Comparison of Anti-SARS-CoV-2 IgG Antibody Responses Generated by the Administration of Ad26.COV2.S, AZD1222, BNT162b2, or CoronaVac: Longitudinal Prospective Cohort Study in the Colombian Population, 2021/2022
Abstract
:1. Introduction
2. Materials and Methods
2.1. Type of Study
2.2. Timeline
2.3. Eligibility Criteria
2.4. Population
2.5. Sociodemographic Characterization and Adverse Reactions
2.6. Biological Sample Collection
2.7. RNA Identification
2.8. Antibody Identification
2.9. Follow-Up
2.10. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics, Vaccination Schedules, and Clinical History
3.2. Adverse Effects
3.3. Incidence of SARS-CoV-2
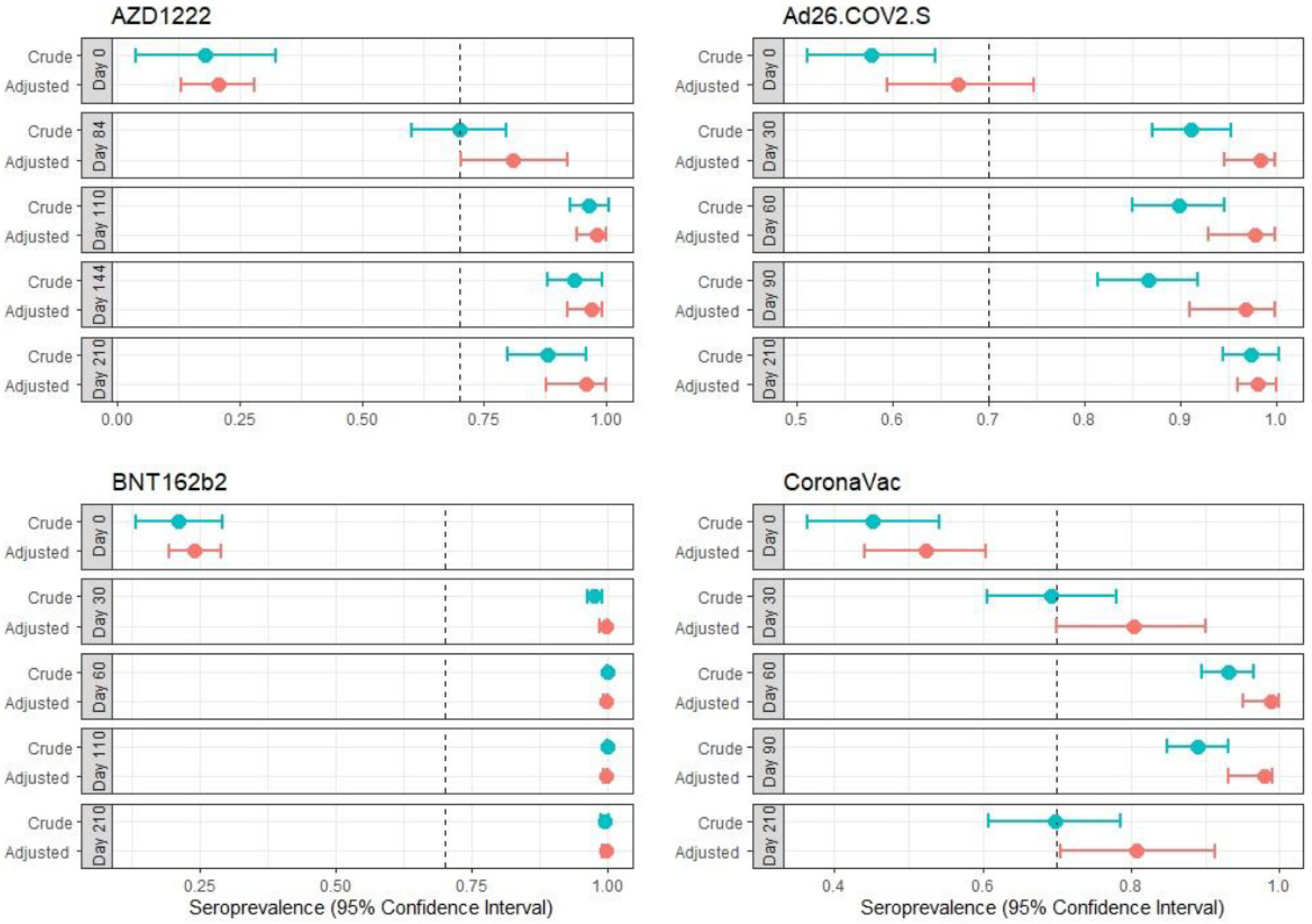
3.4. Seroprevalence
3.5. Changes in Reactivity between Day 0 and Day 210
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Peng, Y.; Xu, H.; Cui, Z.; Iii, R.O.W. The COVID-19 vaccine race: Challenges and opportunities in vaccine formulation. AAPS Pharm. SciTech 2020, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Mathieu, E.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus Pandemic (COVID-19) Vaccination. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 17 May 2022).
