen, I.M.; James, W.; Gordon, S.; Pepper, M.S. SARS-CoV-2 Variants, Vaccines, and Host Immunity. Front. Immunol. 2022, 12, 809244. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
José-Cascón, M.S.; de la Varga-Martínez, R.; Campos-Caro, A.; Rodríguez, C. Dynamics of B-Cell Responses after SARS-CoV-2 Vaccination in Spain. Vaccines 2022, 10, 1615. https://doi.org/10.3390/vaccines10101615
José-Cascón MS, de la Varga-Martínez R, Campos-Caro A, Rodríguez C. Dynamics of B-Cell Responses after SARS-CoV-2 Vaccination in Spain. Vaccines. 2022; 10(10):1615. https://doi.org/10.3390/vaccines10101615
Chicago/Turabian StyleJosé-Cascón, Miriam San, Raquel de la Varga-Martínez, Antonio Campos-Caro, and Carmen Rodríguez. 2022. "Dynamics of B-Cell Responses after SARS-CoV-2 Vaccination in Spain" Vaccines 10, no. 10: 1615. https://doi.org/10.3390/vaccines10101615
APA StyleJosé-Cascón, M. S., de la Varga-Martínez, R., Campos-Caro, A., & Rodríguez, C. (2022). Dynamics of B-Cell Responses after SARS-CoV-2 Vaccination in Spain. Vaccines, 10(10), 1615. https://doi.org/10.3390/vaccines10101615