- Esakandari, H.; Nabi-Afjadi, M.; Fakkari-Afjadi, J.; Farahmandian, N.; Miresmaeili, S.-M.; Bahreini, E. A comprehensive review of COVID-19 characteristics. Biol. Proced. Online 2020, 22, 10. [Google Scholar] [CrossRef]
- Search of: Interventional Studies|COVID-19 Vaccine-Search Details-ClinicalTrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/results/details?type=Intr&cond=covid-19+vaccine (accessed on 17 August 2021).
- Lin, C.; Tu, P.; Beitsch, L.M. Confidence and receptivity for COVID-19 vaccines: A rapid systematic review. Vaccines 2020, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Loe, B.S.; Yu, L.-M.; Freeman, J.; Chadwick, A.; Vaccari, C.; Shanyinde, M.; Harris, V.; Waite, F.; Rosebrock, L.; et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): A single-blind, parallel-group, randomised controlled trial. Lancet Public Health 2021, 6, e416–e427. [Google Scholar] [CrossRef]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. SARS-CoV-2 immunity: Review and applications to phase 3 vaccine candidates. Lancet 2020, 396, 1595–1606. [Google Scholar] [CrossRef]
- Khubchandani, J.; Sharma, S.; Price, J.H.; Wiblishauser, M.J.; Sharma, M.; Webb, F.J. COVID-19 vaccination hesitancy in the United States: A rapid national assessment. J. Community Health 2021, 46, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Craft, J.F.; Travassos, M.A.; Foppiano Palacios, C.; Openshaw, J.J. Inadequate minority representation within SARS-CoV-2 vaccine trials. Am. J. Trop. Med. Hyg. 2021, 104, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Tercer Estudio de Efectividad de Vacunación Anti SARS-CoV-2 en Uruguay al 30 de Junio de 2021. Available online: https://www.gub.uy/ministerio-salud-publica/comunicacion/noticias/tercer-estudio-efectividad-vacunacion-anti-sars-cov-2-uruguay-30-junio-2021 (accessed on 17 August 2021).
- Zeng, B.; Gao, L.; Zhou, Q.; Yu, K.; Sun, F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: A systematic review and meta-analysis. BMC Med. 2022, 20, 200. [Google Scholar] [CrossRef]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Mortaz, E.; Jamaati, H.; Tabarsi, P.; Bayram, H.; Varahram, M.; Adcock, I.M. COVID-19: Molecular and cellular response. Front. Cell. Infect. Microbiol. 2021, 11, 563085. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D. Mix-and-match COVID Vaccines: The case is growing, but questions remain. Nature 2021, 595, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, Tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Vacunación Contra COVID-19. Available online: https://www.minsalud.gov.co/salud/publica/Vacunacion/Paginas/Vacunacion-covid-19.aspx (accessed on 17 August 2021).
- NHS Regulatory Approval of Spikevax (Formerly COVID-19 Vaccine Moderna). Available online: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-moderna (accessed on 22 June 2022).
- NHS Information for UK Recipients on COVID-19 Vaccine AstraZeneca (Regulation 174). Available online: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca/information-for-uk-recipients-on-covid-19-vaccine-astrazeneca (accessed on 22 June 2022).
- NHS Information for UK Recipients on Pfizer/BioNTech COVID-19 Vaccine (Regulation 174). Available online: https://www.gov.uk/government/publications/regulatory-approval-of-pfizer-biontech-vaccine-for-covid-19/information-for-uk-recipients-on-pfizerbiontech-covid-19-vaccine (accessed on 22 June 2022).
- Mercado-Reyes, M.; Malagón-Rojas, J.; Rodríguez-Barraquer, I.; Zapata-Bedoya, S.; Wiesner, M.; Cucunubá, Z.; Toloza-Pérez, Y.G.; Hernández-Ortiz, J.P.; Acosta-Reyes, J.; Parra-Barrera, E.; et al. Seroprevalence of anti-SARS-CoV-2 antibodies in Colombia, 2020: A population-based study. Lancet Reg. Health–Am. 2022, 9, 100195. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Xing, C. Adjusting for covariates in logistic regression models. Genet. Epidemiol. 2010, 34, 769–771. [Google Scholar] [CrossRef]
- Malagón-Rojas, J.N.; Mercado-Reyes, M.; Toloza-Pérez, Y.G.; Parra Barrera, E.L.; Palma, M.; Muñoz, E.; López, R.; Almentero, J.; Rubio, V.V.; Ibáñez, E.; et al. Seroprevalence of the SARS-CoV-2 antibody in healthcare workers: A multicentre cross-sectional study in 10 colombian cities. Occup. Environ. Med. 2021, 79, 388–395. [Google Scholar] [CrossRef]
- Lombardi, A.; Bozzi, G.; Ungaro, R.; Villa, S.; Castelli, V.; Mangioni, D.; Muscatello, A.; Gori, A.; Bandera, A. Mini review immunological consequences of immunization with COVID-19 mRNA vaccines: Preliminary results. Front. Immunol. 2021, 12, 657711. [Google Scholar] [CrossRef]
- Instituto Nacional de Salud Genoma SARS-CoV-2. Available online: https://www.ins.gov.co/Noticias/Paginas/coronavirus-genoma.aspx (accessed on 12 April 2022).
- Instituto Nacional de Salud Modelos de Estimación COVID-19. Available online: https://www.ins.gov.co/Direcciones/ONS/modelos-de-estimacion (accessed on 12 April 2022).
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Van Dromme, I.; Spiessens, B.; et al. Final analysis of efficacy and safety of single-dose Ad26.COV2.S. N. Engl. J. Med. 2022, 386, 847–860. [Google Scholar] [CrossRef]
- Sablerolles, R.S.G.; Rietdijk, W.J.R.; Goorhuis, A.; Postma, D.F.; Visser, L.G.; Geers, D.; Schmitz, K.S.; Garcia Garrido, H.M.; Koopmans, M.P.G.; Dalm, V.A.S.H.; et al. Immunogenicity and reactogenicity of vaccine boosters after Ad26.COV2.S priming. N. Engl. J. Med. 2022, 386, 951–963. [Google Scholar] [CrossRef]
- Barin, B.; Kasap, U.; Selçuk, F.; Volkan, E.; Uluçkan, Ö. Comparison of SARS-CoV-2 anti-spike receptor binding domain IgG antibody responses after CoronaVac, BNT162b2, ChAdOx1 COVID-19 vaccines, and a single booster dose: A prospective, longitudinal population-based study. Lancet Microbe 2022, 3, e274–e283. [Google Scholar] [CrossRef]
- Zhong, D.; Xiao, S.; Debes, A.K.; Egbert, E.R.; Caturegli, P.; Colantuoni, E.; Milstone, A.M. Durability of antibody levels after vaccination With MRNA SARS-CoV-2 vaccine in individuals with or without prior infection. JAMA 2021, 326, 2524–2526. [Google Scholar] [CrossRef] [PubMed]
- Suah, J.L.; Tng, B.H.; Tok, P.S.K.; Husin, M.; Thevananthan, T.; Peariasamy, K.M.; Sivasampu, S. Real-world effectiveness of homologous and heterologous BNT162b2, CoronaVac, and AZD1222 booster vaccination against delta and omicron SARS-CoV-2 infection. Emerg. Microbes Infect. 2022, 11, 1343–1345. [Google Scholar] [CrossRef] [PubMed]
- Arregocés-Castillo, L.; Fernández-Niño, J.; Rojas-Botero, M.; Palacios-Clavijo, A.; Galvis-Pedraza, M.; Rincón-Medrano, L.; Pinto-Álvarez, M.; Ruiz-Gómez, F.; Trejo-Valdivia, B. Effectiveness of COVID-19 vaccines in older adults in Colombia: A retrospective, population-based study of the ESPERANZA cohort. Lancet Healthy Longev. 2022, 3, e242–e252. [Google Scholar] [CrossRef]
- Ranzani, O.T.; Hitchings, M.D.T.; Dorion, M.; D’Agostini, T.L.; de Paula, R.C.; de Paula, O.F.P.; de Moura Villela, E.F.; Torres, M.S.S.; de Oliveira, S.B.; Schulz, W.; et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of Covid-19 in Brazil: Test negative case-control study. BMJ 2021, 374, n2015. [Google Scholar] [CrossRef]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Áñez, G.; Adelglass, J.M.; Barrat Hernández, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef]
- Álvarez-Díaz, D.A.; Muñoz, A.L.; Tavera-Rodríguez, P.; Herrera-Sepúlveda, M.T.; Ruiz-Moreno, H.A.; Laiton-Donato, K.; Franco-Muñoz, C.; Pelaez-Carvajal, D.; Cuellar, D.; Muñoz-Suarez, A.M.; et al. Low neutralizing antibody titers against the Mu variant of SARS-CoV-2 in 31 BNT162b2 vaccinated individuals in Colombia. Vaccines 2022, 10, 180. [Google Scholar] [CrossRef]
- Amodio, D.; Ruggiero, A.; Sgrulletti, M.; Pighi, C.; Cotugno, N.; Medri, C.; Morrocchi, E.; Colagrossi, L.; Russo, C.; Zaffina, S.; et al. Humoral and cellular response following vaccination with the BNT162b2 MRNA COVID-19 vaccine in patients affected by primary immunodeficiencies. Front. Immunol. 2021, 12, 727850. [Google Scholar] [CrossRef]
- Escobar, A.; Reyes-López, F.E.; Acevedo, M.L.; Alonso-Palomares, L.; Valiente-Echeverría, F.; Soto-Rifo, R.; Portillo, H.; Gatica, J.; Flores, I.; Nova-Lamperti, E.; et al. Evaluation of the immune response induced by CoronaVac 28-day schedule vaccination in a healthy population group. Front. Immunol. 2022, 12, 766278. [Google Scholar] [CrossRef]

| COVID-19 Prior to the Enrollment? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes % (n) | No % (n) | |||||||||
| Variable | Total (n) | AZD1222 | Ad26.COV2. S | BNT162b2 | CoronaVac | AZD1222 | Ad26.COV2. S | BNT162b2 | CoronaVac | |
| Sex | Male | 461 | 8.89 (16) | 41.67 (75) | 22.78 (41) | 26.67 (48) | 16.67 (45) | 22.59 (61) | 32.59 (88) | 28.15 (76) |
| Female | 844 | 9.12 (29) | 40.57 (129) | 27.67 (88) | 22.64 (72) | 12.93 (68) | 16.73 (88) | 47.91 (252) | 22.43 (118) | |
| Age group | 18–26 | 98 | - | 18.42 (7) | 23.68 (9) | 57.89 (22) | - | 21.67 (13) | 31.67 (19) | 46.67 (28) |
| 27–59 | 916 | 0.26 (1) | 46.56 (176) | 29.89 (113) | 23.28 (88) | 0.37 (2) | 24.35 (131) | 56.32 (303) | 18.96 (102) | |
| >60 | 313 | 49.44 (44) | 24.72 (22) | 7.87 (7) | 17.98 (16) | 49.55 (111) | 4.02 (9) | 12.95 (29) | 33.48 (75) | |
| Blood type | A | 349 | 7.74 (12) | 39.35 (61) | 26.45 (41) | 26.45 (41) | 15.43 (27) | 18.86 (33) | 38.29 (67) | 27.43 (48) |
| AB | 23 | 12.5 (1) | 12.5 (1) | 50 (4) | 25 (2) | 13.33 (2) | 20 (3) | 33.33 (5) | 33.33 (5) | |
| B | 116 | 6.67 (2) | 36.67 (11) | 33.33 (10) | 23.33 (7) | 9.3 (8) | 10.47 (9) | 47.67 (41) | 32.56 (28) | |
| O | 817 | 9.84 (30) | 42.95 (131) | 24.26 (74) | 22.95 (70) | 14.84 (76) | 20.31 (104) | 42.77 (219) | 22.07 (113) | |
| Without data | 22 | - | 14.29 (1) | - | 85.71 (6) | - | - | - | 100 (11) | |
| Socioeconomic stratum | Low | 601 | 10.79 (30) | 43.17 (120) | 12.95 (36) | 33.09 (92) | 19.81 (64) | 29.41 (95) | 21.98 (71) | 28.79 (93) |
| Medium | 642 | 6.76 (14) | 37.68 (78) | 44.44 (92) | 11.11 (23) | 11.26 (49) | 11.95 (52) | 60.69 (264) | 16.09 (70) | |
| High | 47 | - | - | 50 (1) | 50 (1) | - | - | 36.36 (16) | 63.64 (28) | |
| Without data | 37 | 5.88 (1) | 41.18 (7) | 0 () | 52.94 (9) | - | 30 (6) | - | 70 (14) | |
| Educational level | None | 7 | - | 66.67 (2) | - | 33.33 (1) | - | 75 (3) | - | 25 (1) |
| Primary | 166 | 21.74 (20) | 61.9 6(57) | - | 16.3 (15) | 80.77 (42) | - | - | 19.23 (10) | |
| High school | 373 | 11.41 (21) | 50 (92) | 9.78 (18) | 28.8 (53) | 27.13 (51) | 26.6 (50) | 12.77 (24) | 33.51 (63) | |
| College | 225 | 1.12 (1) | 32.58 (29) | 31.46 (28) | 34.83 (31) | 2.21 (3) | 25 (34) | 32.35 (44) | 40.44 (55) | |
| University | 205 | 3.51 (2) | 36.84 (21) | 33.33 (19) | 26.32 (15) | 6.76 (10) | 21.62 (32) | 40.54 (60) | 31.08 (46) | |
| Post-graduate | 322 | - | 4.23 (3) | 90.14 (64) | 5.63 (4) | 1.21 (3) | 3.24 (8) | 90.28 (223) | 5.26 (13) | |
| Without data | 29 | 11.11 (1) | 11.11 (1) | - | 77.78 (7) | 20 (4) | 20 (4) | - | 60 (12) | |
| Variable | p | PR (CI 0.95) (Unadjusted) | p | PR (CI 0.95) (Adjusted) | |
|---|---|---|---|---|---|
| Sex | Female | 0.018 | 2.343 (1.208–5.003) | 0.033 | 2.182 (1.109–4.712) |
| Male | 1 | 1 | |||
| Age group | >60 | 0.024 | 0.368 (0.139–0.811) | 0.730 | 1.188 (0.397–2.922) |
| <60 | 1 | 1 | |||
| Ad26.COV2.S | 0.059 | 0.399 (0.135–0.947) | 0.076 | 0.420 (0.142–1.001) | |
| AZD1222 | 0.008 | 0.068 (0.004–0.317) | 0.012 | 0.062 (0.003–0.392) | |
| BNT162b2 | 1 | 1 | |||
| CoronaVac | 0.155 | 0.548 (0.220–1.183) | 0.814 | 1.293 (0.068–7.750) | |
| Socioeconomic level | High | 1 | |||
| Low | 0.459 | 0.719 (0.270–1.601) | |||
| Booster | Yes | 0.678 | 0.10 (0.06–0.17) | ||
| No | 1 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malagón-Rojas, J.; Mercado-Reyes, M.; Toloza-Pérez, Y.G.; Galindo, M.; Palma, R.M.; Catama, J.; Bedoya, J.F.; Parra-Barrera, E.L.; Meneses, X.; Barbosa, J.; et al. Comparison of Anti-SARS-CoV-2 IgG Antibody Responses Generated by the Administration of Ad26.COV2.S, AZD1222, BNT162b2, or CoronaVac: Longitudinal Prospective Cohort Study in the Colombian Population, 2021/2022. Vaccines 2022, 10, 1609. https://doi.org/10.3390/vaccines10101609
Malagón-Rojas J, Mercado-Reyes M, Toloza-Pérez YG, Galindo M, Palma RM, Catama J, Bedoya JF, Parra-Barrera EL, Meneses X, Barbosa J, et al. Comparison of Anti-SARS-CoV-2 IgG Antibody Responses Generated by the Administration of Ad26.COV2.S, AZD1222, BNT162b2, or CoronaVac: Longitudinal Prospective Cohort Study in the Colombian Population, 2021/2022. Vaccines. 2022; 10(10):1609. https://doi.org/10.3390/vaccines10101609
Chicago/Turabian StyleMalagón-Rojas, Jeadran, Marcela Mercado-Reyes, Yesith G. Toloza-Pérez, Marisol Galindo, Ruth M. Palma, Jenssy Catama, Juan F. Bedoya, Eliana L. Parra-Barrera, Ximena Meneses, Juliana Barbosa, and et al. 2022. "Comparison of Anti-SARS-CoV-2 IgG Antibody Responses Generated by the Administration of Ad26.COV2.S, AZD1222, BNT162b2, or CoronaVac: Longitudinal Prospective Cohort Study in the Colombian Population, 2021/2022" Vaccines 10, no. 10: 1609. https://doi.org/10.3390/vaccines10101609






